Abstract
The secreted autotransporter toxin (Sat) of uropathogenic Escherichia coli exhibits cytopathic activity upon incubation with HEp-2 cells. We further investigated the effects of Sat on cell lines more relevant to the urinary tract, namely, those derived from bladder and kidney epithelium. Sat elicited elongation of cells and apparent loosening of cellular junctions upon incubation with Vero kidney cells. Additionally, incubation with Sat triggered significant vacuolation within the cytoplasm of both human bladder (CRL-1749) and kidney (CRL-1573) cell lines. This activity has been associated with only a few other known toxins. Following transurethral infection of CBA mice with a sat mutant, no reduction of CFU in urine, bladder, or kidney tissue was seen compared to that in mice infected with wild-type E. coli CFT073. However, significant histological changes were observed within the kidneys of mice infected with wild-type E. coli CFT073, including dissolution of the glomerular membrane and vacuolation of proximal tubule cells. Such damage was not observed in kidney sections of mice infected with a Sat-deficient mutant. These results indicate that Sat, a vacuolating cytotoxin expressed by uropathogenic E. coli CFT073, elicits defined damage to kidney epithelium during upper urinary tract infection and thus contributes to pathogenesis of urinary tract infection.
The urinary tract is a common site of bacterial infection, and Escherichia coli is by far the most common infecting agent at this site (22). In 1991, the last year for which a large database is available, urinary tract infections (UTI) were found to be responsible for nearly 10 million physician visits (unpublished data from the 1991 National Ambulatory Medical Care Survey, National Center for Health Statistics). UTI can involve the bladder, producing cystitis, or the kidneys, producing pyelonephritis. Acute suppurative inflammation of the kidneys occurs, usually via an ascending UTI. In otherwise healthy women, E. coli is the predominant cause of uncomplicated cystitis. Indeed, it is estimated that 40% of adult women will suffer symptoms of cystitis during their lifetimes, and E. coli will be identified as the etiologic agent in 75 to 80% of these cases. Acute pyelonephritis, a complication that involves the kidney, is also most commonly caused by strains of E. coli (39).
Virulent strains of E. coli that cause these infections display specific phenotypic traits. For example, P fimbriae, necessary for attachment to uroepithelial cells, and other fimbriae including S, F1C, and type 1, are commonly produced by uropathogenic strains. Such isolates also typically carry large blocks of genes, called pathogenicity-associated islands, not found in fecal isolates. These strains also secrete specific proteins that may contribute to pathogenesis of UTI. Among these proteins are the well-characterized hemolysin and the more recently described secreted autotransporter toxin (Sat). This serine protease, which is found predominantly in uropathogenic strains of E. coli (17), was previously shown by us to elicit cytopathic effects on cultured epithelial cells. We hypothesized that this autotransported toxin represented a new virulence determinant of uropathogenic E. coli.
Sat appears to fall within one subgroup of autotransporters recently classified as the SPATE (serine protease autotransporters of Enterobacteriaceae) family. There are eight known SPATE proteins: Sat of uropathogenic E. coli (17), Pet of enteroaggregative E. coli (12), EspC of enteropathogenic E. coli (37), Pic of enteroaggregative E. coli and Shigella (18), SigA of Shigella (2), SepA of Shigella (3), Tsh of avian E. coli (31), and EspP of enterohemorrhagic E. coli (6). SPATE autotransporters are identified by the presence of a serine protease active site motif in the passenger domain. It has been shown that the motif is necessary for phenotypic functions (e.g., adhesin, invasin, protease, or cytotoxin) as demonstrated by site-directed mutagenesis with Pet and Tsh (27, 36).
Interestingly, there have been no SPATE proteins identified in nonpathogenic organisms. The phenotypes of the known SPATEs represent functions typically associated with bacterial infection and imply the involvement of the proteins in bacterial pathogenesis. The first known SPATE, Tsh, was identified as a temperature-sensitive protein that displayed hemagglutination of chicken red blood cells and cleavage of hemoglobin (31). Recently, Tsh has been shown to be associated with lethal avian pathogenic E. coli isolates and to contribute to the development of lesions and fibrin deposition in the air sacs of chickens (11). The plasmid-encoded toxin Pet has been shown to induce damage to the intestinal mucosa as indicated by cell rounding and elongation. Similar cytopathic phenotypes have been seen with SigA and EspP. In addition, a SepA mutant lost the ability to elicit histological damage in a rabbit ligated-loop model (3). Pic has been shown to display mucinase activity as well as to cleave human complement (18). Finally, enterotoxic activity has been shown to be associated with the EspC autotransporter (21). All phenotypes associated with the SPATE proteins appear to be related to mechanisms that enable bacteria to damage the host or avoid an immune response.
Sat has been shown to elicit a vigorous antibody response (serum can be diluted 1:5,000 for detection of Sat by Western blotting) in mice transurethrally infected with the parent strain E. coli CFT073 (17). In addition, Sat exhibited cytopathic activity on HEp-2 cells (17). In this report, we demonstrate that Sat is a vacuolating cytotoxin for cultured mammalian bladder and kidney cells. Vacuolation and glomerular damage can also be assigned to Sat as demonstrated in an experimental infection using the CBA mouse model of ascending UTI.
MATERIALS AND METHODS
Bacterial strains.
E. coli CFT073 was isolated from the blood and urine of a female patient admitted to the University of Maryland Medical System for the treatment of acute pyelonephritis (25). This hly+ pap+ sfa+ pil+ strain is highly virulent in the CBA mouse model of ascending UTI (26) and is cytotoxic for cultured human renal proximal tubular epithelial cells (26). It is phenotypically positive for the production of P fimbriae, hemolysin, and type 1 fimbriae. Derivatives of E. coli CFT073 used in this study include E. coli CFT073 hlyD::TnphoA, a hemolysin-negative mutant (25), and E. coli CFT073 sat::pGP704, a Sat-deficient mutant for which Western blotting with rabbit antiserum to Sat confirms lack of synthesis of the protein (reference 17 and data not shown). E. coli HB101(pDG4) is a Sat overexpression clone (17), and E. coli HB101[pBluescript SK(−)] is a negative control strain.
Sat preparations.
Overnight broth cultures (100 ml) of E. coli CFT073 hlyD::TnphoA and its isogenic sat mutant were centrifuged (12,000 × g, 10 min, 4°C), and supernatants were filtered through 0.22-μm-pore-size filters. Supernatants were concentrated using 50,000-molecular weight-cutoff Centricon Plus-80 filters (Millipore) to a volume of 200 μl. Whole-protein concentration was determined using the bicinchoninic acid assay (Pierce).
Determination of cytopathic effects.
HEp-2 cells were propagated in humidified 5% CO2-95% air at 37°C in Dulbecco's modified Eagle's medium. Subconfluent cells were resuspended with 0.25% trypsin and 0.03% EDTA, plated (250 μl) into eight-well slides, and allowed to grow to 60% confluency. Vero primary kidney (CRL-1586) cells were cultured in Dulbecco's modified Eagle's medium-10% phosphate-buffered saline-4 mM l-glutamine adjusted to contain 1.5 g of sodium bicarbonate per liter and 4.5 g of glucose per liter. Human bladder epithelial (CRL-1749) cells were cultured in minimal essential medium containing Earle's balanced salt solution with 1 mM sodium pyruvate and 10% fetal bovine serum. Human kidney epithelial (CRL-1573) cells were cultured in minimal essential medium containing 10% fetal bovine serum, 2 mM l-glutamine, and Earle's balanced salt solution adjusted to contain 1.5 g of sodium bicarbonate per liter, 0.1 mM nonessential amino acids, and 1.0 mM sodium pyruvate.
Twenty-microliter volumes of concentrated supernatants containing Sat (5 to 100 μg of protein) were added directly to monolayers and incubated for 1 to 5 h at 37°C in a humidified atmosphere of 10% CO2-90% air. Medium was aspirated, and cell monolayers were washed three times with Hanks' balanced salt solution and fixed with 70% methanol. The cells were Giemsa stained, and an average of five fields per slide were analyzed by light microscopy.
Lactate dehydrogenase activity.
Concentrated supernatants of E. coli HB101(pDG4), HB101[pBluescript SK(−)], or CFT073 were incubated with various cell lines for 5 h. Supernatants were removed from the monolayer, and lysis was quantified by measuring lactate dehydrogenase release as outlined by the manufacturer (Roche).
Mouse model of ascending UTI.
Female CBA mice (6 to 8 weeks old) were transurethrally inoculated over a 30-s interval with 50 μl containing 108 CFU of either E. coli CFT073 or its isogenic sat mutant (17) through a sterile polyethylene catheter (0.28-mm internal diameter, 0.61-mm outer diameter, 25-mm length) which had been inserted into the bladder via the urethra while mice were anesthetized. Mice were sacrificed with an overdose of methoxyflurane at 4 days, 1 week, or 2 weeks after challenge. Kidney and bladder tissues were sectioned (5-μm thickness) and stained with hematoxylin and eosin for histological analysis by light microscopy.
RESULTS
Assessment of cytopathic activity of Sat on Vero cells.
As shown previously, the incubation of concentrated supernatants of E. coli HB101 overexpressing Sat with HEp-2 cells for 1 h or longer resulted in cytopathic changes, including elongation, rounding, and detachment of a proportion of the cells from the monolayer (17). Based upon the association of sat and its protein product with pyelonephritis isolates, we chose to further investigate the cytopathic effects of Sat by using cell lines more relevant to the urinary tract. Concentrated supernatants of positive control E. coli CFT073 hlyD::TnphoA (which does not secrete an active cytotoxic HlyA hemolysin [25] but does produce Sat), negative control E. coli HB101[pBluescript SK(−)], E. coli CFT073 sat::pGP704 (a Sat-deficient mutant), and E. coli HB101(pDG4) (which overexpresses Sat) (sodium dodecyl sulfate-polyacrylamide gel electrophoresis for each concentrated supernatant is shown in the inset in Fig. 1) were incubated with confluent monolayers of Vero kidney (ATCC CRL-1587), human adult bladder (ATCC CRL-1749), and human adult kidney (ATCC CRL-1573) epithelial cells for 2 h at 37°C. For Vero cells, the structural features of the cellular damage comprised swelling of the cell body and fragmentation of membranes upon incubation of supernatants of E. coli HB101(pDG4) (data not shown). A similar effect was seen when cells were incubated with supernatant of wild-type E. coli CFT073 hlyD::TnphoA but was absent upon incubation with supernatants of E. coli HB101[pBluescript SK(−)] or the Sat-deficient mutant E. coli CFT073 sat::pGP704. In contrast, cells appeared normal and showed no cytopathic effects when incubated with medium alone (data not shown).
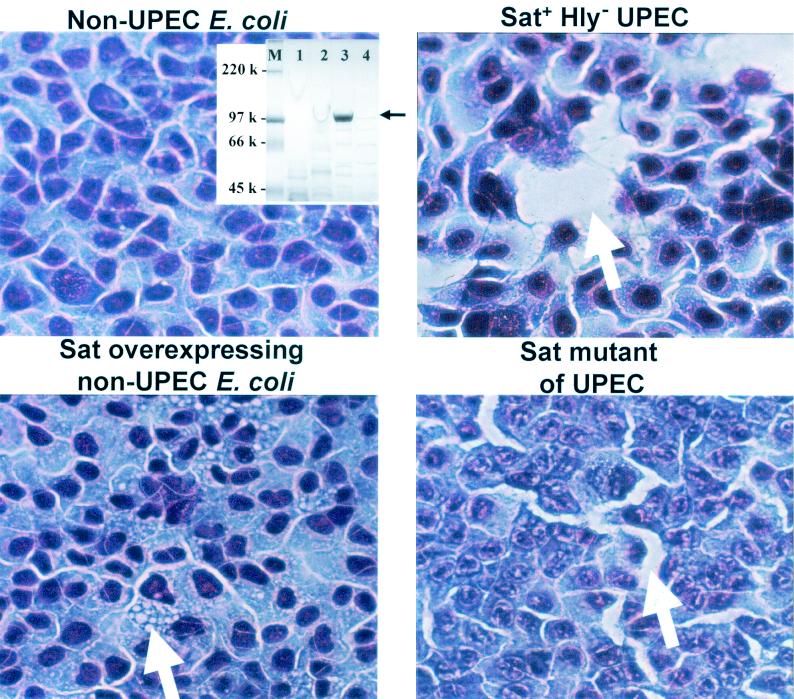
FIG. 1.
Cytopathic activity of Sat on human bladder cells. Concentrated culture supernatants (electrophoresed on sodium dodecyl sulfate-polyacrylamide gels [inset]) of E. coli HB101[pBluescript SK(−)] (lane 4, inset) (nonuropathogenic E. coli [non-UPEC]), E. coli CFT073 hlyD::TnphoA (lane 1, inset) (Sat+ Hly− UPEC), E. coli HB101(pDG4) (lane 3, inset) (Sat-overexpressing non-UPEC), and E. coli CFT073 sat::pGP704 (lane 2, inset) (Sat mutant of UPEC) were incubated with monolayers of human bladder cells for 2 h at 37°C. Supernatant was concentrated 100-fold and added in 20-μl volumes to 250-μl cell monolayers. The monolayers were washed, fixed, stained with Giemsa stain, and photographed at a magnification of ×100. Vacuolation (bottom left panel) and loosening of cellular junctions are indicated by arrows.
Cytoplasmic vacuolation of human bladder and kidney epithelial cells.
The incubation of human bladder (ATCC 1748) (Fig. 1) or human kidney (ATCC CRL-1573) (Fig. 2) cells with concentrated supernatant of E. coli HB101(pDG4) resulted in the formation of vacuoles within the cytoplasm of the urinary tract-derived epithelial cells. Vacuolation was also seen in the cytoplasm of cells incubated with wild-type E. coli CFT073 hlyD::TnphoA, although to a lesser degree (Fig. 1). In addition, an apparent loosening of cellular junctions was seen in cells incubated with wild-type E. coli CFT073 hlyD::TnphoA supernatant but not in cells incubated with E. coli HB101(pDG4) supernatant. Cells appeared as a normal monolayer when incubated with medium alone (data not shown) or E. coli HB101[pBluescript SK(−)] supernatant, but loosening of cellular junctions appeared to occur upon incubation with E. coli CFT073 sat::pGP704 supernatant (Fig. 1). These results suggest that the apparent loosening of cellular junctions is attributable to the activity of a factor(s) of E. coli CFT073 other than Sat (this activity must be confirmed, however, by electron microscopy). Cytoplasmic vacuolation of human bladder and kidney cells, however, appears to be due to the function of Sat alone.
FIG. 2.
Cytopathic activity of Sat on human kidney cells. Concentrated culture supernatants of E. coli HB101(pDG4) overexpressing Sat were incubated with monolayers of human kidney cells for 1 h at 37°C. Supernatant was concentrated 100-fold and added in 20-μl volumes to 250-μl cell monolayers. The monolayers were washed, fixed, stained with Giemsa stain, and photographed at a magnification of ×100. Vacuolation is indicated by arrows. No cytopathic effects were noted for supernatants from E. coli HB101[pBluescript SK(−)] and E. coli CFT073 sat::pGP704 (Sat mutant) (data not shown).
Time and dose dependence of Sat cytopathic activity.
We assessed the cytopathic activity of Sat over time upon incubation with Vero, bladder, and kidney cells. The results showed that after only 1 h of incubation of E. coli HB101(pDG4) supernatant with Vero cells, the monolayer was no longer intact, and cells displayed cellular elongation and cytoplasmic shrinking (data not shown). A similar effect was seen when cells were incubated with wild-type E. coli CFT073 hlyD::TnphoA supernatant but was absent upon incubation with medium alone, supernatant of E. coli HB101[pBluescript SK(−)], or supernatant of E. coli CFT073 sat::pGP704 (data not shown). After 5 h, cells incubated with E. coli HB101(pDG4) supernatant exhibited significant cellular detachment, as no monolayer remained attached. No significant changes were seen after overnight incubation compared to 5 h (data not shown).
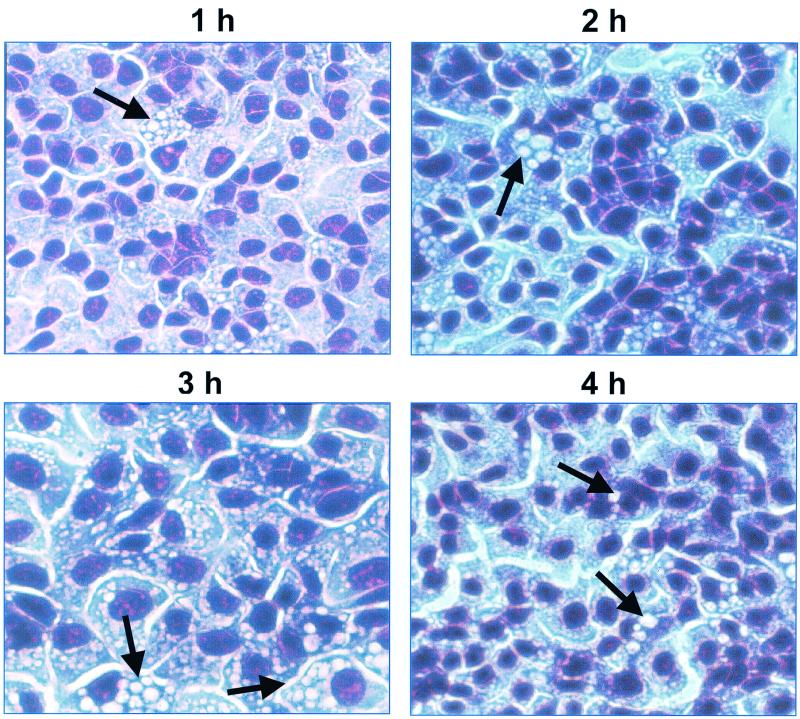
Incubation of Sat-containing culture filtrate from E. coli HB101(pDG4) with human bladder cells showed numerous small-sized vacuoles within the cytoplasm after 1 h and progressively larger and denser regions of vacuoles after 2 to 4 h (Fig. 3). Beyond 4 h, no additional cytopathic effects were evident (data not shown).
FIG. 3.
Time course of cytopathic effects of Sat on human bladder cells. Concentrated culture supernatants of E. coli HB101(pDG4) were incubated with monolayers of human bladder cells for 1 to 4 h at 37°C. The supernatant was concentrated 100-fold and added in 20-μl volumes to 250-μl cell monolayers. The monolayers were washed, fixed, stained with Giemsa stain, and photographed at a magnification of ×100. Arrows indicate areas of prominent vacuolation.
Incubation of Sat-containing culture filtrate from E. coli HB101(pDG4) with human kidney cells triggered formation of small vacuoles that were present throughout the entire cytoplasm after only 1 h of incubation. After 2 h, the majority of cells were detached but the remaining cells displayed denser regions of vacuoles that were much larger, similar to that seen with bladder cells (data not shown). These data suggest that Sat elicits toxicity at a much higher rate in kidney cells of human origin than in Vero cells. Although Sat elicited a similar vacuolating phenotype in both human kidney and bladder cells, the level of vacuolation and cellular damage occurred more rapidly in kidney cells.
We further investigated the cytopathic effects of Sat upon incubation with cells for 1 h at various Sat-containing culture filtrate E. coli HB101(pDG4) concentrations (5, 10, 25, 50, and 100 μg of protein/ml). Sat is the predominant protein (Fig. 1, inset, lane 3) in the filtrate. Incubation of Vero cells with Sat at 25 μg/ml or higher showed an apparent loosening of junctions and elongation (data not shown). Cells incubated with lower concentrations of Sat (5 or 10 μg/ml) appeared to be normal with no morphological changes (data not shown). Cells incubated with positive control wild-type E. coli CFT073 hlyD::TnphoA displayed an apparent loosening of cellular junctions and elongation (data not shown). Cells incubated with negative controls E. coli HB101[pBluescript SK(−)], E. coli CFT073 sat::pGP704, or medium alone appeared to be normal (data not shown). Vacuolation was seen in bladder and kidney epithelial cells incubated with all concentrations of Sat-containing culture filtrate (data not shown). The results indicate that Sat elicits vacuolization at lower concentrations in human bladder and kidney cells than in Vero cells.
Assessment of cell lysis after exposure to Sat.
To determine whether cellular lysis occurs upon incubation of Sat-containing culture filtrate with Vero, human bladder, and human kidney cells, the release of lactate dehydrogenase activity was measured after 5 h of incubation. No activity was seen in the supernatants of any of the three cell lines (data not shown), suggesting that Sat does not cause lysis of the target cell.
Histological analysis of mouse kidney tissue following infection with E. coli CFT073 and a sat::pGP704 mutant.
We determined whether histological changes of bladder or kidney cells occurred following experimental UTI of CBA mice with wild-type E. coli CFT073 compared to a sat::pGP704 mutant of E. coli CFT073. Mice were sacrificed at 4 days, 1 week, and 2 weeks postinfection, and bladder and kidney tissues were sectioned for microscopic examination using hematoxylin and eosin stain. The results showed significant histological changes, including dissolution of the glomerular membrane, loss of tubular epithelial cells, and cytoplasmic vacuolation in kidney tissue from mice following a 4-day infection with wild-type CFT073 (Fig. 4). Comparatively, the histological features of kidney tissue from mice infected with the sat::pGP704 mutant were similar to those of normal sections (Fig. 4). Similar results were seen after 1- and 2-week infections (data not shown). Bladder tissue from mice infected with wild-type E. coli CFT073 or the sat::pGP704 mutant appeared to be normal (data not shown). These data indicate that Sat elicits significant cytopathic changes specific to kidney cells following an experimental upper UTI.
FIG. 4.
Histological damage attributed to Sat following a 4-day infection of CBA mice with wild-type E. coli CFT073 and a sat::pGP704 mutant. Kidney tissue sections were prepared from mice following a 4-day infection with E. coli CFT073 or a sat mutant. normal, tissue isolated from uninfected mice. Sat mutant, kidney tissue from mice infected with a sat::pGP704 mutant. CFT073, kidney tissue from mice infected with E. coli CFT073. The glomerular membrane (G) is present in normal uninfected control mice (upper left panel) and mice infected with the sat mutant (upper right panel). Proximal tubules (PT) show a loss of epithelial cells in tissue from mice infected with CFT073 (lower right panel) but not in normal tissue or tissue from mice infected with the sat mutant (upper panels). Vacuolation (V) is faintly visible in kidney sections of mice infected with CFT073 (lower right panel).
DISCUSSION
Autotransporters such as Sat possess properties commonly associated with bacterial virulence (26). Examples include adhesins such as AIDA-1 (4) and Tsh (31), invasins such as Hap (38), proteases such as EspP (6) and SepA (3), and toxins such as VacA (9, 23), EspC (21), and Pet (12, 27). In this study, we demonstrate that Sat, the 107-kDa autotransported serine protease of uropathogenic E. coli CFT073, elicits cytopathic effects on human bladder, human kidney, and Vero kidney epithelial cells. The minimum effective concentrations of supernatant filtrate of the Sat-overexpressing clone E. coli HB101(pDG4) on the cell types used in this study were 5, 5, and 25 μg/ml for human bladder, human kidney, and Vero kidney epithelial cells, respectively. In these preparations, Sat was the predominant protein (Fig. 1, inset, lane 3). In cell culture, Sat triggers dramatic vacuolation within the cytoplasm of the urinary tract-derived epithelial cells. In experimental UTI involving the upper urinary tract, demonstrable histological damage attributable to Sat, particularly to glomeruli and proximal tubules, can be identified. These phenotypes support Sat as an important virulence factor in uropathogenic E. coli.
The cytopathic phenotype of Sat suggests that it may be a key player in the pathogenicity of E. coli CFT073 in the urinary tract. The ability of Sat to induce vacuolization within the cytoplasm of human cell lines of urinary tract origin is a novel discovery, considering that there is no other known vacuolating toxin of E. coli strains isolated from humans. Of particular interest is the induction of vacuolation following an experimental infection of CBA mice with E. coli CFT073. These results strongly suggest that Sat is involved in the pathogenesis of UTI caused by uropathogenic E. coli. Also interesting is the difference in the rate of appearance of vacuolation in human bladder versus human kidney cells upon incubation with Sat. Vacuolation was much more prominent in human kidney cells after only 1 hour, suggesting that kidney cells may be a preferred or more sensitive target of Sat. This is further supported by the lack of histological changes in bladder tissue compared to kidney tissue following an experimental infection of mice with E. coli CFT073. Based upon the significant cytopathic effects elicited both in vitro and following experimental infection, it seems likely that Sat is important in the ability of E. coli CFT073 to promote infection.
Based upon the association of the sat gene and its protein product with uropathogenic strains, the display of cytopathic activity on various cell lines, and the histological changes within kidney tissue incurred following an experimental infection, it appears justified to conclude that the 107-kDa autotransporter protein Sat is a virulence determinant which contributes to the pathogenicity of E. coli UTI. This is further supported by the ability of Sat to elicit strong antibody response during experimental infection in the CBA mouse model of ascending UTI (17). Although Sat is not required for colonization of the urinary tract, it seems likely that Sat elicits cytopathic activity that damages host tissue and may increase the ability of E. coli CFT073 to propagate. Indeed, one could speculate that specific damage to glomeruli and proximal tubules could facilitate entry of pyelonephritogenic strains into the bloodstream.
Vacuole formation in nucleated mammalian cells has been reported for a broad spectrum of bacterial pathogens. However, direct identification of causative agents for the vacuolation is scarce. By far the most investigated vacuolating agent is the VacA toxin of Helicobacter pylori (8, 30). Within 2 h of addition of H. pylori or purified VacA to sensitive cell types, activated VacA disrupts cytoskeletal architecture and focal adhesion, affecting the proper function of the epithelial barrier (28). Initiating around the nucleus, the vacuoles induced by VacA originate from massive swelling of membranous compartments of late stages of the endocytic pathway (33). The lumen of the vacuoles are acidified, as signified by uptake of the weak base neutral red (10), and acidification is through the activity of a membrane-bound vacuolar-type ATPase, which can be inhibited by the actions of bafilomycin A1 (29). Vibrio cholerae HlyA, a hemolysin, also acts upon susceptible cell membranes initially as a monomer, leading to formation of oligomeric pores in the membrane (13). Unlike VacA vacuoles, those caused by HlyA can appear within 15 min, are not inhibited by bafilomycin, and resist neutral red staining (13, 24). HlyA activity is similar to that of the aerolysin of Aeromonas hydrophila, which interacts with the cell membrane and forms heptameric pores but does not enter the cell cytosol. The vacuoles formed were derived from the endoplasmic reticulum, which appeared to be fragmented and vacuolated (1). Interestingly, aerolysin was reported to interact with a glycosylphosphatidylinositol-anchored protein, as defined its by lack of activity on phosphatidylinositol-specific phospholipase C-treated cells (1), a phenomenon also observed with VacA on treated HeLa cells (32). Serratia marcescens also produces a hemolysin, ShlA, which, once secreted via ShlB in the outer membrane and converted to the active form, caused vacuoles in several epithelial cell lines, which eventually led to cell lysis (19). Like aerolysin-induced vacuoles, ShlA vacuoles are visible within 15 min, are not acidic, and do not appear to be derived from late endosomes (19). ShlA not only formed pores in plasma membranes but at sublytic doses led to decreased levels of intracellular potassium ions and cellular ATP depletion (19). Other organisms known to cause vacuolation of nucleated cells include Alcaligenes faecalis (16), Vibrio parahaemolyticus (20), and various strains of E. coli (E. coli 0119 [34], E. coli 0157:H7 [14], and avian pathogen E. coli [5, 35]; however, the agent responsible for vacuolating activity was not identified. Many of the reported cases of vacuolation have activity only in specific cell types. In 1975, Fry and colleagues first published data indicating involvement of a pyelonephritis strain of E. coli in the formation of cytoplasmic vacuoles in renal tissue sections of experimentally infected rats. Although the direct cause of vacuolation was not identified, the lack of actual bacteria around sites of activity led to the assumption that a bacterial toxin was involved (15).
As previously reported, in 4-day, 1-week, and 2-week assessments of colonization in a murine model of ascending UTI, we measured no differences between the number of bacteria in the urine, bladders, and kidneys of mice infected with the wild-type and sat mutant strains (17). These results were not surprising considering previous data which showed a lack of attenuation with a hlyD::TnphoA mutant (25) and with a double mutant of both copies of the pap operon (encodes P fimbriae) in E. coli CFT073 (26). Furthermore, the only virulence factor of E. coli to date that has shown attenuation in the CBA mouse model is type 1 fimbriae (7). The ascending model shows similarity to human infection, including the infiltration of polymorphonuclear monocytes and establishment of bacteremia. However, it is possible that the mouse model does not completely reproduce all of the components of human disease. As a result, it cannot be conclusively determined if a lack of attenuation is based upon the insignificant contribution of the putative virulence factor to infection or the inadequacy of the mouse model.
Acknowledgments
This work was supported in part by Public Health Service grant AI43363 from the National Institutes of Health.
Editor: D. L. Burns
REFERENCES
- 1.Abrami, L., M. Fivaz, P. E. Glauser, R. G. Parton, and F. G. van der Goot. 1998. A pore-forming toxin interacts with a GPI-anchored protein and causes vacuolation of the endoplasmic reticulum. J. Cell Biol. 140:525-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al Hasani, K., I. R. Henderson, H. Sakellaris, K. Rajakumar, T. Grant, J. P. Nataro, R. Robins-Browne, and B. Adler. 2000. The sigA gene which is borne on the pathogenicity island of Shigella flexneri 2a encodes an exported cytopathic protease involved in intestinal fluid accumulation. Infect. Immun. 68:2457-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjelloun-Touimi, Z., P. J. Sansonetti, and C. Parsot. 1995. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol. Microbiol. 17:123-135. [DOI] [PubMed] [Google Scholar]
- 4.Benz, I., and M. A. Schmidt. 1989. Cloning and expression of an adhesin (AIDA-I) involved in diffuse adherence of enteropathogenic Escherichia coli. Infect. Immun. 57:1506-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanco, J. E., M. Blanco, A. Mora, and J. Blanco. 1997. Production of toxins (enterotoxins, verotoxins, and necrotoxins) and colicins by Escherichia coli strains isolated from septicemic and healthy chickens: relationship with in vivo pathogenicity. J. Clin. Microbiol. 35:2953-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunder, W., H. Schmidt, and H. Karch. 1997. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol. Microbiol. 24:767-778. [DOI] [PubMed] [Google Scholar]
- 7.Connell, I., W. Agace, P. Klemm, M. Schembri, S. Marild, and C. Svanborg. 1996. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl. Acad. Sci. USA 93:9827-9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cover, T. L. 1996. The vacuolating cytotoxin of Helicobacter pylori. Mol. Microbiol. 20:241-246. [DOI] [PubMed] [Google Scholar]
- 9.Cover, T. L., and M. J. Blaser. 1992. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J. Biol. Chem. 267:10570-10575. [PubMed] [Google Scholar]
- 10.Cover, T. L., W. Puryear, G. I. Perez-Perez, and M. J. Blaser. 1991. Effect of urease on HeLa cell vacuolation induced by Helicobacter pylori cytotoxin. Infect. Immun. 59:1264-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dozois, C. M., M. Dho-Moulin, A. Bree, J. M. Fairbrother, C. Desautels, and R. Curtiss III. 2000. Relationship between the Tsh autotransporter and pathogenicity of avian Escherichia coli and localization and analysis of the Tsh genetic region. Infect. Immun. 68:4145-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eslava, C., F. Navarro-Garcia, J. R. Czeczulin, I. R. Henderson, A. Cravioto, and J. P. Nataro. 1998. Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect. Immun. 66:3155-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Figueroa-Arredondo, P., J. E. Heuser, N. S. Akopyants, J. H. Morisaki, S. Giono-Cerezo, F. Enriquez-Rincon, and D. E. Berg. 2001. Cell vacuolation caused by Vibrio cholerae hemolysin. Infect. Immun. 69:1613-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fratamico, P. M., R. L. Buchanan, and P. H. Cooke. 1993. Virulence of an Escherichia coli O157:H7 sorbitol-positive mutant. Appl. Environ. Microbiol. 59:4245-4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fry, T. L., F. A. Fried, and B. A. Goven. 1975. Pathogenesis of pyelonephritis. Escherichia coli-induced renal ultrastructural changes. Investig. Urol. 13:47-51. [PubMed] [Google Scholar]
- 16.Gray, J. G., J. F. Roberts, R. C. Dillman, and D. G. Simmons. 1981. Cytotoxic activity of pathogenic Alcaligenes faecalis in turkey tracheal organ cultures. Am. J. Vet. Res. 42:2184-2186. [PubMed] [Google Scholar]
- 17.Guyer, D. M., I. R. Henderson, J. P. Nataro, and H. L. Mobley. 2000. Identification of Sat, an autotransporter toxin produced by uropathogenic Escherichia coli. Mol. Microbiol. 38:53-66. [DOI] [PubMed] [Google Scholar]
- 18.Henderson, I. R., J. Czeczulin, C. Eslava, F. Noriega, and J. P. Nataro. 1999. Characterization of Pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect. Immun. 67:5587-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hertle, R., M. Hilger, S. Weingardt-Kocher, and I. Walev. 1999. Cytotoxic action of Serratia marcescens hemolysin on human epithelial cells. Infect. Immun. 67:817-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoashi, K., K. Ogata, H. Taniguchi, H. Yamashita, K. Tsuji, Y. Mizuguchi, and N. Ohtomo. 1990. Pathogenesis of Vibrio parahaemolyticus: intraperitoneal and orogastric challenge experiments in mice. Microbiol. Immunol. 34:355-366. [DOI] [PubMed] [Google Scholar]
- 21.Kaper, J. B., J. L. Mellies, and J. P. Nataro. 1999. Pathogenicity islands and other mobile genetic elements of diarrheagenic Escherichia coli, p. 33-58. In J. B. Kaper and J. Hacker (ed.), Pathogenicity islands and other mobile virulence elements. ASM Press, Washington, D.C.
- 22.Kunin, C. M. 1987. Detection, prevention and management of urinary tract infections, 4th ed. Lea & Febiger, Philadelphia, Pa.
- 23.Leunk, R. D. 1991. Production of a cytotoxin by Helicobacter pylori. Rev. Infect. Dis. 13:S686-S689. [DOI] [PubMed] [Google Scholar]
- 24.Mitra, R., P. Figueroa, A. K. Mukhopadhyay, T. Shimada, Y. Takeda, D. E. Berg, and G. B. Nair. 2000. Cell vacuolation, a manifestation of the El tor hemolysin of Vibrio cholerae. Infect. Immun. 68:1928-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mobley, H. L., D. M. Green, A. L. Trifillis, D. E. Johnson, G. R. Chippendale, C. V. Lockatell, B. D. Jones, and J. W. Warren. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 58:1281-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mobley, H. L., K. G. Jarvis, J. P. Elwood, D. I. Whittle, C. V. Lockatell, R. G. Russell, D. E. Johnson, M. S. Donnenberg, and J. W. Warren. 1993. Isogenic P-fimbrial deletion mutants of pyelonephritogenic Escherichia coli: the role of αGal(1-4) βGal binding in virulence of a wild-type strain. Mol. Microbiol. 10:143-155. [DOI] [PubMed] [Google Scholar]
- 27.Navarro-Garcia, F., C. Sears, C. Eslava, A. Cravioto, and J. P. Nataro. 1999. Cytoskeletal effects induced by Pet, the serine protease enterotoxin of enteroaggregative Escherichia coli. Infect. Immun. 67:2184-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pai, R., T. L. Cover, and A. S. Tarnawski. 1999. Helicobacter pylori vacuolating cytotoxin (VacA) disorganizes the cytoskeletal architecture of gastric epithelial cells. Biochem. Biophys. Res. Commun. 262:245-250. [DOI] [PubMed] [Google Scholar]
- 29.Papini, E., M. Bugnoli, M. de Bernard, N. Figura, R. Rappuoli, and C. Montecucco. 1993. Bafilomycin A1 inhibits Helicobacter pylori-induced vacuolization of HeLa cells. Mol. Microbiol. 7:323-327. [DOI] [PubMed] [Google Scholar]
- 30.Papini, E., M. Zoratti, and T. L. Cover. 2001. In search of the Helicobacter pylori VacA mechanism of action. Toxicon 39:1757-1767. [DOI] [PubMed] [Google Scholar]
- 31.Provence, D. L., and R. Curtiss, III. 1994. Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect. Immun. 62:1369-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ricci, V., A. Galmiche, A. Doye, V. Necchi, E. Solcia, and P. Boquet. 2000. High cell sensitivity to Helicobacter pylori VacA toxin depends on a GPI-anchored protein and is not blocked by inhibition of the clathrin-mediated pathway of endocytosis. Mol. Biol. Cell 11:3897-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ricci, V., P. Sommi, R. Fiocca, M. Romano, E. Solcia, and U. Ventura. 1997. Helicobacter pylori vacuolating toxin accumulates within the endosomal-vacuolar compartment of cultured gastric cells and potentiates the vacuolating activity of ammonia. J. Pathol. 183:453-459. [DOI] [PubMed] [Google Scholar]
- 34.Rothbaum, R. J., J. C. Partin, K. Saalfield, and A. J. McAdams. 1983. An ultrastructural study of enteropathogenic Escherichia coli infection in human infants. Ultrastruct. Pathol. 4:291-304. [DOI] [PubMed] [Google Scholar]
- 35.Salvadori, M. R., T. Yano, H. E. Carvalho, V. R. Parreira, and C. L. Gyles. 2001. Vacuolating cytotoxin produced by avian pathogenic Escherichia coli. Avian Dis. 45:43-51. [PubMed] [Google Scholar]
- 36.Stathopoulos, C., D. L. Provence, and R. Curtiss, III. 1999. Characterization of the avian pathogenic Escherichia coli hemagglutinin Tsh, a member of the immunoglobulin A protease-type family of autotransporters. Infect. Immun. 67:772-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stein, M., B. Kenny, M. A. Stein, and B. B. Finlay. 1996. Characterization of EspC, a 110-kilodalton protein secreted by enteropathogenic Escherichia coli which is homologous to members of the immunoglobulin A protease-like family of secreted proteins. J. Bacteriol. 178:6546-6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.St. Geme, J. W., III, M. L. de la Morena, and S. Falkow. 1994. A Haemophilus influenzae IgA protease-like protein promotes intimate interaction with human epithelial cells. Mol. Microbiol. 14:217-233. [DOI] [PubMed] [Google Scholar]
- 39.Warren, J. W. 1996. Clinical presentations and epidemiology of urinary tract infections, p. 3-27. In H. L. T. Mobley and J. W. Warren (ed.), Urinary tract infections: molecular pathogenesis and clinical management. ASM Press, Washington, D.C.