Abstract
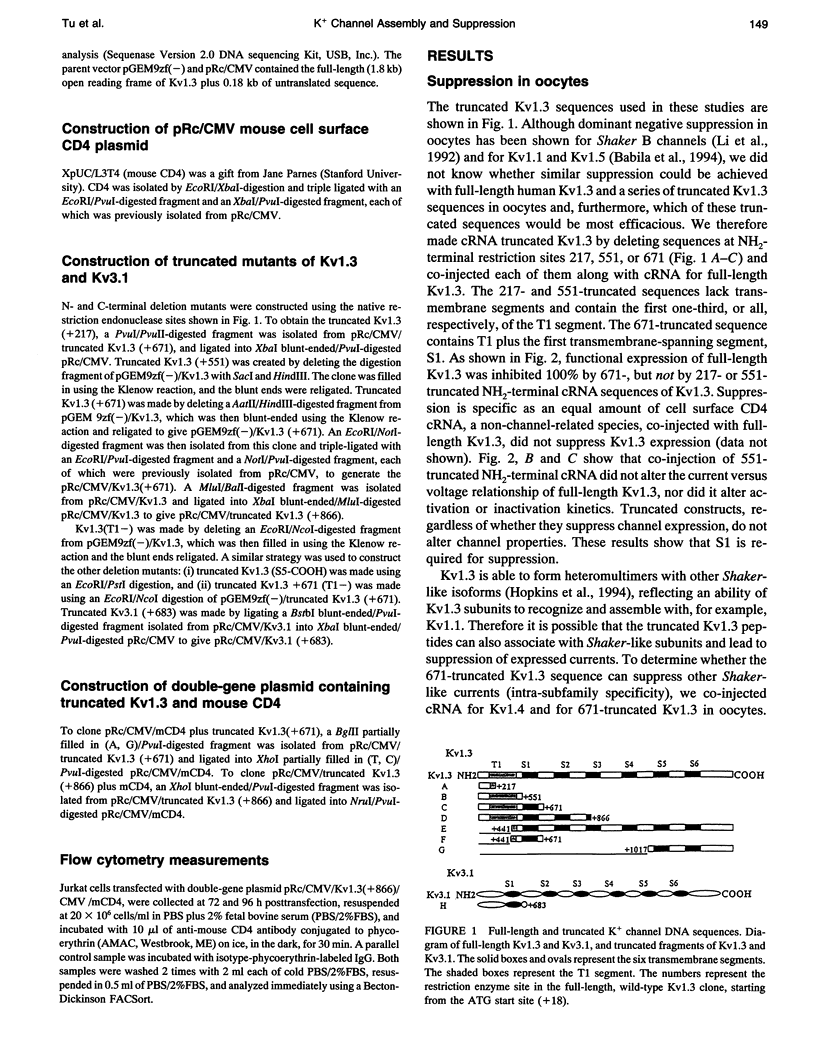
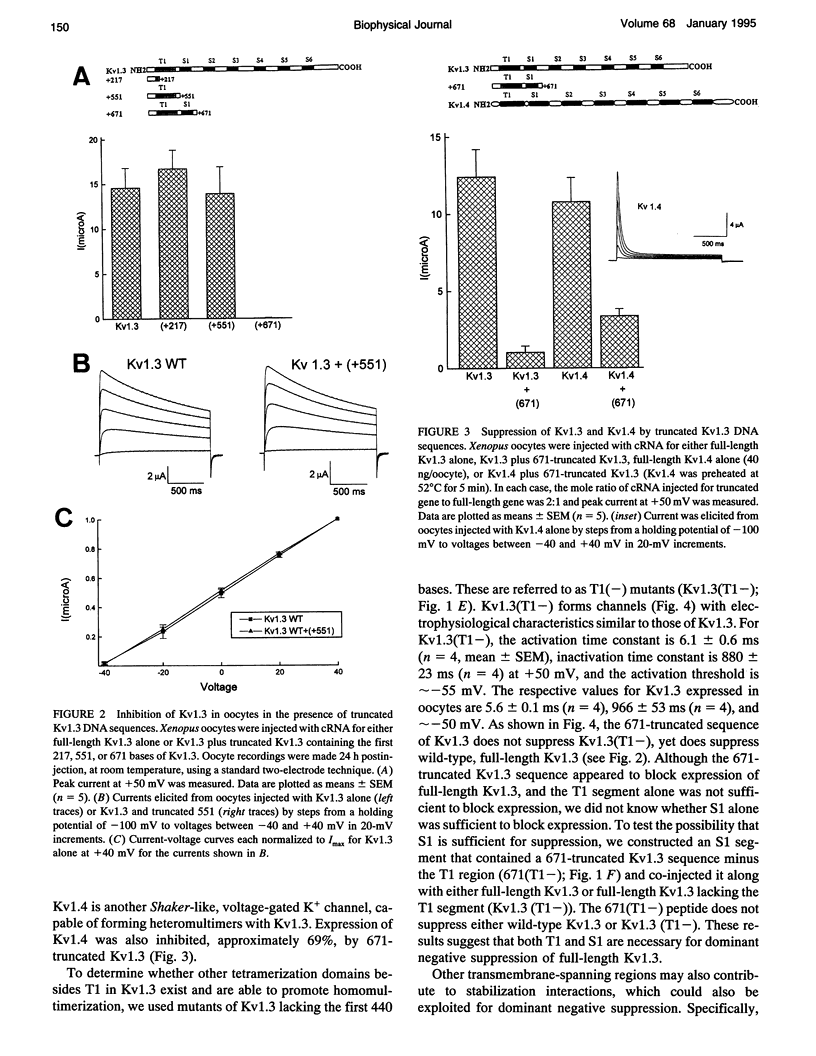
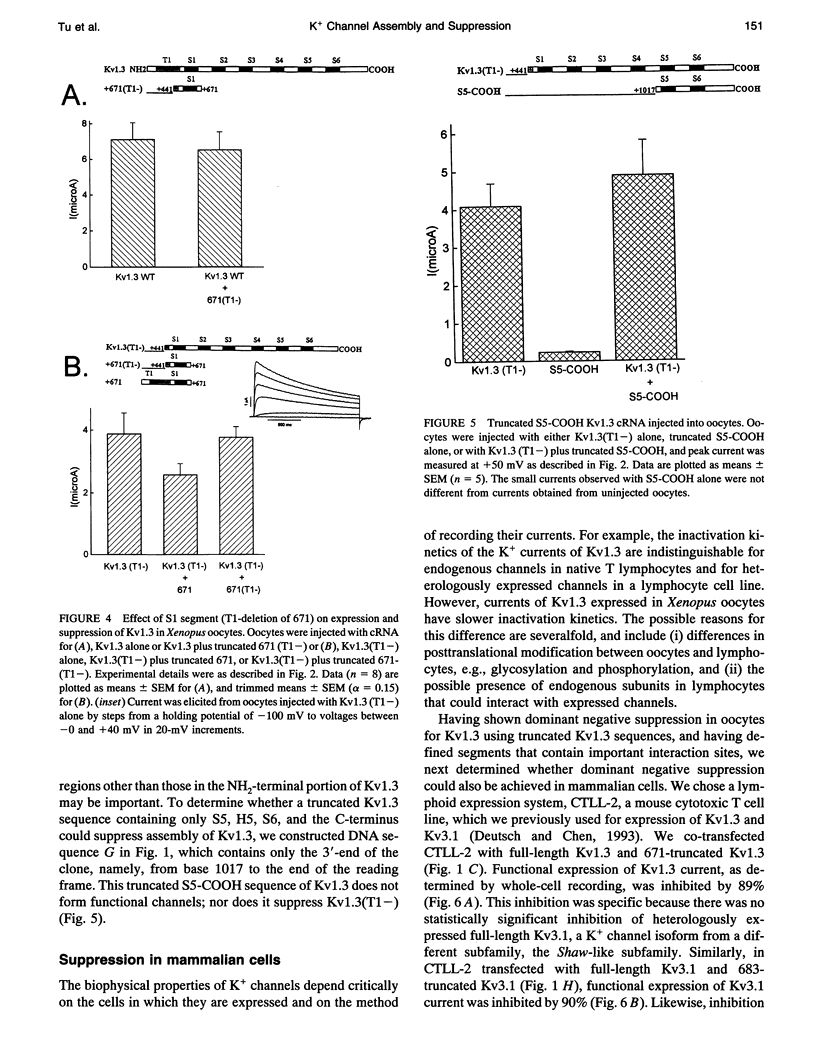
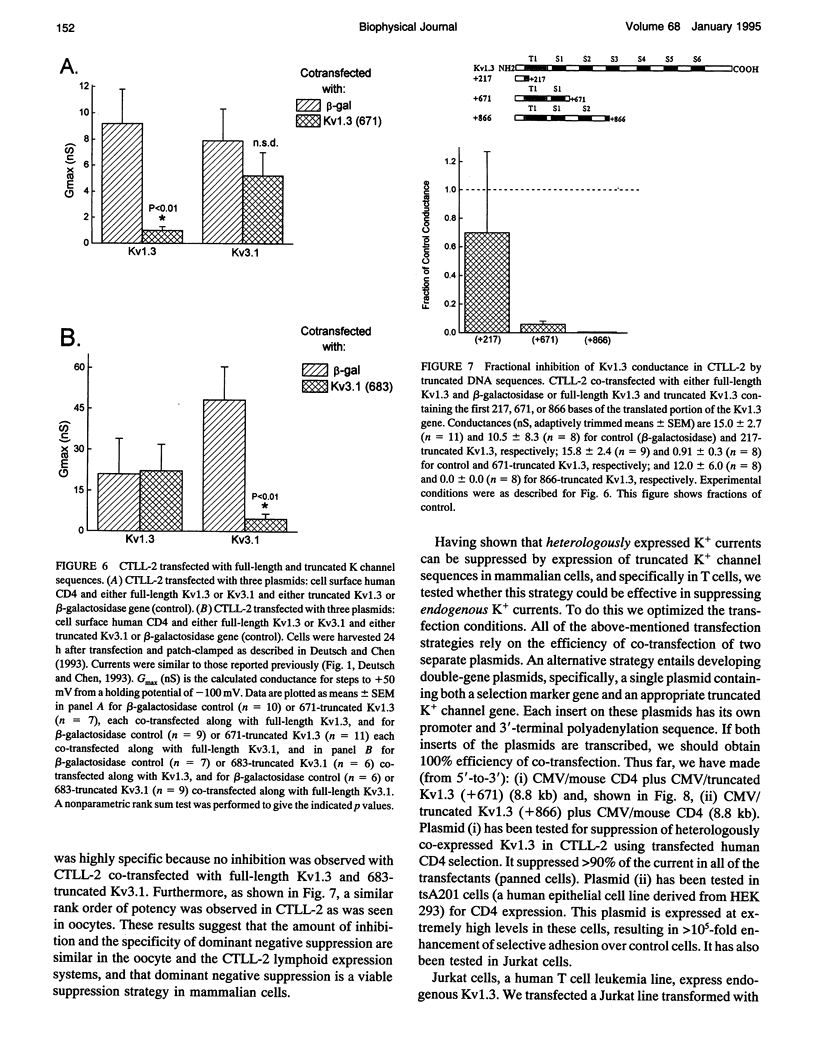
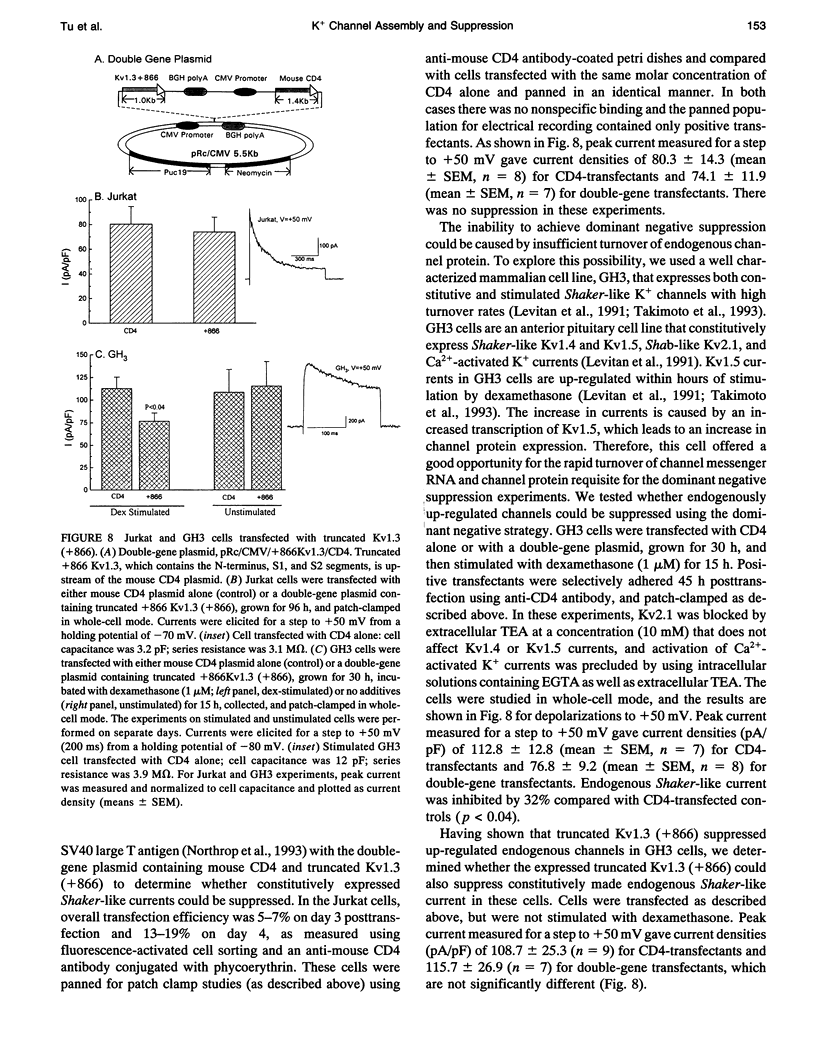
We have constructed a series of deletion mutants of Kv1.3, a Shaker-like, voltage-gated K+ channel, and examined the ability of these truncated mutants to form channels and to specifically suppress full-length Kv1.3 currents. These constructs were expressed heterologously in both Xenopus oocytes and a mouse cytotoxic T cell line. Our results show that a truncated mutant Kv1.3 must contain both the amino terminus and the first transmembrane-spanning segment, S1, to suppress full-length Kv1.3 currents. Amino-terminal-truncated DNA sequences from one subfamily suppress K+ channel expression of members of only the same subfamily. The first 141 amino acids of the amino-terminal of Kv1.3 are not necessary for channel formation. Deletion of these amino acids yields a current identical to that of full-length Kv1.3, except that it cannot be suppressed by a truncated Kv1.3 containing the amino terminus and S1. To test the ability of truncated Kv1.3 to suppress endogenous K+ currents, we constructed a plasmid that contained both truncated Kv1.3 and a selection marker gene (mouse CD4). Although constitutively expressed K+ currents in Jurkat (a human T cell leukemia line) and GH3 (an anterior pituitary cell line) cells cannot be suppressed by this double-gene plasmid, stimulated (up-regulated) Shaker-like K+ currents in GH3 cells can be suppressed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyar J., Grissmer S., Chandy K. G. Full-length and truncated Kv1.3 K+ channels are modulated by 5-HT1c receptor activation and independently by PKC. Am J Physiol. 1993 Dec;265(6 Pt 1):C1571–C1578. doi: 10.1152/ajpcell.1993.265.6.C1571. [DOI] [PubMed] [Google Scholar]
- Attali B., Lesage F., Ziliani P., Guillemare E., Honoré E., Waldmann R., Hugnot J. P., Mattéi M. G., Lazdunski M., Barhanin J. Multiple mRNA isoforms encoding the mouse cardiac Kv1-5 delayed rectifier K+ channel. J Biol Chem. 1993 Nov 15;268(32):24283–24289. [PubMed] [Google Scholar]
- Babila T., Moscucci A., Wang H., Weaver F. E., Koren G. Assembly of mammalian voltage-gated potassium channels: evidence for an important role of the first transmembrane segment. Neuron. 1994 Mar;12(3):615–626. doi: 10.1016/0896-6273(94)90217-8. [DOI] [PubMed] [Google Scholar]
- Chahine M., Chen L. Q., Barchi R. L., Kallen R. G., Horn R. Lidocaine block of human heart sodium channels expressed in Xenopus oocytes. J Mol Cell Cardiol. 1992 Nov;24(11):1231–1236. doi: 10.1016/0022-2828(92)93090-7. [DOI] [PubMed] [Google Scholar]
- Deutsch C., Chen L. Q. Heterologous expression of specific K+ channels in T lymphocytes: functional consequences for volume regulation. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10036–10040. doi: 10.1073/pnas.90.21.10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins W. F., Demas V., Tempel B. L. Both N- and C-terminal regions contribute to the assembly and functional expression of homo- and heteromultimeric voltage-gated K+ channels. J Neurosci. 1994 Mar;14(3 Pt 1):1385–1393. doi: 10.1523/JNEUROSCI.14-03-01385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacoff E. Y., Jan Y. N., Jan L. Y. Evidence for the formation of heteromultimeric potassium channels in Xenopus oocytes. Nature. 1990 Jun 7;345(6275):530–534. doi: 10.1038/345530a0. [DOI] [PubMed] [Google Scholar]
- Lee T. E., Philipson L. H., Kuznetsov A., Nelson D. J. Structural determinant for assembly of mammalian K+ channels. Biophys J. 1994 Mar;66(3 Pt 1):667–673. doi: 10.1016/s0006-3495(94)80840-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan E. S., Hemmick L. M., Birnberg N. C., Kaczmarek L. K. Dexamethasone increases potassium channel messenger RNA and activity in clonal pituitary cells. Mol Endocrinol. 1991 Dec;5(12):1903–1908. doi: 10.1210/mend-5-12-1903. [DOI] [PubMed] [Google Scholar]
- Li M., Jan Y. N., Jan L. Y. Specification of subunit assembly by the hydrophilic amino-terminal domain of the Shaker potassium channel. Science. 1992 Aug 28;257(5074):1225–1230. doi: 10.1126/science.1519059. [DOI] [PubMed] [Google Scholar]
- Li M., Unwin N., Stauffer K. A., Jan Y. N., Jan L. Y. Images of purified Shaker potassium channels. Curr Biol. 1994 Feb 1;4(2):110–115. doi: 10.1016/s0960-9822(94)00026-6. [DOI] [PubMed] [Google Scholar]
- Liman E. R., Tytgat J., Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992 Nov;9(5):861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991 Mar 21;350(6315):232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- Margolskee R. F., McHendry-Rinde B., Horn R. Panning transfected cells for electrophysiological studies. Biotechniques. 1993 Nov;15(5):906–911. [PubMed] [Google Scholar]
- Matsubara H., Liman E. R., Hess P., Koren G. Pretranslational mechanisms determine the type of potassium channels expressed in the rat skeletal and cardiac muscles. J Biol Chem. 1991 Jul 15;266(20):13324–13328. [PubMed] [Google Scholar]
- Matteson D. R., Deutsch C. K channels in T lymphocytes: a patch clamp study using monoclonal antibody adhesion. Nature. 1984 Feb 2;307(5950):468–471. doi: 10.1038/307468a0. [DOI] [PubMed] [Google Scholar]
- Northrop J. P., Ullman K. S., Crabtree G. R. Characterization of the nuclear and cytoplasmic components of the lymphoid-specific nuclear factor of activated T cells (NF-AT) complex. J Biol Chem. 1993 Feb 5;268(4):2917–2923. [PubMed] [Google Scholar]
- Rettig J., Heinemann S. H., Wunder F., Lorra C., Parcej D. N., Dolly J. O., Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature. 1994 May 26;369(6478):289–294. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- Shen N. V., Chen X., Boyer M. M., Pfaffinger P. J. Deletion analysis of K+ channel assembly. Neuron. 1993 Jul;11(1):67–76. doi: 10.1016/0896-6273(93)90271-r. [DOI] [PubMed] [Google Scholar]
- Takimoto K., Fomina A. F., Gealy R., Trimmer J. S., Levitan E. S. Dexamethasone rapidly induces Kv1.5 K+ channel gene transcription and expression in clonal pituitary cells. Neuron. 1993 Aug;11(2):359–369. doi: 10.1016/0896-6273(93)90191-s. [DOI] [PubMed] [Google Scholar]
- VanDongen A. M., Frech G. C., Drewe J. A., Joho R. H., Brown A. M. Alteration and restoration of K+ channel function by deletions at the N- and C-termini. Neuron. 1990 Oct;5(4):433–443. doi: 10.1016/0896-6273(90)90082-q. [DOI] [PubMed] [Google Scholar]


