Abstract
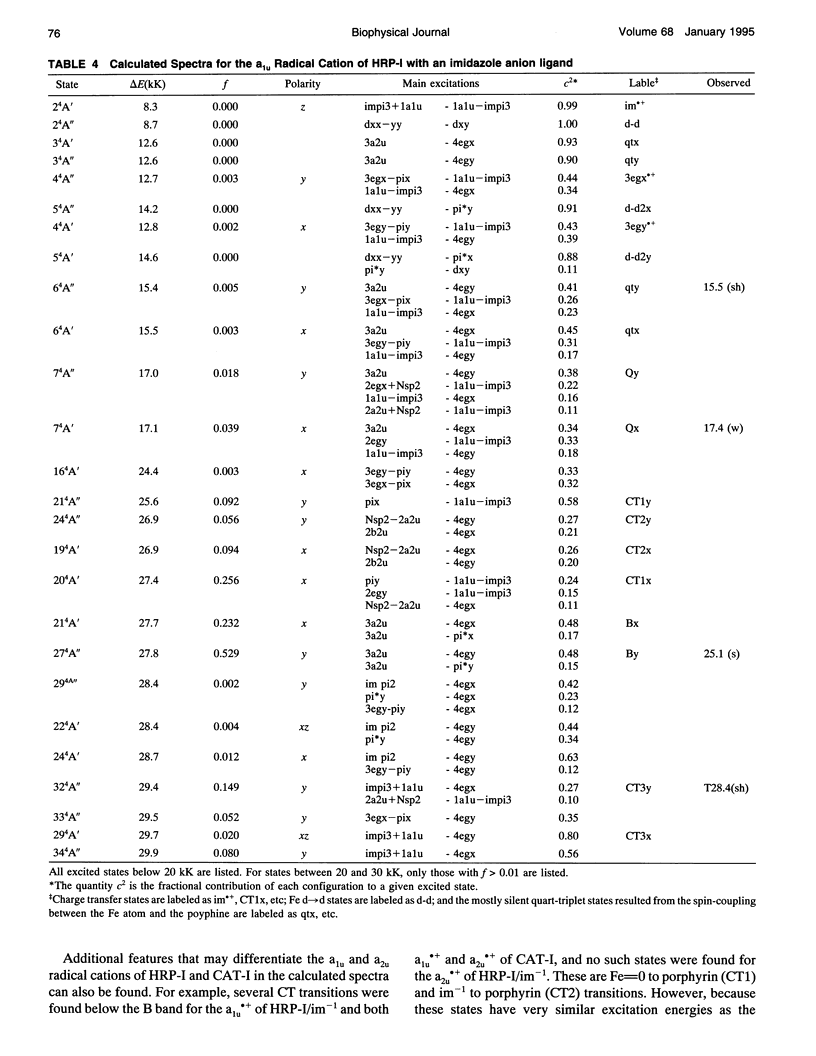
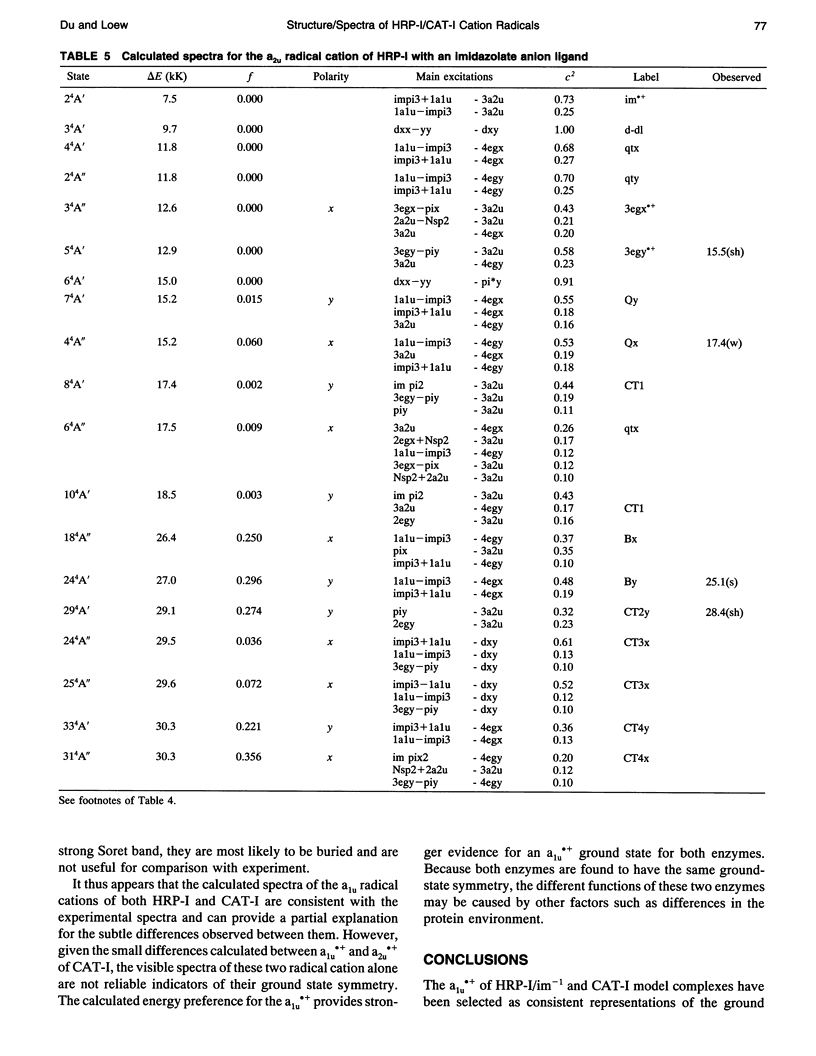
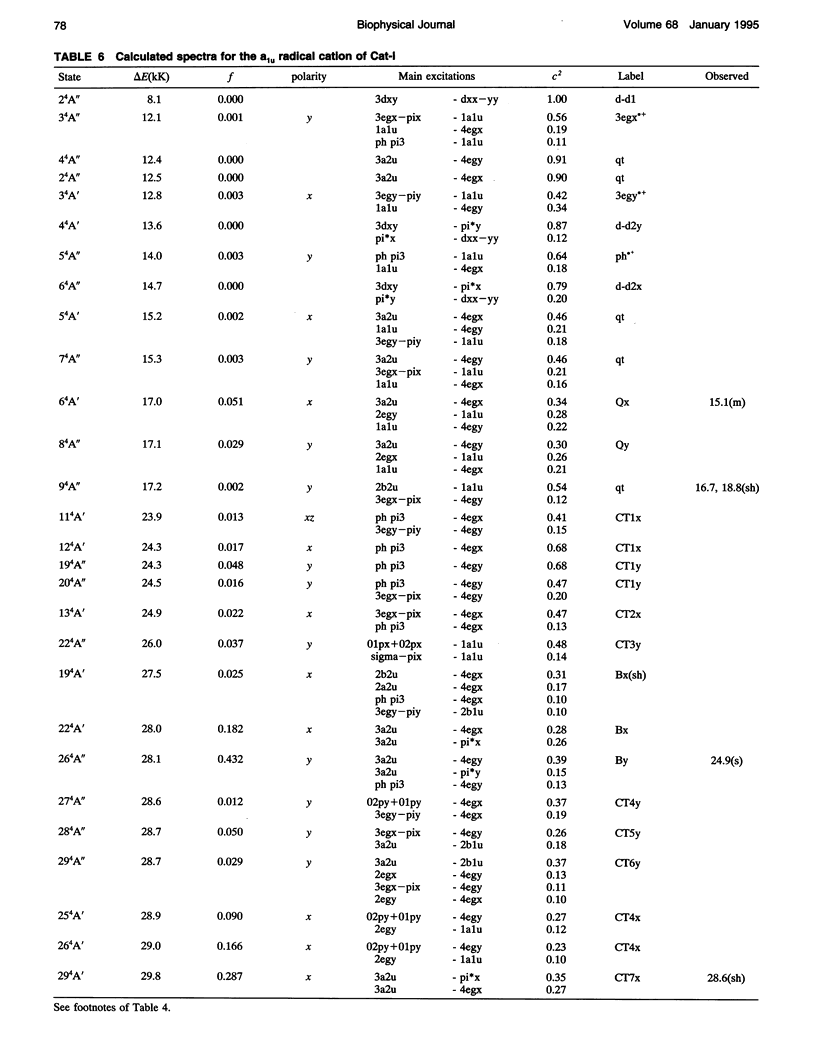
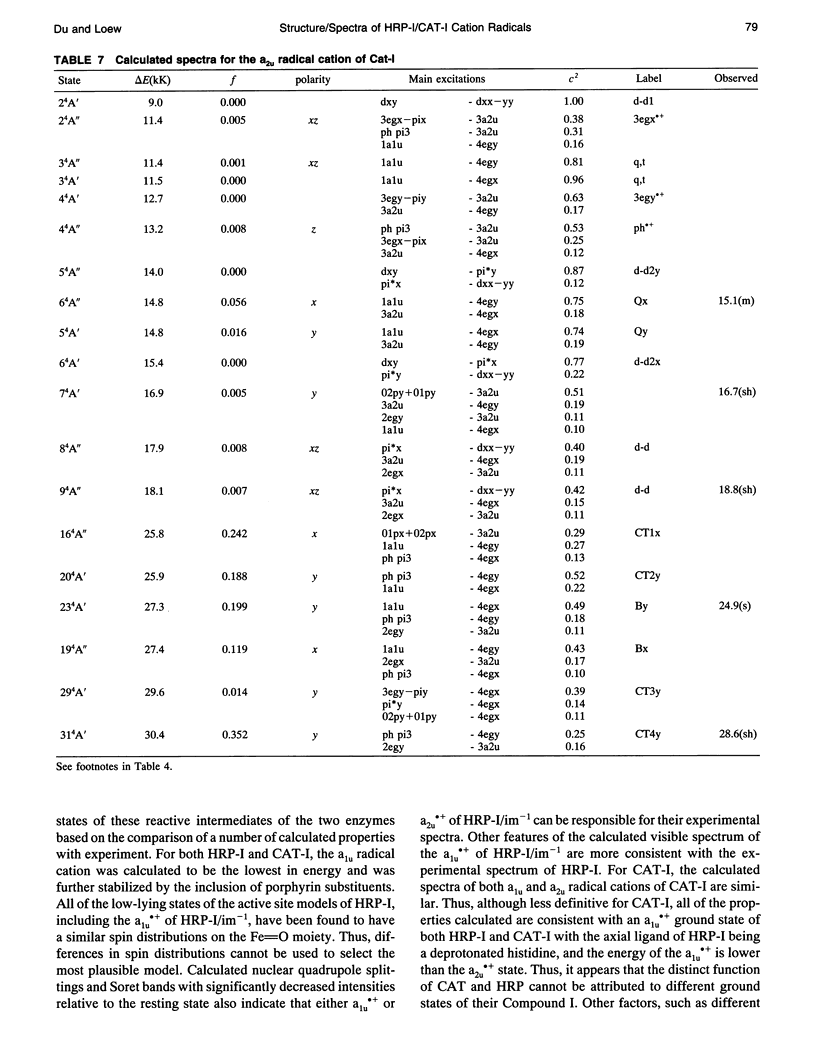
Theoretical studies of the electronic structure and spectra of models for the ferric resting state and Compound I intermediates of horseradish peroxidase (HRP-I) and catalase (CAT-I) have been performed using the INDO-RHF/CI method. The goals of these studies were twofold: i) to determine whether the axial ligand of HRP is best described as imidazole or imidazolate, and ii) to address the long-standing question of whether HRP-I and CAT-I are a1u and a2u tau cation radicals. Only the imidazolate HRP-I model led to a calculated electronic spectra consistent with the experimentally observed significant reduction in the intensity of the Soret band compared with the ferric resting state. These results provide compelling evidence for significant proton transfer to the conserved Asp residue by the proximal histidine. The origin of the observed reduction of the Soret band intensity in HRP-I and CAT-I spectra has been examined and found to be caused by the mixing of charge transfer transitions into the predominantly porphyrin tau-tau transitions. For both HRP-I and CAT-I, the a1u porphyrin tau cation state is the lowest energy, and it is further stabilized by both the anionic form of the ligand and the porphyrin ring substituents of protoporphyrin-IX. The calculated values of quadrupole-splitting observed in the Mossbauer resonance of HRP-I and CAT-I are similar for the a1u and a2u tau cation radicals. Electronic spectrum of the a1u tau cation radical of HRP-I are more similar to the observed spectra, whereas the spectra of both a1u tau and a2u tau cation radicals of CAT-I resemble the observed spectra. These results also indicate the limitations of using any one observable property to try to distinguish between these states. Taken together, comparison of calculated and observed properties indicate that there is no compelling reason to invoke the higher energy a2u tau cation radical as the favored state in HRP-I and CAT-I. Both ground-state properties and electronic spectra are consistent with the a1u tau cation radical.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Browlett W. R., Stillman M. J. Evidence for heme pi cation radical species in compound I of horseradish peroxidase and catalase. Biochim Biophys Acta. 1981 Jul 24;660(1):1–7. doi: 10.1016/0005-2744(81)90100-5. [DOI] [PubMed] [Google Scholar]
- Chance B., Powers L., Ching Y., Poulos T., Schonbaum G. R., Yamazaki I., Paul K. G. X-ray absorption studies of intermediates in peroxidase activity. Arch Biochem Biophys. 1984 Dec;235(2):596–611. doi: 10.1016/0003-9861(84)90234-0. [DOI] [PubMed] [Google Scholar]
- Chuang W. J., Van Wart H. E. Resonance Raman spectra of horseradish peroxidase and bovine liver catalase compound I species. Evidence for predominant 2A2u pi-cation radical ground state configurations. J Biol Chem. 1992 Jul 5;267(19):13293–13301. [PubMed] [Google Scholar]
- Dolphin D., Forman A., Borg D. C., Fajer J., Felton R. H. Compounds I of catalase and horse radish peroxidase: pi-cation radicals. Proc Natl Acad Sci U S A. 1971 Mar;68(3):614–618. doi: 10.1073/pnas.68.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fita I., Rossmann M. G. The NADPH binding site on beef liver catalase. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1604–1608. doi: 10.1073/pnas.82.6.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager L. P., Doubek D. L., Silverstein R. M., Hargis J. H., Martin J. C. Chloroperoxidase. IX. The structure of compound I. J Am Chem Soc. 1972 Jun 14;94(12):4364–4366. doi: 10.1021/ja00767a068. [DOI] [PubMed] [Google Scholar]
- Harrison J. E., Araiso T., Palcic M. M., Dunford H. B. Compound I of myeloperoxidase. Biochem Biophys Res Commun. 1980 May 14;94(1):34–40. doi: 10.1016/s0006-291x(80)80183-5. [DOI] [PubMed] [Google Scholar]
- La Mar G. N., Thanabal V., Johnson R. D., Smith K. M., Parish D. W. Deuterium NMR study of structural and dynamic properties of horseradish peroxidase. J Biol Chem. 1989 Apr 5;264(10):5428–5434. [PubMed] [Google Scholar]
- Moss T. H., Ehrenberg A., Bearden A. J. Mössbauer spectroscopic evidence for the electronic configuration of iron in horseradish peroxidase and its peroxide derivatives. Biochemistry. 1969 Oct;8(10):4159–4162. doi: 10.1021/bi00838a037. [DOI] [PubMed] [Google Scholar]
- Palcic M. M., Dunford H. B. The reaction of human erythrocyte catalase with hydroperoxides to form compound I. J Biol Chem. 1980 Jul 10;255(13):6128–6132. [PubMed] [Google Scholar]
- Palcic M. M., Rutter R., Araiso T., Hager L. P., Dunford H. B. Spectrum of chloroperoxidase compound I. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1123–1127. doi: 10.1016/0006-291x(80)90535-5. [DOI] [PubMed] [Google Scholar]
- Roberts J. E., Hoffman B. M., Rutter R., Hager L. P. Electron-nuclear double resonance of horseradish peroxidase compound I. Detection of the porphyrin pi-cation radical. J Biol Chem. 1981 Mar 10;256(5):2118–2121. [PubMed] [Google Scholar]
- Schulz C. E., Devaney P. W., Winkler H., Debrunner P. G., Doan N., Chiang R., Rutter R., Hager L. P. Horseradish peroxidase compound I: evidence for spin coupling between the heme iron and a 'free' radical. FEBS Lett. 1979 Jul 1;103(1):102–105. doi: 10.1016/0014-5793(79)81259-4. [DOI] [PubMed] [Google Scholar]
- Schulz C. E., Rutter R., Sage J. T., Debrunner P. G., Hager L. P. Mössbauer and electron paramagnetic resonance studies of horseradish peroxidase and its catalytic intermediates. Biochemistry. 1984 Sep 25;23(20):4743–4754. doi: 10.1021/bi00315a033. [DOI] [PubMed] [Google Scholar]


