Abstract
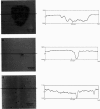
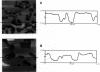
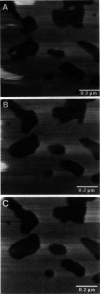
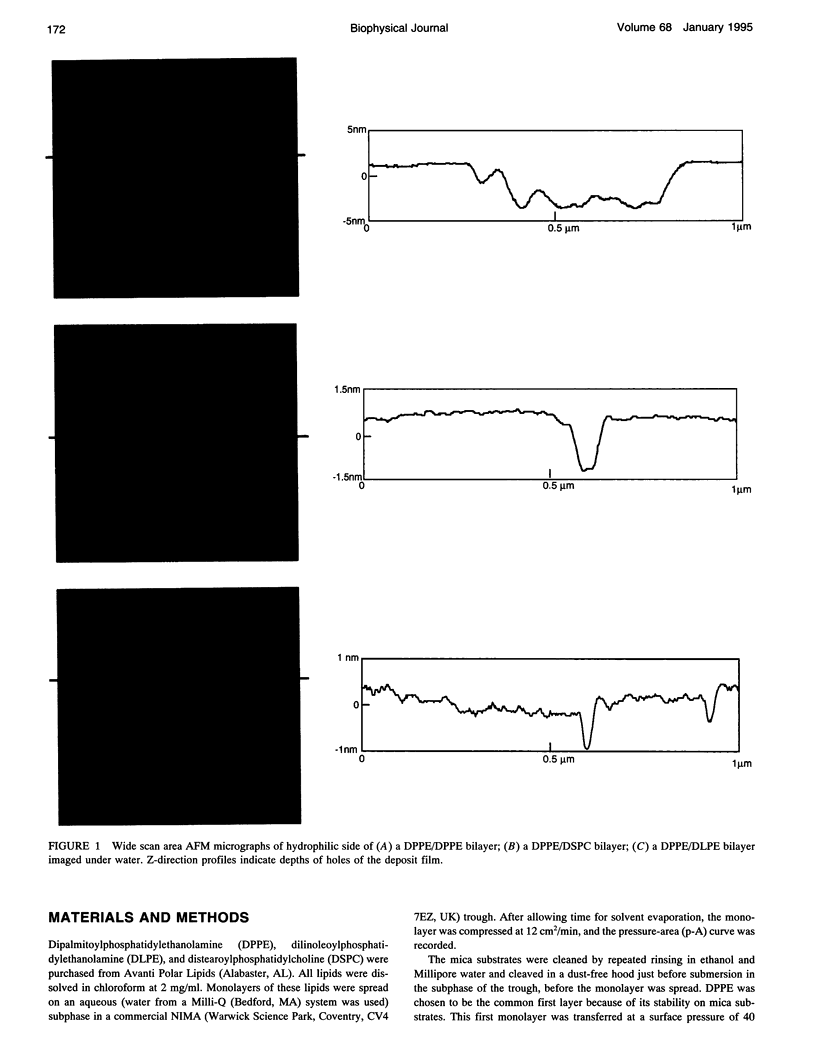
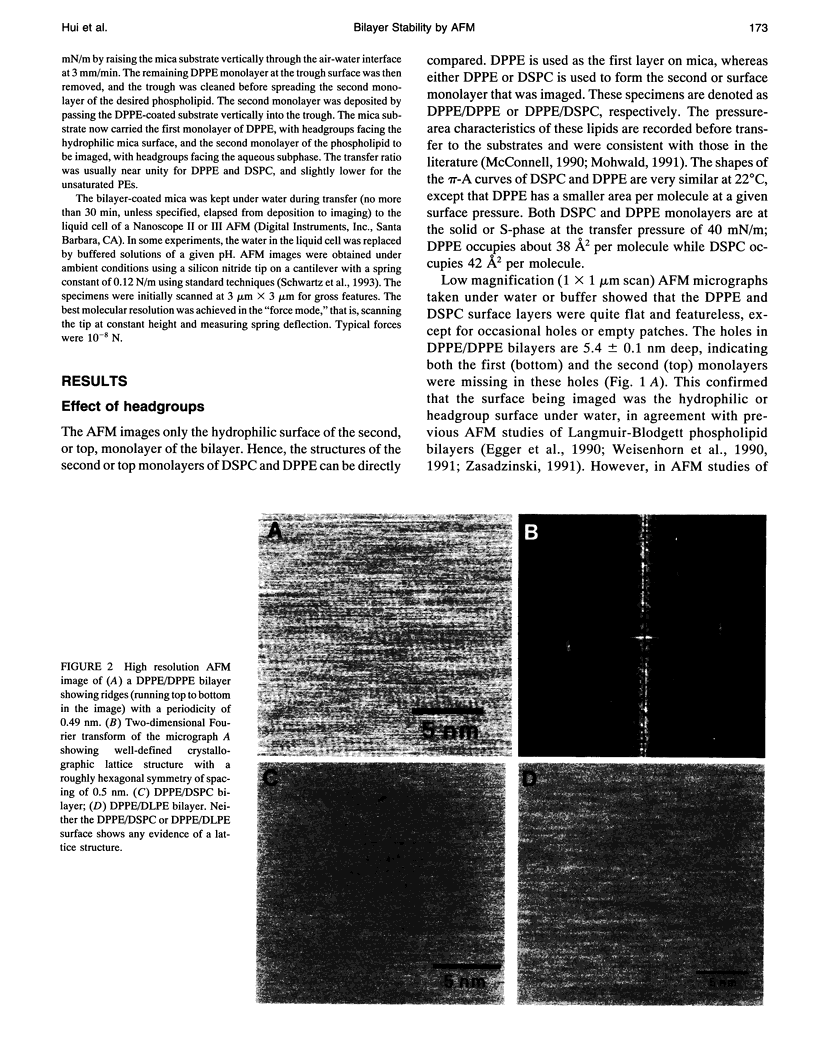
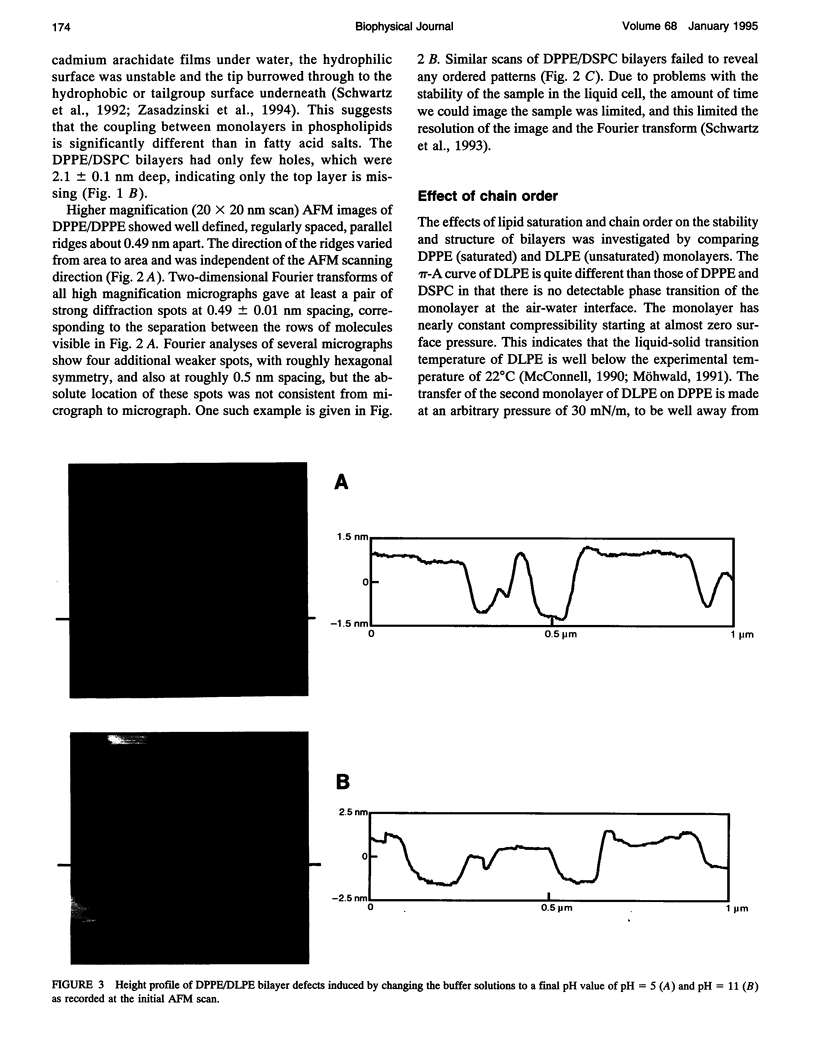
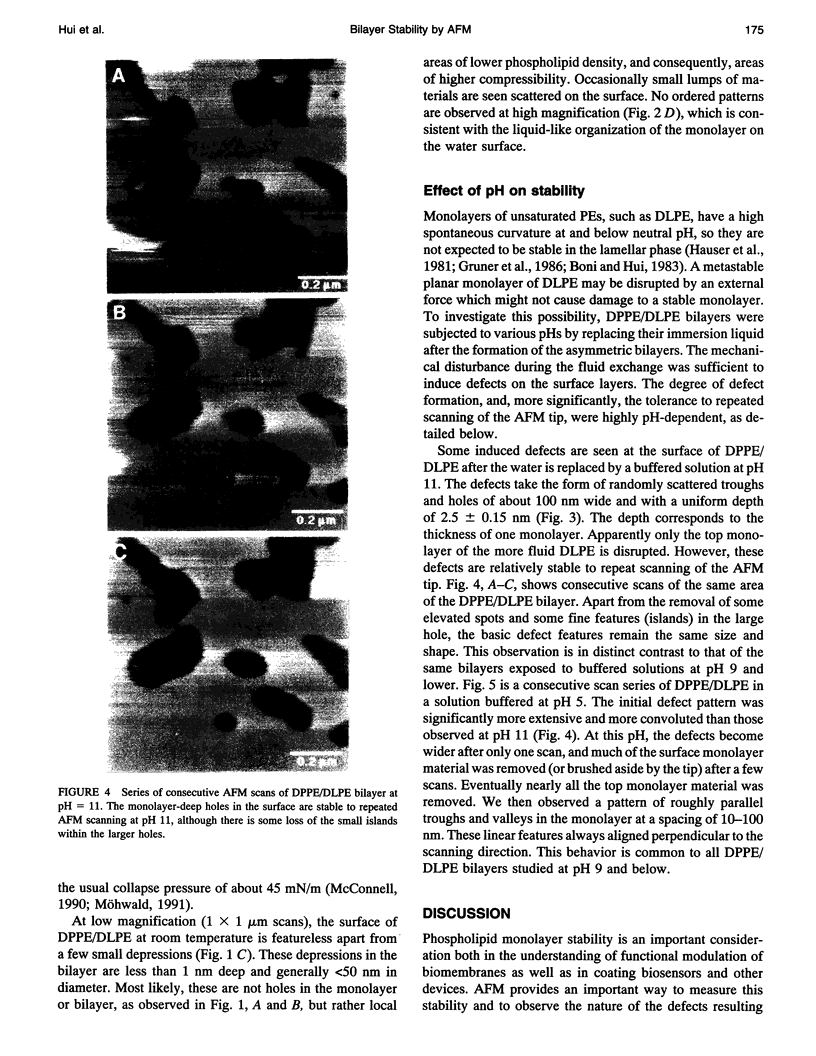
Atomic force microscopy (AFM) was used to investigate the structure, stability, and defects of the hydrophilic surfaces of Langmuir-Blodgett bilayer films of distearoylphosphatidylcholine (DSPC) and dipalmitoylphosphatidylethanolamine (DPPE) in the solid phase, and dilinoleoylphosphatidylethanolamine (DLPE) in the fluid phase. Their relative resilience to external mechanical stress by the scanning tip and by fluid exchange were also investigated. DPPE monolayers showed parallel ridges at the surface with a period of 0.49 nm, corresponding to the rows of aligned headgroups consistent with the known crystallographic structure. DSPC and DLPE monolayers did not show any periodic order. The solid DSPC and DPPE monolayers were stable to continued rastering by the AFM tip; however, the stability of DLPE monolayers depended on the pH of the aqueous environment. Structural defects in the form of monolayer gaps and holes were observed after fluid exchange, but the defects in DLPE monolayer at pH 11 were stable during consecutive scanning. At pH 9 and below, the defects induced by fluid exchange over DLPE monolayers were more extensive and were deformed easily by consecutive scanning of the AFM tip at a force of 10 nN. The pH dependence of resilience was explained by the increasing bending energy or frustration due to the high spontaneous curvature of DLPE monolayers at low pH. The tangential stress exerted by the AFM tip on the deformable monolayers eventually produced a ripple pattern, which could be described as a periodic buckling known as Shallamach waves.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Gruner S. M., Parsegian V. A., Rand R. P. Directly measured deformation energy of phospholipid HII hexagonal phases. Faraday Discuss Chem Soc. 1986;(81):29–37. doi: 10.1039/dc9868100029. [DOI] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Pearson R. H., Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981 Jun 16;650(1):21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Hui S. W., Sen A. Effects of lipid packing on polymorphic phase behavior and membrane properties. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5825–5829. doi: 10.1073/pnas.86.15.5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung O. M., Goh M. C. Orientational ordering of polymers by atomic force microscope tip-surface interaction. Science. 1992 Jan 3;255(5040):64–66. doi: 10.1126/science.255.5040.64. [DOI] [PubMed] [Google Scholar]
- Möhwald H. Phospholipid and phospholipid-protein monolayers at the air/water interface. Annu Rev Phys Chem. 1990;41:441–476. doi: 10.1146/annurev.pc.41.100190.002301. [DOI] [PubMed] [Google Scholar]
- Schwartz D. K., Garnaes J., Viswanathan R., Zasadzinski J. A. Surface order and stability of langmuir-blodgett films. Science. 1992 Jul 24;257(5069):508–511. doi: 10.1126/science.257.5069.508. [DOI] [PubMed] [Google Scholar]
- Schwartz DK, Garnaes J, Viswanathan R, Chiruvolu S, Zasadzinski JA. Quantitative lattice measurement of thin Langmuir-Blodgett films by atomic-force microscopy. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1993 Jan;47(1):452–460. doi: 10.1103/physreve.47.452. [DOI] [PubMed] [Google Scholar]
- Seddon J. M. Structure of the inverted hexagonal (HII) phase, and non-lamellar phase transitions of lipids. Biochim Biophys Acta. 1990 Feb 28;1031(1):1–69. doi: 10.1016/0304-4157(90)90002-t. [DOI] [PubMed] [Google Scholar]
- Sen A., Isac T. V., Hui S. W. Bilayer packing stress and defects in mixed dilinoleoylphosphatidylethanolamine and palmitoyloleoylphosphatidylcholine and their susceptibility to phospholipase A2. Biochemistry. 1991 May 7;30(18):4516–4521. doi: 10.1021/bi00232a021. [DOI] [PubMed] [Google Scholar]
- Sen A., Yang P. W., Mantsch H. H., Hui S. W. Extended hydrogen-bonded structures of phosphatidylethanolamine. Chem Phys Lipids. 1988 Jun;47(2):109–116. doi: 10.1016/0009-3084(88)90079-5. [DOI] [PubMed] [Google Scholar]
- Weisenhorn A. L., Drake B., Prater C. B., Gould S. A., Hansma P. K., Ohnesorge F., Egger M., Heyn S. P., Gaub H. E. Immobilized proteins in buffer imaged at molecular resolution by atomic force microscopy. Biophys J. 1990 Nov;58(5):1251–1258. doi: 10.1016/S0006-3495(90)82465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasadzinski J. A., Helm C. A., Longo M. L., Weisenhorn A. L., Gould S. A., Hansma P. K. Atomic force microscopy of hydrated phosphatidylethanolamine bilayers. Biophys J. 1991 Mar;59(3):755–760. doi: 10.1016/S0006-3495(91)82288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasadzinski J. A., Viswanathan R., Madsen L., Garnaes J., Schwartz D. K. Langmuir-Blodgett films. Science. 1994 Mar 25;263(5154):1726–1733. doi: 10.1126/science.8134836. [DOI] [PubMed] [Google Scholar]