Abstract
Neurocysticercosis, a parasitic infection of the human central nervous system caused by Taenia solium, is a leading cause of seizures. Seizures associated with neurocysticercosis are caused mainly by the host inflammatory responses to dying parasites in the brain parenchyma. We previously demonstrated sequential expression of Th1 cytokines in early-stage granulomas, followed by expression of Th2 cytokines in later-stage granulomas in murine cysticercosis. However, the mechanism leading to this shift in cytokine response in the granulomas is unknown. Neuropeptides modulate cytokine responses and granuloma formation in murine schistosomiasis. Substance P (SP) induces Th1 cytokine expression and granuloma formation, whereas somatostatin inhibits the granulomatous response. We hypothesized that neuropeptides might play a role in regulation of the granulomatous response in cysticercosis. To test this hypothesis, we compared expression of SP and expression of somatostatin in murine cysticercal granulomas by using in situ hybridization and immunohistochemistry. We also compared expression with granuloma stage. Expression of SP mRNA was more frequent in the early-stage granulomas than in the late-stage granulomas (34 of 35 early-stage granulomas versus 1 of 13 late-stage granulomas). By contrast, somatostatin was expressed primarily in later-stage granulomas (13 of 14 late-stage granulomas versus 2 of 35 early-stage granulomas). The median light microscope grade of SP mRNA expression in the early-stage granulomas was significantly higher than that in the late-stage granulomas (P = 0.008, as determined by the Wilcoxon signed rank test). By contrast, somatostatin mRNA expression was higher at later stages (P = 0.008, as determined by the Wilcoxon signed rank test). SP and somatostatin are therefore temporally expressed in granulomas associated with murine cysticercosis, which may be related to differential expression of Th1 and Th2 cytokines.
Neurocysticercosis is a parasitic infection of the human central nervous system caused by the helminth Taenia solium. The most common clinical manifestation of neurocysticercosis is seizures. Neurocysticercosis is now recognized as a leading cause of seizures worldwide (6, 22, 23, 40). Although there are cases of human neurocysticercosis in which symptoms occur after a short asymptomatic period, seizures in neurocysticercosis most often occur after an asymptomatic period lasting 4 to 5 years (8). Thus, infection of the central nervous system alone does not explain the symptoms. Individuals who die of other causes can have viable cysticerci in their central nervous systems. By contrast, cysticerci from patients with seizures invariably demonstrate a prominent inflammatory infiltrate. Thus, seizures likely result not from parasitic infection per se but from the chronic granulomatous host response to dying cysts (7, 12, 14, 27, 40). Antiparasite drugs can be used to kill the parasites, but they also may worsen symptoms by stimulating the host inflammatory response in response to the death of the organisms.
Murine Taenia crassiceps cysticercosis has been used as an experimental model for human T. solium cysticercosis (18, 20, 32, 36, 37). As in the human infection, live parasites produce little or no inflammation, whereas dying parasites initiate a chronic granulomatous reaction. We have previously studied and grouped granulomas that are associated with murine cysticercosis into four stages based on the histologic appearance of the degenerating parasite; the early stages are characterized by dying degenerating parasite remnants, and in the later stages there are no clearly identifiable parasites (29). Early-stage granulomas were also found to predominantly express Th1 cytokines, whereas interleukin-4 (IL-4) was expressed in the later granulomas (29). The mediators leading to initiation and control of the granulomatous inflammation are unknown.
Recent studies have shown that neuropeptides modulate the host immune response to parasites. Substance P (SP) is a short polypeptide involved in pain transmission (it is especially associated with inflammation) (17). SP is made by nerves, endothelial cells, and cells of the immune system. Receptors for SP are widely distributed throughout the body on neurons, endothelial cells, and immunocytes, such as lymphocytes and macrophages (5, 38). Binding of SP to its specific receptor is required for the maximal granulomatous response in murine schistosomiasis (3). In addition, SP stimulates the production of cytokines, including gamma interferon (IFN-γ) and proinflammatory cytokines like IL-1β, and tumor necrosis factor alpha (TNF-α) (1, 2, 13, 26, 31).
Somatostatin, another neuropeptide, counteracts the effects of SP and mediates analgesic effects by inhibiting the pain induced by noxious agents (15, 16, 25, 28, 30). Macrophages, after stimulation by IFN-γ and TNF-α, produce large amounts of somatostatin, while lymphocytes express somatostatin receptors (4, 9, 10, 39). Somatostatin analogues have anti-inflammatory activities and suppress the production of proinflammatory Th1 cytokines (19, 31). Schistosome-infected animals treated with somatostatin analogues form smaller granulomas (11). We therefore hypothesized that neuropeptides might play a role in regulation of the granulomatous response in cysticercosis. To test this hypothesis, we compared expression of the neuropeptides SP and somatostatin after infection at different granuloma stages.
MATERIALS AND METHODS
In vivo animal studies.
Six- to 10-week-old female BALB/c mice were inoculated with 10 cysts of the ORF strain of T. crassiceps suspended in Hanks balanced salt solution (T. crassiceps cysts were kindly provided by Raymond E. Kuhn, Wake Forest University). After 3 to 9 months, eight heavily parasitized mice were sacrificed. Granulomas associated with parasites were identified visually and removed from the peritoneal cavity. Each granuloma was sectioned into two portions. One portion was immediately fixed in 4% paraformaldehyde for histological staging and examination of mRNA of the different bioactive mediators. The other portion was frozen immediately in freezing media for examination of the SP and somatostatin protein. The portion of each granuloma that was fixed with 4% paraformaldehyde (prepared in diethyl pyrocarbonate-treated phosphate-buffered saline) was stored in 70% alcohol until it was sectioned. The sections were used for in situ hybridization studies and histological staging analysis performed by a previously described method (29). This study was approved by the Animal Research Committee at Baylor College of Medicine.
Granuloma staging.
Histologic stages of the granulomas were determined as follows. Stage 1 granulomas showed areas of histologically intact tegument of dead parasites and other areas where there was infiltration of host cells. Stage 2 granulomas displayed no areas of normal tegument of the parasite with intact morphology of dead parasites, including a cyst cavity with infiltration of lymphocytes. In stage 3 granulomas there was no evidence of a parasitic cyst cavity, but there was a suggestion of the underlying degenerating parasite morphology and there was complete infiltration of host mononuclear cells. Stage 4 granulomas had no clearly identifiable parasite elements and consisted of only host cells and debris.
Cytokine cDNAs, plasmids, and preparation of 35S-labeled riboprobes.
pBluescript SK(−) plasmids containing cDNA for murine SP precursor (preprotachykinin) and somatostatin (provided by J. V. Weinstock, University of Iowa) were prepared by using ion-exchange chromatography (Qiagen Inc., Chatsworth, Calif.) and a previously standardized protocol (29, 41). The plasmid cDNA were linearized with appropriate restriction enzymes. Antisense and sense RNA probes were synthesized by in vitro transcription by using T3 or T7 polymerases along with 250 μCi of 35S-labeled UTP, 20 U of RNasin, 0.5 mM unlabeled ATP, 0.5 mM unlabeled GTP, 0.5 mM unlabeled CTP, 0.1 M dithiothreitol, and transcription buffers in diethyl pyrocarbonate-treated water, using a commercial kit (Amersham Life Science, Inc., Arlington Heights, Ill.) (29, 41). The template was digested with RNase-free DNase; the labeled probe was precipitated with ethanol and salt, washed with alcohol, and suspended in dithiothreitol.
In situ hybridization.
In situ hybridization was performed with sections made from granulomas obtained from T. crassiceps-infected BALB/c mice as described previously (29, 41). The paraffin in paraffin-embedded sections of granulomas was removed by treatment with xylene, and the sections were rehydrated with decreasing concentrations of ethanol (90 to 70% ethanol). Sections were incubated with the prehybridization buffer for 1 h and then with the 35S-UTP-labeled probe in an in situ hybridization cocktail for 3 h. Adjacent serial sections were examined with antisense (experimental) and sense (negative control) probes. After hybridization, the slides were washed twice with 2× SSC (1× SSC is 0.15 M sodium chloride plus 0.015 M sodium citrate), incubated for 20 min with 50% formamide in 2× SSC, washed six times in decreasing concentrations of 2× SSC, and digested with RNase (37°C, 30 min) to remove nonhybridized probe. The slides were then immersed in autoradiographic emulsion NTB2 (Kodak Eastman Co., Rochester, N.Y.) for 48 h at 24°C, developed with Kodak Dektol developer, fixed with Kodak fixer, and counterstained with Giemsa stain. The optimal concentration of probe that gave a positive signal with minimal background was assessed for each probe by using murine brain sections as a positive control. The sense strand of each probe was used as a negative control. Following in situ hybridization, the slides were examined by bright-field microscopy, and the number of cells overlaid with numerous silver granules was expressed as follows: 1+ (one or two positive cells per slide), 2+ (more than two positive cells per slide but less than one positive cell per low-power field [×20 lens]), 3+ (about one positive cell per low-power field but less than one positive cell per high-power field [×40 lens]), or 4+ (one or more positive cells per high-power field).
Immunohistochemistry.
Immunoperoxidase staining was performed on 5-μm-thick frozen or paraformaldehyde-fixed granuloma sections by using the avidin-biotin method, an automated immunostainer (Biogenex), and polyclonal rabbit antibody to murine SP (1:20; Chemicon, Temecula, Calif.) and somatostatin (1:50; Chemicon). Murine brain tissue sections were used as a positive control. Slides were considered positive if brown staining was noted within the cytoplasm of cells at a level above the level of the nonspecific signal in tissue cells. Positive slides were graded on a scale from 1+ to 3+ (1+, <10% of the leukocytes were stained; 2+, 10 to 20% of the leukocytes were stained; 3+, >20% of the leukocytes were stained).
RESULTS
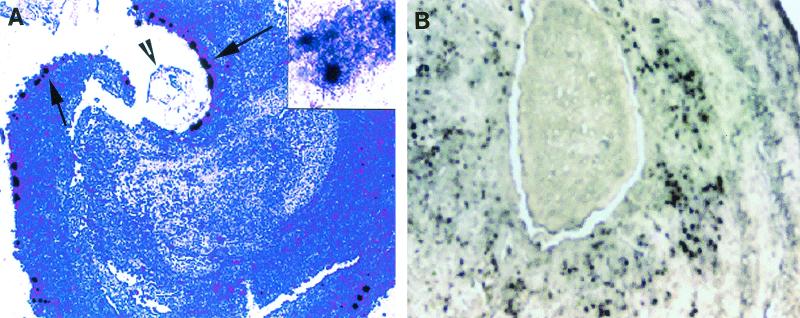
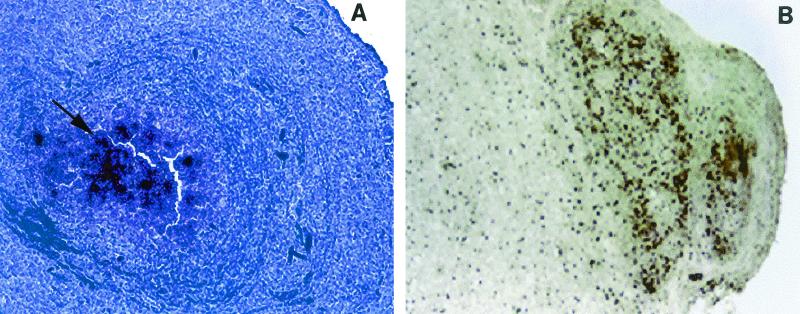
Eight T. crassiceps-infected mice with 49 granulomas were studied. Nineteen granulomas were at stage 1, 16 granulomas were at stage 2, 6 granulomas were at stage 3, and 8 granulomas were at stage 4. In the present study, since the neuropeptide expression patterns seen in stage 1 and 2 granulomas do not differ significantly from one another and, similarly, the neuropeptide expression patterns seen in stage 3 and 4 granulomas do not differ significantly from one another, we grouped stage 1 and 2 granulomas together as early-stage granulomas and stage 3 and 4 granulomas together as late-stage granulomas. SP and somatostatin mRNA were expressed in the granulomatous inflammations surrounding the dying cysts of T. crassiceps. SP mRNA was located primarily in the cells along the interface between the parasite and the host in early-stage granulomas (Fig 1A). Somatostatin was found primarily in the late-stage granulomas in cells resembling monocytes (Fig 2A).
FIG. 1.
Granuloma sections probed by in situ hybridization with 35S-labeled riboprobes for SP precursor. (A) Stage 1 granuloma surrounding a section of a T. crassiceps cyst, showing numerous positive cells after the section was probed with an antisense probe for SP precursor mRNA. The arrows indicate positive cells overlaid with multiple silver granules; the arrowhead indicates an intact cyst showing intact tegument, loose subtegmental tissue, and a central cyst cavity. Original magnification, ×100. The inset is a close-up of a few positive cells overlaid with multiple silver granules (original magnification, ×400). (B) Early-stage granuloma (stage 1), showing numerous positive cells expressing SP protein as revealed by immunohistochemistry. Original magnification, ×400.
FIG. 2.
Granuloma sections probed by in situ hybridization with 35S-labeled riboprobes for somatostatin. (A) Late-stage granuloma (stage 3), showing numerous positive cells after the section was probed with an antisense probe for somatostatin mRNA. The arrow indicates positive cells overlaid with multiple silver granules. Magnification, ×100. (B) Late-stage granuloma (stage 3), showing numerous positive cells expressing somatostatin protein as revealed by immunohistochemistry. Original magnification, ×200.
Expression of SP mRNA was significantly more frequent in the early granuloma stages than in the late granuloma stages; 34 of the 35 early-stage granulomas expressed SP precursor mRNA, whereas only 1 of the 13 late-stage granulomas that were studied for expression of SP precursor was positive. The median light microscope grade for SP mRNA expression in the early granulomas (stages 1 and 2) was significantly higher than the median light microscope grade for SP mRNA expression in the late granulomas (stages 3 and 4) (P = 0.008, as determined by the Wilcoxon signed rank test) (Table 1). In contrast, somatostatin mRNA was more frequent in late granuloma stages than in early granuloma stages. Of 14 late-stage granulomas, 13 expressed somatostatin, compared to 2 of 35 early-stage granulomas. The median light microscope grade for somatostatin mRNA expression in the late-stage granulomas was significantly higher than the median light microscope grade for somatostatin mRNA expression in the early-stage granulomas (P = 0.008, as determined by the Wilcoxon signed rank test) (Table 1).
TABLE 1.
Correlation of SP and somatostatin mRNA expression with granuloma stagea
| Animal | SP mRNA expression score
|
Somatostatin mRNA expression score
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stages 1 and 2
|
Stages 3 and 4
|
Stages 1 and 2
|
Stages 3 and 4
|
|||||||||
| Median | n | Range | Median | n | Range | Median | n | Range | Median | n | Range | |
| 1 | 2b | 5 | 0-2 | 0 | 2 | 0 | 0 | 5 | 0 | 2.5c | 2 | 1-4 |
| 2 | 3 | 11 | 1-4 | 0 | 2 | 0 | 0 | 11 | 0-1 | 2.0 | 2 | 0-4 |
| 3 | 1 | 3 | 1-2 | 0 | 1 | 0 | 0 | 3 | 0 | 1 | 1 | 1 |
| 4 | 1.5 | 4 | 1-2 | 0.5 | 2 | 0-1 | 0 | 4 | 0-1 | 1 | 2 | 1 |
| 5 | 1 | 5 | 1-4 | 0 | 1 | 0 | 0 | 5 | 0 | 1 | 1 | 1 |
| 6 | 2.5 | 2 | 1-4 | 0 | 2 | 0 | 0 | 2 | 0 | 1 | 2 | 1 |
| 7 | 4 | 2 | 4 | 0 | 2 | 0 | 0 | 2 | 0 | 2.5 | 2 | 1-4 |
| 8 | 3 | 3 | 2-4 | 0 | 1 | 0 | 0 | 3 | 0 | 1 | 2 | 1 |
Slides were examined by bright-field microscopy, and the number of positive cells expressing SP or somatostatin mRNA were counted and scored as follows: 0, no positive cells on the entire slide; 1+, one or two positive cells per slide; 2+, more than two positive cells per slide but less than one positive cell per low-power field (×20 lens); 3+, about one positive cell per low-power field but less than one positive cell per high-power field (×40 lens); or 4+, one or more positive cells per high-power field. The median light microscope scores for SP and somatostatin mRNA expression were calculated for the early stages (stages 1 and 2) and the late stages (stages 3 and 4) for each animal. The differences between the median scores for the early and late stages were compared by using the Wilcoxon signed rank test.
P = 0.008 (Wilcoxon signed rank test) for expression of SP mRNA in early versus late stages.
P = 0.008 (Wilcoxon signed rank test) for expression of somatostatin mRNA in late versus early stages.
To confirm that mRNA expression correlated with synthesis of the mature protein, representative frozen sections of granulomas from each of the four stages were studied by immunohistochemistry by using polyclonal antibody to SP or somatostatin (Chemicon). The two early-stage granulomas both expressed SP (Fig. 1B), whereas the two late-stage granulomas did not express SP. By contrast, both the late-stage granulomas expressed somatostatin protein (Fig. 2B), whereas both the early-stage granulomas did not.
DISCUSSION
We observed differential expression of the neuropeptides SP and somatostatin in the granulomatous inflammations surrounding dying cysts of T. crassiceps. SP was preferentially expressed in early-stage granulomas, whereas somatostatin was expressed mainly in the late stages. These results suggest that SP and somatostatin may modify granulomatous inflammatory responses in cysticercosis. In murine schistosomiasis, normal induction of granulomatous inflammation requires binding of SP to its specific receptor (3). SP stimulates the production of cytokines, including IFN-γ, and proinflammatory cytokines like IL-1β, as well as TNF-α (1, 2, 13, 26, 31). Thus, SP is thought to be an important upregulator of the granulomatous response. By contrast, somatostatin analogues suppress the production of proinflammatory Th1 cytokines (19, 31). Infected animals treated with somatostatin analogues form smaller granulomas (11). This leads to a paradigm in which early expression of SP causes enhancement of the granulomatous response. As this response progresses, somatostatin is expressed, which in turn downregulates the response.
Significantly, a similar pattern of sequential expression of these neuropeptides occurred in our cysticercosis model. The association of SP with early-stage granulomas suggests that SP may play a similar role in cysticercosis by inducing early granuloma formation. The expression of somatostatin later is consistent with an anti-inflammatory role in the late stages of granuloma formation. While the present study was performed with heavily parasitized mice, we expect the same results for mice that are less heavily parasitized. In our previous studies, we noted that early-stage granulomas expressed predominantly Th1 cytokines, whereas Th2 cytokines were expressed only in the late stages (29). It is tempting to speculate that differential expression of SP and somatostatin in the early and late stages of granulomas may promote the Th1-to-Th2 cytokine shift in the granulomas surrounding dying T. crassiceps cysts.
Neuropeptides are known modulators of epileptogenic responses. SP can evoke epileptiform responses in neurons (24). Intrahippocampal administration of SP triggered status epilepticus in a process resembling human epilepsy (21). In contrast, somatostatin modulates classical neurotransmission and has anticonvulsant properties in experimental models of seizures (33-35). At present, the relevance of neuropeptides for the pathophysiology of neurocysticercosis is unknown. In preliminary studies, we have also observed induction of seizures in rodents by extracts derived from early murine cysticercal granulomas. Understanding which inflammatory modulators are present in the different stages of the granulomas, which mediators are epileptogenic, and which modulators inhibit seizure responses may open up possibilities for treatment of neurocysticercosis seizures with specific antagonists or analogues. Further studies are also required to demonstrate expression of neuropeptides in response to human infection with T. solium, within the central nervous system, and in association with seizures.
Acknowledgments
We thank Sherita Daniel for help with immunohistochemistry.
This study was supported by the Baylor Center for AIDS Research Core Support (grant AI36211 from the National Institute of Allergy and Infectious Diseases).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Berczi, I., I. M. Chalmers, E. Nagy, and R. J. Warrington. 1996. The immune effects of neuropeptides. Baillieres Clin. Rheumatol. 10:227-257. [DOI] [PubMed] [Google Scholar]
- 2.Blum, A. M., D. E. Elliott, A. Metwali, J. Li, K. Qadir, and J. V. Weinstock. 1998. Substance P regulates somatostatin expression in inflammation. J. Immunol. 161:6316-6322. [PubMed] [Google Scholar]
- 3.Blum, A. M., A. Metwali, M. Kim-Miller, J. Li, K. Qadir, D. E. Elliott, B. Lu, Z. Fabry, N. Gerard, and J. V. Weinstock. 1999. The substance P receptor is necessary for a normal granulomatous response in murine schistosomiasis mansoni. J. Immunol. 162:6080-6085. [PubMed] [Google Scholar]
- 4.Blum, A. M., A. Metwali, R. C. Mathew, G. Cook, D. Elliott, and J. V. Weinstock. 1992. Granuloma T lymphocytes in murine schistosomiasis mansoni have somatostatin receptors and respond to somatostatin with decreased IFN-gamma secretion. J. Immunol. 149:3621-3626. [PubMed] [Google Scholar]
- 5.Cook, G. A., D. Elliott, A. Metwali, A. M. Blum, M. Sandor, R. Lynch, and J. V. Weinstock. 1994. Molecular evidence that granuloma T lymphocytes in murine schistosomiasis mansoni express an authentic substance P (NK-1) receptor. J. Immunol. 152:1830-1835. [PubMed] [Google Scholar]
- 6.Cruz, M. E., P. M. Schantz, I. Cruz, P. Espinosa, P. M. Preux, A. Cruz, W. Benitez, V. C. Tsang, J. Fermoso, and M. Dumas. 1999. Epilepsy and neurocysticercosis in an Andean community. Int J. Epidemiol. 28:799-803. [DOI] [PubMed] [Google Scholar]
- 7.Cuetter, A. C., J. Garcia-Bobadilla, L. G. Guerra, F. M. Martinez, and B. Kaim. 1997. Neurocysticercosis: focus on intraventricular disease. Clin. Infect Dis. 24:157-164. [DOI] [PubMed] [Google Scholar]
- 8.Dixon, H. B. F., and F. M. Lipscomb. 1961. Cysticercosis: an analysis and follow-up of 450 cases, vol. 299. Her Majesty's Stationery Service, London, England.
- 9.Elliott, D. E., A. M. Blum, J. Li, A. Metwali, and J. V. Weinstock. 1998. Preprosomatostatin messenger RNA is expressed by inflammatory cells and induced by inflammatory mediators and cytokines. J. Immunol. 160:3997-4003. [PubMed] [Google Scholar]
- 10.Elliott, D. E., A. Metwali, A. M. Blum, M. Sandor, R. Lynch, and J. V. Weinstock. 1994. T lymphocytes isolated from the hepatic granulomas of schistosome-infected mice express somatostatin receptor subtype II (SSTR2) messenger RNA. J. Immunol. 153:1180-1186. [PubMed] [Google Scholar]
- 11.Elliott, D. E., and J. V. Weinstock. 1996. Granulomas in murine schistosomiasis mansoni have a somatostatin immunoregulatory circuit. Metabolism 45:88-90. [DOI] [PubMed] [Google Scholar]
- 12.Escobar, A. 1983. The pathology of neurocysticercosis, p. 27-54. In E. Palacios, J. Rodriguez-Carbajal, and J. Taveras (ed.), Cysticercosis of the central nervous system. Charles C Thomas, Publisher, Springfield, Ill.
- 13.Gordon, D. J., L. S. Ostlere, and C. A. Holden. 1997. Neuropeptide modulation of Th1 and Th2 cytokines in peripheral blood mononuclear leucocytes in atopic dermatitis and non-atopic controls. Br. J. Dermatol. 137:921-927. [PubMed] [Google Scholar]
- 14.Gutierrez, Y. 1990. Diagnostic pathology of parasitic infection with clinical correlation, p. 432-459. Lea and Febiger, Philadelphia, Pa.
- 15.Helyes, Z., E. Pinter, J. Szolcsanyi, and J. Horvath. 1996. Anti-inflammatory and antinociceptive effect of different somatostatin-analogs. Neurobiology 4:115-117. [PubMed] [Google Scholar]
- 16.Helyes, Z., M. Than, G. Oroszi, E. Pinter, J. Nemeth, G. Keri, and J. Szolcsanyi. 2000. Anti-nociceptive effect induced by somatostatin released from sensory nerve terminals and by synthetic somatostatin analogues in the rat. Neurosci. Lett. 278:185-188. [DOI] [PubMed] [Google Scholar]
- 17.Honore, P., S. D. Rogers, M. J. Schwei, J. L. Salak-Johnson, N. M. Luger, M. C. Sabino, D. R. Clohisy, and P. W. Mantyh. 2000. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience 98:585-598. [DOI] [PubMed] [Google Scholar]
- 18.Kunz, J., B. Kalinna, V. Watschke, and E. Geyer. 1989. Taenia crassiceps metacestode vesicular fluid antigens shared with the Taenia solium larval stage and reactive with serum antibodies from patients with neurocysticercosis. Zentralbl. Bakteriol. 271:510-520. [DOI] [PubMed] [Google Scholar]
- 19.Lamrani, A., M. Tulliez, L. Chauvelot-Moachon, S. Chaussade, C. Mauprivez, A. M. Hagnere, and N. Vidon. 1999. Effects of octreotide treatment on early TNF-alpha production and localization in experimental chronic colitis. Aliment. Pharmacol. Ther. 13:583-594. [DOI] [PubMed] [Google Scholar]
- 20.Larralde, C., R. M. Montoya, J. Sotelo, J. Hayunga, E. Sciutto, G. Palencia, A. Padilla, T. Govensky, and M. L. Diaz. 1990. Murine T. crassiceps antigens in immunodiagnosis of T. solium human neurocysticercosis, T. saginata bovine cysticercosis, and human E. granulosus hydatidosis. Bull. Soc. Fr. Parasitol. 8:6-8.
- 21.Liu, H., A. M. Mazarati, H. Katsumori, R. Sankar, and C. G. Wasterlain. 1999. Substance P is expressed in hippocampal principal neurons during status epilepticus and plays a critical role in the maintenance of status epilepticus. Proc. Natl. Acad. Sci. USA 96:5286-5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medina, M. T., E. Rosas, F. Rubio-Donnadieu, and J. Sotelo. 1990. Neurocysticercosis as the main cause of late-onset epilepsy in Mexico. Arch. Intern. Med. 150:325-327. [PubMed] [Google Scholar]
- 23.Murthy, J. M., and R. Yangala. 1998. Etiological spectrum of symptomatic localization related epilepsies: a study from South India. J. Neurol. Sci. 158:65-70. [DOI] [PubMed] [Google Scholar]
- 24.Nalivaiko, E., J. C. Michaud, P. Soubrie, G. Le Fur, and P. Feltz. 1997. Tachykinin neurokinin-1 and neurokinin-3 receptor-mediated responses in guinea-pig substantia nigra: an in vitro electrophysiological study. Neuroscience 78:745-757. [DOI] [PubMed] [Google Scholar]
- 25.Paice, J. A., R. D. Penn, and J. S. Kroin. 1996. Intrathecal octreotide for relief of intractable nonmalignant pain: 5-year experience with two cases. Neurosurgery 38:203-207. [DOI] [PubMed] [Google Scholar]
- 26.Rameshwar, P., and P. Gascon. 1995. Substance P (SP) mediates production of stem cell factor and interleukin-1 in bone marrow stroma: potential autoregulatory role for these cytokines in SP receptor expression and induction. Blood 86:482-490. [PubMed] [Google Scholar]
- 27.Riduara Sanz, C. 1987. Host response in childhood neurocysticercosis. Child's Nerv. Syst. 3:206-207. [DOI] [PubMed] [Google Scholar]
- 28.Ripamonti, C., S. Mercadante, L. Groff, E. Zecca, F. De Conno, and A. Casuccio. 2000. Role of octreotide, scopolamine butylbromide, and hydration in symptom control of patients with inoperable bowel obstruction and nasogastric tubes: a prospective randomized trial. J. Pain Symptom Manag. 19:23-34. [DOI] [PubMed] [Google Scholar]
- 29.Robinson, P., R. L. Atmar, D. E. Lewis, and A. C. White, Jr. 1997. Granuloma cytokines in murine cysticercosis. Infect. Immun. 65:2925-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruan, H. Z., X. C. Li, and W. Q. Cai. 1997. Somatostatin inhibited pain modulation action of substance P in spinal cord. Sheng Li Hsueh Pao 49:13-17. [PubMed] [Google Scholar]
- 31.Ryu, S., K. Jeong, W. Yoon, S. Park, B. Kang, S. Kim, B. Park, and S. Cho. 2000. Somatostatin and substance P induced in vivo by lipopolysaccharide and in peritoneal macrophages stimulated with lipopolysaccharide or interferon-gamma have differential effects on murine cytokine production. Neuroimmunomodulation 8:25-30. [DOI] [PubMed] [Google Scholar]
- 32.Sciutto, E., G. Fragoso, M. Baca, V. De la Cruz, L. Lemus, and E. Lamoyi. 1995. Depressed T-cell proliferation associated with susceptibility to experimental Taenia crassiceps infection. Infect. Immun. 63:2277-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tallent, M. K., and G. R. Siggins. 1999. Somatostatin acts in CA1 and CA3 to reduce hippocampal epileptiform activity. J. Neurophysiol. 81:1626-1635. [DOI] [PubMed] [Google Scholar]
- 34.Vezzani, A., and D. Hoyer. 1999. Brain somatostatin: a candidate inhibitory role in seizures and epileptogenesis. Eur J. Neurosci. 11:3767-3776. [DOI] [PubMed] [Google Scholar]
- 35.Vezzani, A., M. Rizzi, M. Conti, and R. Samanin. 2000. Modulatory role of neuropeptides in seizures induced in rats by stimulation of glutamate receptors. J. Nutr. 130:1046S-1048S. [DOI] [PubMed] [Google Scholar]
- 36.Villa, O., and R. Kuhn. 1991. Antigenic and immunogenic analysis of Taenia crassiceps and Taenia solium using sera from their natural intermediate hosts. ASB Bull. 39:99. [Google Scholar]
- 37.Villa, O. F., and R. E. Kuhn. 1996. Mice infected with the larvae of Taenia crassiceps exhibit a Th2-like immune response with concomitant anergy and downregulation of Th1-associated phenomena. Parasitology 112:561-570. [DOI] [PubMed] [Google Scholar]
- 38.Weinstock, J. V., A. Blum, J. Walder, and R. Walder. 1988. Eosinophils from granulomas in murine schistosomiasis mansoni produce substance P. J. Immunol. 141:961-966. [PubMed] [Google Scholar]
- 39.Weinstock, J. V., A. M. Blum, and T. Malloy. 1990. Macrophages within the granulomas of murine Schistosoma mansoni are a source of a somatostatin 1-14-like molecule. Cell. Immunol. 131:381-390. [DOI] [PubMed] [Google Scholar]
- 40.White, A. C., Jr. 2000. Neurocysticercosis: updates on epidemiology, pathogenesis, diagnosis, and management. Annu. Rev. Med. 51:187-206. [DOI] [PubMed] [Google Scholar]
- 41.White, A. C., P. Robinson, P. C. Okhuysen, D. E. Lewis, I. Shahab, S. Lahoti, H. L. DuPont, and C. L. Chappell. 2000. Interferon-gamma expression in jejunal biopsies in experimental human cryptosporidiosis correlates with prior sensitization and control of oocyst excretion. J. Infect. Dis. 181:701-709. [DOI] [PubMed] [Google Scholar]