Abstract
Burkholderia cepacia has emerged as a serious respiratory pathogen in cystic fibrosis (CF) patients. The clinical course of B. cepacia infections is variable, but ∼20% of patients eventually succumb to the cepacia syndrome, which is characterized as a fatal necrotizing pneumonia with bacteremia. The mechanisms that permit B. cepacia to cause bacteremia are not yet known but probably involve sequential penetration of airway barriers. This study evaluated the abilities of different species of the B. cepacia complex, including a strain from the ET12 lineage (BC-7, genomovar III, cblA+), which is associated with most cepacia syndrome fatalities among CF populations, a genomovar IV strain (HI2258), and a genomovar II strain (J-1) to penetrate polarized, well-differentiated human airway epithelial cell cultures. As revealed by light and electron microscopy, all three B. cepacia strains tested circumvented the mechanical barriers of mucus and ciliary transport to penetrate the airway epithelium but they used different routes. The BC-7 strain (genomovar III) formed biofilms in close proximity to the apical cell surface, followed by invasion and destruction of epithelial cells. This process involved disruption of the glycocalyx and rearrangements of the actin cytoskeleton. The HI2258 strain (genomovar IV) did not form biofilms, and the majority of bacteria that penetrated the epithelium were located between epithelial cells, suggesting paracytosis. Strain J-1 penetrated the epithelium both by cell destruction and paracytosis. These studies suggest that the distinct invasion pathways employed by B. cepacia may account for differences in virulence between B. cepacia genomovars.
In cystic fibrosis (CF), chronic bacterial infection results in bronchiectasis and ultimately respiratory failure. The initial infective organisms may include Haemophilus influenzae and Staphylococcus aureus. Then, chronic persistent infections with mucoid strains of Pseudomonas aeruginosa develop in the majority of CF patients (5, 8, 12). Despite chronic infections with these organisms, necrotizing pneumonia and bacteremia are rare occurrences in CF. Recently, Burkholderia cepacia has emerged as an important pathogen for CF patients (9). Although not as prevalent as P. aeruginosa, B. cepacia can be isolated from as many as 13.3% of CF patients (Cystic Fibrosis Foundation Patient Registry, Annual data report, 2000: all cystic fibrosis patients currently under care at 117 CF care centers in the United States, 2001), and highly transmissible strains have been reported (32). Most patients harboring B. cepacia exhibit a chronic infection associated with periods of acute exacerbations. Importantly, ∼20% of patients harboring B. cepacia eventually succumb to the cepacia syndrome, a rapidly fatal clinical deterioration with acute necrotizing pneumonia and bacteremia (13). Up to nine genomovars are known to occur in CF patients (4, 34). Of these, genomovar III strains occur in approximately 50% of CF patients infected with B. cepacia and are associated with most cases of cepacia syndrome (18).
The mechanism(s) that permits B. cepacia to cause chronic versus invasive infections with bacteremia is not yet known. One possibility is that, during chronic infection, B. cepacia forms biofilms confined to the intraluminal mucus layer. This notion is supported in part by the observation that B. cepacia is able to form biofilms in vitro (2, 6, 11, 22, 25), and an outbreak of B. cepacia in hemodialysis patients has been reported to result from biofilm-forming organisms in catheters (15). In addition Nielsen et al. (22) showed that both P. aeruginosa and B. cepacia grown in flow chambers formed coaggregated or single-species biofilms depending on growth conditions, suggesting that P. aeruginosa and B. cepacia may coexist in CF airways. However, localization of B. cepacia to intraluminal mucus in CF lungs has yet not been reported.
In contrast, it appears likely that invasion and bacteremia are associated with penetration of airway epithelia and transmission via macrophages. For example, several investigators have shown that B. cepacia can survive in A549 human epithelial cells and macrophages (3, 16, 19, 26). Recently, B. cepacia has been localized by immunohistology in patients with cepacia syndrome in the exudates within bronchial lumens and between airway epithelial cells (27). These CF patients were infected by the transmissible clonal ET12 (Edinburgh-Toronto) lineage belonging to genomovar III. The isolates of this clone carry the cblA gene, which encodes the major subunit for surface cable pili, which have been shown to mediate binding to respiratory mucins (28), and thus may facilitate invasion by this bacterium. It is not known whether the immunohistological findings in the report of Sajjan et al. (27) represent features characteristic of this ET12 strain or of late-stage pulmonary infection with B. cepacia in general.
In the present study, we examined different species of the B. cepacia complex invasion pathways in well-differentiated human airway epithelial cell cultures. In this in vitro model system, we tested whether different strains of B. cepacia developed different invasion pathways, i.e., biofilm formation associated with invasion, single-cell entry, and/or paracytosis. In addition, we asked whether biofilm-mediated invasion involved glycocalyx disruption and rearrangement of the actin filaments on the apical cell surface.
MATERIALS AND METHODS
Human airway epithelial cell cultures.
Well-differentiated human airway epithelial cell cultures were grown on Costar (Cambridge, Mass.) 12-mm-diameter Transwell-Col (T-Col) supports as previously described (1). Cells were removed by protease dissociation from portions of the main stem or lobar bronchi from excess donor tissue obtained at the time of lung transplantation under the auspices of the University of North Carolina Institutional Committee on the Protection of The Rights of Human Subjects. Dissociated primary airway epithelial cells were grown on 100-mm-diameter tissue culture dishes in bronchial epithelial culture (BEC) medium. Passage 2 cells were seeded on the T-Col membrane supports (Costar) under air-liquid interface conditions at a density of about 106 cells/cm2 and were grown in BEC medium modified from that in reference 1 by the use of LHC basal medium-high-glucose Dulbecco's modified Eagle's medium (1:1) and bovine pituitary extract (10 mg/liter; Upstate Biotechnology, Inc., Lake Placid, N.Y.) in the absence of antibiotics. When the cultures reached confluence (∼5 days), only the medium in the serosal chamber was replaced. Epithelial cell cultures were studied 2 to 4 weeks after achieving confluence when they were fully differentiated into ciliated and mucus-producing cells (determined by light microscopy).
Bacterial strains and growth conditions.
The following B. cepacia strains were analyzed for their abilities to invade airway epithelial cultures: BC-7 (genomovar III, cblA+ major CF lineage ET12; provided by Richard Goldstein, Boston University), J-1 (Burkholderia multivorans, genomovar II; provided by Peter Gilligan), HI2258 (Burkholderia stabilis, genomovar IV; provided by John LiPuma, Michigan University), and K56-2 (genomovar III, cblA+ major CF lineage ET12; provided by Pamela Sokol) and its derivative K56-I2, which does not produce N-acylhomoserine lactones (cepI::tmp [17]; provided by Pamela Sokol). Strains HI2258 and J-1 were isolates from CF patients without bacteremic infection. Stocks of the bacteria were kept at −70°C in skim milk. For all studies, bacteria were grown at 37°C on sheep blood agar plates.
Infection of airway epithelial cell cultures.
For infection of airway epithelial cell cultures, several colonies of B. cepacia grown overnight on agar plates were inoculated into 5 ml of BEC medium and grown for 6 to 8 h to reach an optical density at 470 nm of ∼0.25. The suspension was pelleted and resuspended in 200 μl of medium, and 50 μl was directly transferred to the apical cell surfaces of the airway epithelial cell cultures. In some experiments the bacterial suspension was diluted 1:100 prior to infection of the airway epithelium. The inoculum size was determined for each infection by serial dilutions and plating and ranged between approximately 4 × 107 to 6 × 107 CFU/50 μl and 4 × 105 to 6 × 105 CFU/50 μl when the suspension was diluted 1:100. We used log phase bacteria, since it is not yet clear whether the organisms are in log or stationary phase during acquisition of infection, which is the stage of the infection process that we have investigated.
To confirm the functional integrity of the epithelial cell layers, transepithelial resistance (Rt) was measured with an EVOM epithelial volt-ohmmeter (World Precision Instruments, Berlin, Germany).
Pretreatment of bacteria.
To test whether viability was required for epithelium penetration, we infected airway epithelial cell cultures with either killed organisms or live organisms at an inoculum of ∼5 × 107 CFU/50 μl. Two methods were used to kill bacteria. In the first, bacteria were exposed to UV light for 15 min. In the second method, bacteria were exposed to 56°C for 40 min. For both methods, bacteria were pelleted and resuspended in 200 μl of culture medium and 50 μl of this suspension was transferred to the mucus layer of airway epithelial cell cultures. Both treatments reduced the number of viable organisms to <10 CFU/50 μl.
Preparation of infected airway epithelial cell cultures for light, scanning, and electron microscopy.
Infected and noninfected epithelial cell cultures were fixed with 1% OsO4-perfluorocarbon for 30 min, a fixation method which does not perturb the airway surface liquid layer (see Fig. 1A) (20). For light microscopy (LM), the fixed cultures were embedded in epoxy and sectioned. Semithin sections from the center of the cell culture preparation were stained with Richardson's stain. The following characteristics were determined in sections encompassing the overall length of airway epithelial cell cultures (12 mm): presence of biofilms, single bacteria found between cells or within cells, and damage of the airway epithelium, evaluated as mild, moderate, or severe. For transmission electron microscopy (TEM), ultrathin sections (90 nm) were cut from blocks and mounted on grids and stained with uranyl acetate and lead citrate and areas of interest were examined with an electron microscope. For scanning electron microscopy (SEM), the cultures were fixed with 4% paraformaldehyde for 10 min and postfixed in 1% OsO4 prior to dehydration through a graded ethanol series. After being coated with gold-palladium the specimens were viewed by SEM.
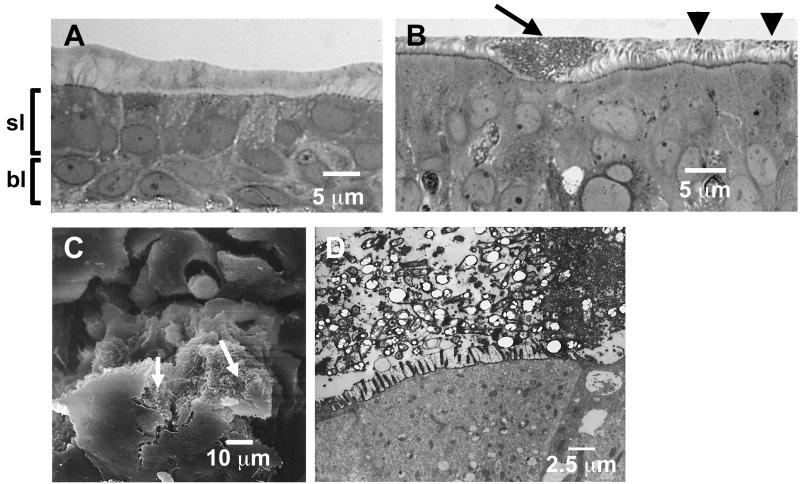
FIG. 1.
Noninfected and B. cepacia BC-7-infected airway epithelial cell cultures. (A) Well-differentiated human airway epithelial cell culture grown on a T-Col membrane support for 3 weeks under air-liquid interface conditions and fixed by the perfluorocarbon-osmium tetroxide technique (20). Semithin sections were stained with Richardson's stain. Note the preservation of the mucus layer. sl, superficial cell layer; bl, basal cell layer. (B to D) Small biofilms closely associated with the apical cell surface formed by B. cepacia BC-7 (∼5 × 107 CFU/airway epithelial cell culture) in airway epithelial cell cultures after 24 h of incubation. Cultures were examined by LM (B) (semithin section stained with Richardson's stain), SEM (C), and TEM (ultrathin section from ruthenium red-stained culture) (D), which show bacteria being buried in the residue of their glycocalyx. Arrows (B and C), small biofilm; arrowheads (B), single (planktonic) bacteria.
Immunohistology.
Infected and noninfected airway epithelial cell cultures were fixed in 4% paraformaldehyde for 15 min and washed in phosphate-buffered saline (PBS). Paraffin-embedded sections were cut, deparaffinized by xylene treatment, rehydrated in graded alcohols, washed in PBS, and then incubated with different dilutions of the rabbit antiserum specific for the B. cepacia complex (Heiner Neubauer, FAF Medical Academy, Institute of Microbiology, Munich, Germany) or normal rabbit serum (negative control). Afterwards, we added sequentially the secondary antibody (biotinylated goat anti-rabbit immunoglobulin G; diluted 1:250), streptavidin-horseradish peroxidase conjugate reagent, and the substrate, diaminobenzidine tetrahydrochloride. The sections were counterstained with light green and examined by LM. The strongest signal was obtained by using the B. cepacia antiserum at a 1:500 dilution, and this dilution was used for all infected cultures.
Apical cell surface glycocalyx staining.
Ruthenium red oxychloride is an inorganic, synthetically prepared, intensely colored crystalline compound that displays a high affinity for sulfated mucopolysaccharides, chondroitin sulfate, heparan sulfate, and keratan sulfate (23). Because of its size, ruthenium red does not penetrate undamaged plasma membranes. To assess the integrity of the glycocalyx in infected airway epithelial cell cultures, cultures were immersed in 4% glutaraldehyde containing 1,500 ppm ruthenium red (10). After being rinsed in 0.1 M cacodylate buffer, the tissues underwent immersion fixation in a solution of 5% osmium tetroxide containing 1,500 ppm ruthenium red for 3 h. Specimens were then dehydrated and embedded in Epon. Semithin sections stained with Richardson's stain were analyzed for areas of the epithelium associated with bacterial biofilms closely associated with the apical cell surface. Ultrathin sections (90 nm) of these areas were then stained with uranyl acetate and lead citrate and examined by TEM.
Fluorescent staining of B. cepacia and the actin cytoskeleton in well-differentiated human airway epithelial cell cultures for confocal microscopy.
An immunofluorescence staining protocol modified from a previously published method (24) was used. Noninfected and B. cepacia (∼5 × 107 CFU/airway epithelial cell culture for 24 h)-infected airway epithelial cell cultures were fixed with 4% paraformaldehyde for 15 min, washed in PBS, and permeabilized with 1% Triton X-100. The cultures were then blocked overnight with 3% bovine serum albumin, and infected cultures were incubated for 1 h at 37°C with rabbit antiserum (1:250 dilution) specific for the B. cepacia complex, followed by incubation with a goat anti-rabbit fluorescein isothiocyanate-conjugated antibody. For the labeling of the actin cytoskeleton, the immunostained and the noninfected airway epithelial cell cultures were then incubated with BODIPY-phalloidin (Molecular Probes Inc., Eugene, Oreg.) at a 1:25 dilution for 30 min at 25°C (24). Airway cell cultures were then rinsed with PBS and visualized by confocal microscopy (Leica; model TCS 4D confocal microscope; PL APO 63×/NA1.2 water lens).
Quantification of bacteria in mucus of infected airway epithelial cell cultures.
Airway epithelial cell cultures were inoculated with 5 × 105 CFU of the different strains of B. cepacia in parallel experiments for 24, 36, and 48 h. In one set of experiments, the mucus from one culture was harvested by lavage with PBS containing 10 mM dithiothreitol (DTT; Sigma Chemical Co., St. Louis, Mo.) at 24, 36, and 48 h for quantification of bacterial number. In preliminary experiments we could show that DTT at this concentration did not affect bacterial viability. The numbers of bacteria in the fractions harvested with DTT were determined by serial dilution and plating on sheep blood agar plates. For a second set of experiments, we fixed the culture with 1% OsO4-perfluorocarbon to examine the mucus layer for formed biofilms.
RESULTS
Biofilm-dependent invasion of B. cepacia BC-7 (genomovar III, cblA+ major CF lineage ET12).
To determine if B. cepacia BC-7 invades the airway wall, we infected well-differentiated human airway epithelial cell cultures that contained superficial (lumen-facing) and basal epithelial cells (Fig. 1A). This superficial epithelial cell layer contained ciliated and secretory cells that produce mucus in quantities mimicking in vivo conditions. After a 24-h incubation with ∼5 × 107 CFU log phase bacteria of strain BC-7, a few planktonic (single) bacteria were seen in the mucous layer but the majority of bacteria formed aggregates in the mucus layer when analyzed by LM (Fig. 1B) and SEM (Fig. 1C). These aggregates formed in close proximity to the apical surface of cells in defined regions and showed some of the characteristics of a typical biofilm: (i) they had a distinct boundary, (ii) they were attached to a surface, and (iii) the bacteria appeared to be buried in the residue of their glycocalyx (Fig. 1D). The number of biofilms per section ranged between 2 and 12.
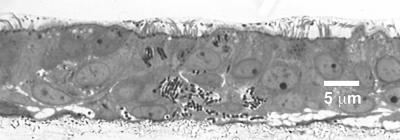
Several other features of these biofilms were noteworthy. In all cultures infected with BC-7, biofilms were formed but they appeared to be in different stages of development. In addition to the smaller biofilms in close proximity to the apical cell surface (Fig. 1B), larger biofilms either at an early (Fig. 2B) or late stage (Fig. 2A) of penetration were observed. Interestingly, even though moderate-to-severe tissue damage of the superficial epithelial cell layer occurred when larger biofilms penetrated tissues, the basal cell layer remained intact in these infected cultures (Fig. 2B). When areas of epithelia subjacent to such biofilms were analyzed by TEM, intracellular (Fig. 2C) and intercellular (Fig. 2D) bacteria were found only in epithelial cells associated with biofilms, suggesting that biofilm formation facilitated invasion into the epithelium. This biofilm-dependent invasion pathway seems to be the predominant one used by the BC-7 strain, since we rarely found single bacteria not associated with biofilms within or between epithelial cells (Table 1).
FIG. 2.
Different stages of biofilm development of B. cepacia BC-7 (∼5 × 107 CFU/airway epithelial cell culture) in well-differentiated airway epithelial cell cultures after 24 h of incubation. Large biofilms at late (A) and early (B) stages of penetration were examined by LM (semithin sections stained with Richardson's stain; see also Table 1). (C and D) Large biofilm at an early stage of penetration examined by TEM revealing intracellular (C) and intercellular (D) bacteria (arrowhead) in epithelial cells associated with this biofilm. Note destruction of adjacent epithelial cells.
TABLE 1.
Biofilm-dependent and -independent invasion of B. cepacia examined by different staining methods
| B. cepacia straina | Staining methodb | No. of infected airway cultures showingc:
|
||
|---|---|---|---|---|
| Biofilm-mediated invasion | Intracellular bacteria | Paracytosis | ||
| BC-7 | RS | 12 (12) | 3 (12) | 3 (12) |
| IS | 2 (2) | 0 (2) | 0 (2) | |
| RR | 4 (4) | 1 (4) | 1 (4) | |
| HI2258 | RS | 0 (3) | 3 (3) | 3 (3) |
| IS | 0 (2) | 2 (2) | 2 (2) | |
| RR | 0 (2) | 2 (2) | 2 (2) | |
| J-1 | RS | 3 (3) | 3 (3) | 3 (3) |
| IS | 3 (3) | 3 (3) | 3 (3) | |
| RR | 2 (2) | 2 (2) | 2 (2) | |
| K56-2 | RS | 3 (3) | 0 (3) | 0 (3) |
| K56-12 | RS | 3 (3) | 0 (3) | 0 (3) |
Approximately 5 × 107 CFU/airway epithelial cell culture for 24 h.
RS, Richardson staining; IS, immunostaining; RR, ruthenium red staining.
Numbers in parentheses are total numbers of infected airway cultures.
Immunostaining and ruthenium red staining of BC-7-infected cultures (Table 1) confirmed our observations of infected tissues stained with Richardson's stain. Interestingly, even though the mucus layer was removed by the fixation methods used for the immunostaining and ruthenium red staining techniques, densely packed biofilms closely associated with the epithelium were still detectable, suggesting that these biofilms are tightly attached to epithelial cells.
When cultures were infected with ∼5 × 107 CFU of UV-irradiated or heat-killed bacteria for 24 h, no invading biofilms were observed.
Biofilm-independent invasion by B. stabilis HI2258 (genomovar IV).
To determine whether biofilm-mediated invasion was a specific feature of the particular BC-7 strain belonging to genomovar III, cblA+ major CF lineage ET12, we investigated whether a different genomovar type, i.e., genomovar IV (B. stabilis), which is seen in fewer than 5% of infected CF patients (18), formed biofilms and invaded the tissue by using the same well-differentiated cell culture preparation.
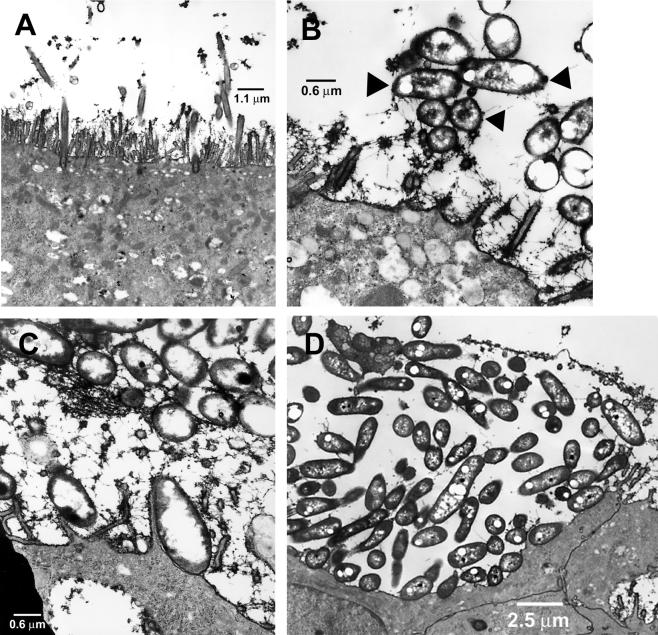
In contrast to BC-7, the B. stabilis HI2258 strain did not form biofilms in any of the cultures examined, as determined by using the different staining methods (Table 1). Instead, this organism was predominantly located between cells and occasionally within cells in infected well-differentiated cultures (Fig. 3). No disruption of the integrity of the epithelium was observed during infection with this strain. Intercellular tight junctions were observed above single bacteria and clusters, indicating that the integrity of the epithelia was not damaged by the bacterial penetration into the epithelium. The integrity of the tight junctions was further assessed by Rt measurements, which showed similar values (means ± standard errors) for Rt for noninfected (884 ± 284 Ω/cm2; n = 3) and HI2258-infected (747 ± 197 Ω/cm2; n = 3) cultures. When cultures were infected with ∼5 × 107 CFU of UV-irradiated or heat-killed bacteria for 24 h, no intra- or intercellular bacteria were detected, suggesting that the penetration of this organism into or between the cells required living cells.
FIG. 3.
Light micrograph of semithin section of a well-differentiated airway epithelial cell culture infected with B. stabilis HI2258 (∼5 × 107 CFU/culture) for 24 h. Note bacteria within and between epithelial cells (see also Table 1).
Biofilm-dependent and -independent invasion by B. multivorans J-1 (genomovar II).
When a more common genomovar type, i.e., genomovar II (B. multivorans), which is seen in about 38% of infected CF patients (18), was tested for invasion of well-differentiated airway epithelia, it was found that the B. multivorans J-1 strain appeared to use multiple invasion routes. This strain invaded the tissue after biofilm formation (one to six biofilms per section), but it also was detected within and between epithelial cells in areas not associated with biofilms (data not shown). Quantitative analysis of multiple cultures for invasion patterns showed that the invasion patterns were seemingly equally distributed (Table 1). Occasionally, J-1 caused extensive damage of the tissue that included the basal cell layer. Again, in cultures infected with UV-irradiated or heat-killed bacteria, no invasion was observed (data not shown).
Biofilm-dependent invasion process involves disruption of the apical cell surface glycocalyx.
Semithin sections of tissue cultures exposed to ∼5 × 107 CFU of B. cepacia BC-7 or vehicle (24-h incubation) were prepared and examined by LM. Areas of the epithelium associated with overlying biofilms were selected and prepared for TEM. In noninfected cultures, the electron-dense ruthenium red stained a clearly defined 70- to 100-nm-thick glycocalyx on the apical membrane of the airway epithelium and the cilia (Fig. 4A). In areas of infected cultures where small biofilms formed and the mucus layer was preserved, the bacteria, mucins contained in the mucus layer, and the cell surface glycocalyx were intensely stained. In some areas, small biofilms in direct contact with the glycocalyx of microvilli were observed (Fig. 4B). However, we also observed bacteria contained within biofilms associated with microvilli on which the glycocalyx was dramatically reduced. Figure 4C shows a bacterium binding to a microvillus with the glycocalyx apparently destroyed, suggesting glycocalyx degradation by the bacteria within biofilms. In Fig. 4D, individual bacteria within a biofilm bind to the cell membrane, and the glycocalyx was absent in regions where this binding occurs. Interestingly, intracellular bacteria were also found selectively in those areas. In regions not associated with biofilms, we did not detect glycocalyx degradation or bacterial binding. This glycocalyx degradation was also found in J-1-infected cultures in areas associated with biofilms but not in cultures infected with HI2258.
FIG. 4.
TEM of ruthenium red-stained glycocalyx on the apical cell surface of noninfected and BC-7 (∼5 × 107 CFU/airway epithelial cell culture for 24 h)-infected airway epithelial cultures (see also Table 1). (A) Glycocalyx (70 to 100 nm thick) on the apical membrane of a noninfected airway epithelium including cilia. (B) Bacteria (arrowheads) closely associated with glycocalyx of microvilli. (C) Single bacterium binding to the extended microvillus with the glycocalyx apparently destroyed. (D) Mature biofilm of BC-7. Note the absence of a glycocalyx on the apical cell surface of the biofilm-associated airway epithelium.
Biofilm-dependent invasion process involves disruption of the actin cytoskeleton.
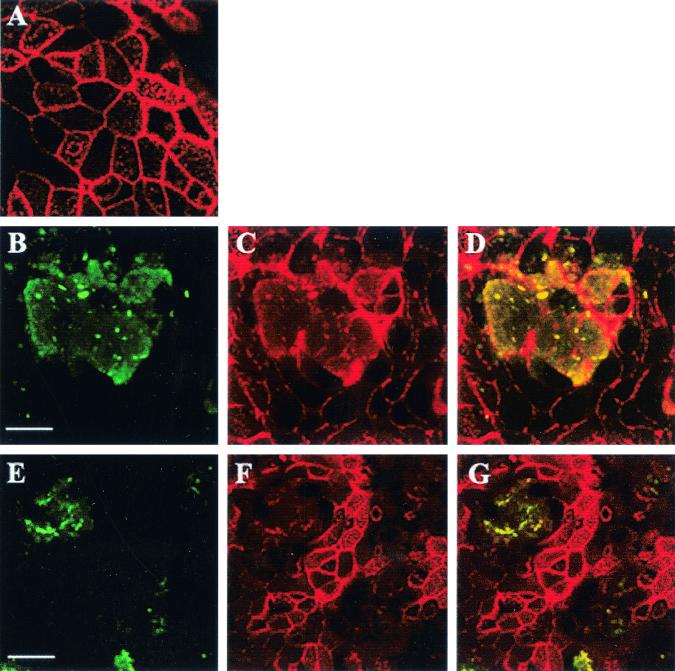
The epithelium beneath biofilms appeared “indented,” suggesting a loss of cytoskeletal function (Fig. 1B). To determine whether biofilms disrupted the actin cytoskeleton, we performed confocal microscopy using BODIPY-phalloidin to specifically label filamentous actin. In noninfected cultures, the airway epithelium showed intact actin filaments (Fig. 5A). In contrast, in J-1- and BC-7-infected cultures immunostained bacteria formed a biofilm-like structure (Fig. 5B and E) and the actin cytoskeleton in this area was rearranged (Fig. 5C and F). This notion was confirmed by an overlay of both signals, demonstrating colocalization of the bacterial and cytoskeletal stains as a yellow-stained area (Fig. 5D and G), suggesting that the invading biofilm rearranged the actin cytoskeleton. In contrast, no actin cytoskeletal changes in HI2258-infected cultures were observed. UV-irradiated and heat-killed bacteria of strains BC-7 and J-1 did not affect the actin component of the cytoskeleton (data not shown).
FIG. 5.
Visualization of B. multivorans J-1 and B. cepacia BC-7 biofilm and structure of filamentous actin in human airway epithelia. Confocal x-y scans of the apical epithelial domain from noninfected airway epithelia (A) and B. multivorans J-1- (B to D) and B. cepacia BC-7 (E to G)-infected (∼5 × 107 CFU/airway epithelial cell culture for 24 h) airway epithelia. (B and E) B. cepacia biofilm (green); (C and F), actin filaments (red; note extensive cytoskeletal rearrangements); (D and G) colocalization of green biofilm and red actin filaments seen as yellow-stained area. For immunostaining noninfected and infected airway epithelial cell cultures were incubated for 1 h at 37°C with B. cepacia antiserum (1:250 dilution), followed by incubation with a goat anti-rabbit fluorescein isothiocyanate (FITC)-conjugated antibody. For labeling the actin cytoskeleton, the immunostained airway epithelial cell cultures were incubated with BODIPY-phalloidin at a 1:25 dilution for 30 min at 25°C. Airway cultures were then visualized by confocal microscopy. Bars, 10 (A to D) and 20 μm (E to G).
Effect of bacterial density on biofilm formation.
To determine the effect of bacterial growth on biofilm formation, six airway epithelial cell cultures were infected with 5 × 105 CFU of one of the biofilm-forming strains, BC-7 or J1, or the non-biofilm-forming strain, HI2258, and incubated for 24, 36, and 48 h. In one set of experiments, the mucus from one infected culture was harvested by PBS-DTT lavage at the appropriate time points for enumeration of bacteria. For a second set of experiments, we fixed the infected culture with OsO4-perfluorocarbon to examine the mucus layer for formed biofilms.
All tested B. cepacia strains grew in mucus. As expected, strain HI2258 did not form biofilms, even after an incubation of 48 h producing >108 CFU/airway epithelial cell culture, whereas strains BC-7 and J-1 formed biofilms after 48 and 36 h of incubation, respectively, when both bacteria reached a density of >108 CFU/airway epithelial cell culture. At shorter incubation periods, the organisms presumably failed to achieve the necessary bacterial density to synthesize the products critical for biofilm formation. Infection of cultures with higher bacterial numbers (∼5 × 107 CFU/airway epithelial cell culture) required shorter incubation times for developing biofilms (24 h; Table 1). Of particular clinical relevance, the bacterial density at which B. cepacia formed biofilms correlated with the number of bacteria (106 to 108 CFU/ml) found in CF patient sputa when heavily infected with B. cepacia (Peter Gilligan, personal communication).
Effect of quorum sensing on biofilm formation.
Recently, it has been shown that biofilm formation by B. cepacia on abiotic surfaces requires a functional cepIR quorum-sensing system (11). We asked whether a cepI mutant strain that does not produce N-acylhomoserine lactones is capable of forming biofilms in the mucus layers of well-differentiated airway epithelial cell cultures. Both K56-2 (wild-type) and the cepI mutant strain formed characteristic biofilms in the mucus layer when ∼5 × 107 bacteria were incubated with the airway epithelium for 24 h (Table 1). Small biofilms growing in mucus and large biofilms growing in the epithelium and degrading the epithelial surface were observed. The number of biofilms per section ranged between 2 and 13. When lower inocula were used for infection, both the parent and the mutant strain reached >108 CFU within 36 h compared to 48 h required for BC-7. At 36 and 48 h of incubation, no apparent morphological differences between biofilms formed by the wild type K56-2 and the mutant K56-I2 strains were observed.
DISCUSSION
Bacterial invasion through the airways and sepsis are not observed with the major CF pathogens, S. aureus, H. influenzae, and P. aeruginosa (8, 12, 30). In contrast, B. cepacia invades airway epithelia and causes sepsis in CF patients. To evaluate the abilities of different species of the B. cepacia complex to penetrate airway epithelia, we conducted the present study of well-differentiated human airway epithelial cell cultures that reflect closely the morphology and function of airway epithelia in vivo (20).
Interestingly, all three B. cepacia strains tested invaded airway epithelia, but they used different invasion pathways. Strain BC-7, belonging to the transmissible genomovar III ET12 lineage, predominantly invaded the epithelium by forming biofilms in mucus that ultimately reached epithelial surfaces (Fig. 1 and 2). Almost all intra- and intercellular organisms of this strain were found associated with biofilms. Immunolocalization studies of CF patient lung tissue infected by strains of the ET12 lineage showed bacterial clusters in the luminal exudates within the larger bronchi, some of which were associated with the epithelial surface (27). Based on our observations, these bacterial clusters could reflect biofilms growing in mucus that extended to the apical cell surface of the epithelium. In addition, the study of Sajjan et al. (27) also reported bacteria in surface epithelial cells and between adjacent epithelial cells, consistent with our findings.
Bacteria that grow in the stationary mucus layer that characterizes CF airway surfaces (20, 36) face a second extracellular barrier, the glycocalyx, on the apical surface of human airway epithelial cells. The glycocalyx is composed of carbohydrate-rich molecules, including proteoglycans, glycolipids, glycoproteins, and, most notably, cell surface (“tethered”) mucins (1, 21). In BC-7-infected cultures, the glycocalyx matrix was disrupted in areas where bacteria contained within biofilms closely associated with the apical cell surface of the epithelium (Fig. 4D). We observed bacteria closely attached to microvilli devoid of glycocalyx (Fig. 4C), suggesting that B. cepacia degraded the glycocalyx. Of interest, this phenomenon was observed only (i) with bacteria within a biofilm and (ii) in areas associated with cellular invasion.
Our data suggest a sequence for BC-7 invasion of the airway epithelium. First, a biofilm forms in stagnant airway mucus. Interestingly, our results argue for a role for bacterial density in biofilm formation but argue against the involvement of the cepIR quorum-sensing system in biofilm formation since an isolate (K56-I2) of the highly transmissible ET12 lineage with a mutation in the cepI autoinducer synthase still formed biofilms. Huber et al. (11) showed by quantitative characterization of B. cepacia H111 biofilm structures on abiotic surfaces that the cep quorum-sensing system may not be important for the initial attachment of bacteria to the surface but is essential for the differentiation of microcolonies, a process that is required for the development of a mature biofilm. Using our descriptive techniques we are not able to differentiate between microcolonies and mature biofilms. However, our data indicate that biofilms formed in mucus rather than on an abiotic surface may not require the cep quorum-sensing system. Second, BC-7 growing in biofilms can apparently degrade the glycocalyx barrier, which appears to be a precursor to cellular invasion. This capacity to degrade the mucin-rich glycocalyx is consistent with previous data. For example, B. cepacia has been shown to possess mucin-sulfatase activity (14), which renders mucins susceptible to degradation by bacterial glycosidases and proteinases. B. cepacia can also grow in mucus, suggesting that it may produce mucin-degrading enzymes to utilize mucins as carbohydrate and energy resources. Consequently, if B. cepacia produces mucin-degrading enzymes (31) under biofilm conditions, those same enzymes could also degrade glycocalyx components, particularly the tethered mucins (1, 21). Third, the biofilms formed by B. cepacia BC-7 induced rearrangements in the actin cytoskeleton. These rearrangements appear to be associated with a loss of cell structural integrity as the cells underneath the biofilm appear to “collapse” (Fig. 1B and 2). At this point we are not yet able to determine which bacterial factors are responsible for actin cytoskeletal disruption and whether these factors are produced uniquely under biofilm conditions. Fourth, following cytoskeletal rearrangements, there appears to be a frank epithelial necrosis that destroys the luminal cells but not the basal cells (Fig. 2).
Observations made by other investigators are consistent with aspects of this scheme. For example, ultrastructural observations of endoscopic biopsy samples from a Helicobacter pylori-infected gastric epithelium showed that adherence to the target cell was initiated by direct contact with the cell surface, leading to the loss of the glycocalyx layer (7). Thus, it seems that H. pylori is able to bridge the gastric epithelial barrier, in part by disrupting the glycocalyx.
The absence of biofilm formation by the B. stabilis HI2258 strain (genomovar IV) in our study is consistent with the data of Conway and Speert (B. D. Conway and D. P. Speert, abstract from the North American Cystic Fibrosis Conference 2001, abstr. no. 323), who reported that several genomovar IV strains did not form biofilms on polypropylene surfaces. Consistent with the scheme described above, there was no detectable degradation of the glycocalyx and no disruption of the actin cytoskeleton in the absence of biofilms in HI2258-infected airway epithelial cell cultures. In contrast, the paracytosis route across airway epithelia that we observed for this strain (Fig. 3) has been reported for bacteria that do not form biofilms. For example, this route is used by H. influenzae when passing through cell layers of human lung epithelial cell line NCI-H292 (35). Although B. stabilis has been recovered from the respiratory tracts of CF patients, there are no data which associate this organism with worsening CF pulmonary disease. The finding of a lack of invasiveness with this organism may be consistent with this observation.
Strain J-1, belonging to genomovar type II, which is observed in CF patients more often than genomovar IV, formed biofilms and showed glycocalyx disruption and rearrangements of the cytoskeleton of epithelial cells associated with biofilms. Whether biofilm formation is a characteristic feature of genomovars III and II, which are the most common genomovar types in CF in vivo (18), remains to be investigated.
Why B. cepacia causes the cepacia syndrome in ∼20% of infected CF patients while, in the majority of infected patients, B. cepacia persists in the airways is not yet clear. Our data suggest that the BC-7 and K56-2 strains, which belong to the ET12 lineage (genomovar III) associated with cepacia syndrome (18), have to reach a relatively high density to form biofilms and invade the tissue. Thus, bacterial growth rate may play an important role in the virulence of these bacteria. The fact that strains belonging to the most common genomovar types associated with sepsis in vivo, genomovars II (29) and III (18), showed a common invasion pattern with biofilm formation and epithelial necrosis supports our notion that this invasion pathway may be important in vivo. Consequently, these studies may help in the development of new strategies for preventive and/or therapeutic intervention against the factors that trigger epithelial penetration and sepsis by these microbes.
Acknowledgments
We thank Tracy Eldred, UNC Histology Core, for immunostaining of infected airway epithelial cell cultures and Scott Randell for technical assistance in the preparation of well-differentiated airway epithelial cultures.
This work was supported by research grant SCHWAB01I0 from the Cystic Fibrosis Foundation.
Editor: D. L. Burns
REFERENCES
- 1.Bernacki, S. H., A. L. Nelson, L. Abdullah, J. K. Sheehan, A. Harris, C. W. Davis, and S. H. Randell. 1999. Mucin gene expression during differentiation of human airway epithelia in vitro. Am. J. Respir. Cell Mol. Biol. 20:595-604. [DOI] [PubMed] [Google Scholar]
- 2.Buhler,T., S. Ballestero, M. Desai, and M. R. Brown. 1998. Generation of a reproducible nutrient-depleted biofilm of Escherichia coli and Burkholderia cepacia. J. Appl. Microbiol. 85:457-462. [DOI] [PubMed] [Google Scholar]
- 3.Burns, J. L., M. Jonas, E. Y. Chi, D. K. Clark, A. Berger, and A. Griffith. 1996. Invasion of respiratory epithelial cells by Burkholderia (Pseudomonas) cepacia. Infect. Immun. 64:4054-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coenye, T., P. Vandamme, J. R. W. Govan, and J. L. LiPuma. 2001. Taxonomy and identification of the Burkholderia cepacia complex. J. Clin. Microbiol. 39:3427-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 6.Desai, M., T. Buhler, P. H. Weller, and M. R. Brown. 1998. Increasing resistance of planktonic and biofilm cultures of Burkholderia cepacia to ciprofloxacin and ceftazidime during exponential growth. J. Antimicrob. Chemother. 42:153-160. [DOI] [PubMed] [Google Scholar]
- 7.el-Shoura, S. M. 1995. Helicobacter pylori: I. Ultrastructural sequences of adherence, attachment, and penetration into the gastric mucosa. Ultrastruct. Pathol. 19:323-333. [DOI] [PubMed] [Google Scholar]
- 8.Gilligan, P. 1999. Microbiology of cystic fibrosis lung disease, p. 69-92. In J. R. Yankaskas and M. R. Knowles (ed.), Cystic fibrosis in adults. Lippincott-Raven Publishers, Philadelphia. Pa.
- 9.Govan, J. R. W., J. E. Hughes, and P. Vandamme. 1996. Burkholderia cepacia: medical, taxonomic and ecological issues. J. Med. Microbiol. 45:395-407. [DOI] [PubMed] [Google Scholar]
- 10.Hayat, M.A. 1975. Positive staining for electron microscopy. Reinhold, New York, N.Y.
- 11.Huber, B., K. Riedel, M. Hentzer, A. Heydorn, A. Gotschlich, M. Givskov, S. Molin, and L. Eberl. 2001. The cep quorum sensing system of Burkholderia cepacia HIII controls biofilm formation and swarming motility. Microbiology 147:2517-2528. [DOI] [PubMed] [Google Scholar]
- 12.Hutchison, M. L., and J. R. W. Govan. 1999. Pathogenicity of microbes associated with cystic fibrosis. Microb. Infect. 1:1005-1014. [DOI] [PubMed] [Google Scholar]
- 13.Isles, A., I. MacLusky, M. Corey, R. Gold, C. Prober, P. Fleming, and H. Levison. 1984. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J. Pediatr. 104:206-221. [DOI] [PubMed] [Google Scholar]
- 14.Jansen, H. J., C. A. Hart, J. M. Rhodes, J. R. Saunders, and J. W. Smalley. 1999. A novel mucin-sulphatase activity found in Burkholderia cepacia and Pseudomonas aeruginosa. J. Med. Microbiol. 48:551-557. [DOI] [PubMed] [Google Scholar]
- 15.Kaitwatcharachai, C., K. Silpapojakul, S. Jitsurong, and S. Kalnauwakul. 2000. An outbreak of Burkholderia cepacia bacteremia in hemodialysis patients: an epidemiologic and molecular study. Am. J. Kidney Dis. 36:199-204. [DOI] [PubMed] [Google Scholar]
- 16.Keig, P. M., E. Ingham, and K. G. Kerr. 2001. Invasion of human type II pneumocytes by Burkholderia cepacia. Microb. Pathog. 30:167-170. [DOI] [PubMed] [Google Scholar]
- 17.Lewenza, S., B. Conway, E. P. Greenberg, and P. A. Sokol. 1999. Quorum sensing in Burkholderia cepacia: identification of the LuxRI homologs Cep-RI. J. Bacteriol. 181:748-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LiPuma, J. J., T. Spilker, L. H. Gill, P. W. Campbell III, L. Liu, and E. Mahenthiralingam. 2001. Disproportionate distribution of Burkholderia cepacia complex species and transmissibility markers in cystic fibrosis. Am. J. Respir. Crit. Care Med. 164:92-96. [DOI] [PubMed] [Google Scholar]
- 19.Martin, D. W., and C. D. Mohr. 2000. Invasion and intracellular survival of Burkholderia cepacia. Infect. Immun. 68:24-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsui, H., B. R. Grubb, R. Tarran, S. H. Randell, J. T. Gatzy, C. W. Davis, and R. C. Boucher. 1998. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 95:1005-1015. [DOI] [PubMed] [Google Scholar]
- 21.Moniaux, N., S. Nollet, N. Porchet, P. Degand, A. Laine, and J. P. Aubert. 1999. Complete sequence of the human mucin MUC4: a putative cell membrane-associated mucin. Biochem. J. 338:325-333. [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen, A. T., T. Toker-Nielsen, K. B. Barken, and S. Molin. 2000. Role of commensal relationships on the spatial structure of a surface-attached microbial consortium. Environ. Microbiol. 2:59-68. [DOI] [PubMed] [Google Scholar]
- 23.Pickles, R. J., J. A. Fahrner, J. M. Petrella, R. C. Boucher, and J. M. Bergelson. 2000. Retargeting the coxsackievirus and adenovirus receptor to the apical surface of polarized epithelial cells reveals the glycocalyx as a barrier to adenovirus-mediated gene transfer. J. Virol. 74:6050-6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribeiro, C. M., J. Reece, and J. W. Putney, Jr. 1997. Role of the cytoskeleton in calcium signaling in NIH 3T3 cells. An intact cytoskeleton is required for agonist-induced [Ca2+]i signaling, but not for capacitative calcium entry. J. Biol. Chem. 272:26555-26561. [DOI] [PubMed] [Google Scholar]
- 25.Riedel, K., M. Hentzer, O. Geisenberger, B. Huber, A. Steidle, H. Wu, N. Hoiby, M. Givskov, S. Molin, and L. Eberl. 2001. N-acylhomoserine-lactone-mediated communication between Pseudomonas aeruginosa and Burkholderia cepacia in mixed biofilms. Microbiology 7:3249-3262. [DOI] [PubMed] [Google Scholar]
- 26.Saini, L. S., S. B. Galsworthy, M. A. John, and M. A. Valvano. 1999. Intracellular survival of Burkholderia cepacia complex isolates in the presence of macrophage cell activation. Microbiology 145:3465-3475. [DOI] [PubMed] [Google Scholar]
- 27.Sajjan, U., M. Corey, A. Humar, E. Tullis, E. Cutz, C. Ackerley, and J. Forstner. 2001. Immunolocalisation of Burkholderia cepacia in the lungs of cystic fibrosis patients. J. Med. Microbiol. 50:535-546. [DOI] [PubMed] [Google Scholar]
- 28.Sajjan, U. S., M. Corey, M. A. Karmali, and J. F. Forstner. 1992. Binding of Pseudomonas cepacia to normal human intestinal mucin and respiratory mucin from patients with cystic fibrosis. J. Clin. Investig. 89:648-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segonds, C., T. Heulin, N. Marty, and G. Chabanon. 1999. Differentiation of Burkholderia species by PCR-restriction fragment length polymorphism analysis of the 16S rRNA gene and application to cystic fibrosis isolates. J. Clin. Microbiol. 37:2201-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, A. 1997. Pathogenesis of bacterial bronchitis in cystic fibrosis. Pediatr. Infect. Dis. J. 16:91-95. [DOI] [PubMed] [Google Scholar]
- 31.Stark, R. M., R. Wiggins, E. Walley, S. J. Hicks, G. A. Gill, S. D. Carrington, and A. P. Corfield. 2000. Mucinase activity, p. 385-392. In A. P. Corfield (ed.), Glycoprotein methods and protocols: the mucins. Humana Press, Inc., Totowa, N.J. [DOI] [PubMed]
- 32.Sun, L., R. Z. Jiang, S. Steinbach, A. Holmes, C. Campanelli, J. Forstner, U. Sajjan, Y. Tan, M. Riley, and R. Goldstein. 1995. The emergence of a highly transmissible lineage of cbl+ Pseudomonas (Burkholderia) cepacia causing CF centre epidemics in North America and Britain. Nat. Med. 1:661-666. [DOI] [PubMed] [Google Scholar]
- 33.Vandamme, P., E. Mahenthiralingam, B. Holmes, T. Coenye, B. Hoste, P. De Vos, D. Henry, and D. P. Speert. 2000. Identification and population structure of Burkholderia stabilis sp. nov. (formerly Burkholderia cepacia genomovar IV). J. Clin. Microbiol. 38:1042-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandamme, P., D. Henry, T. Coenye, S. Nzula, M. Vancanneyt, J. J. LiPuma, D. P. Speert, J. R. W. Govan, and E. Mahenthiralingam. Burkholderia anthina sp. nov. and Burkholderia pyrrocinia, two additional Burkholderia cepacia complex bacteria, may confound test results of new molecular diagnostic tools. FEMS Med. Microbiol. Immunol., in press. [DOI] [PubMed]
- 35.Van Schilfgaarde, M., L. Van Alphen, P. Eijk, V. Everts, and J. Dankert. 1995. Paracytosis of Haemophilus influenzae through cell layers of NCI-H292 lung epithelial cells. Infect. Immun. 63:4729-4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worlitzsch, D., R. Tarran, M. Ulrich, U. Schwab, A. Cekici, K. Meyer, P. Birrer, G. Bellon, J. Berger, T. Weiss, K. Botzenhart, J. Yankaskas, S. Randell, R. Boucher, and G. Doring. 2001. Effects of reduced mucus oxygen concentrations in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Investig. 109:317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]