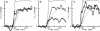Abstract
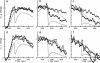
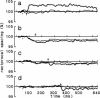
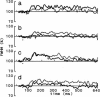
Time-resolved intensity measurements of the x-ray equatorial reflections were made during twitch contractions of frog skeletal muscles, to which stretches or releases were applied at various times. A ramp stretch applied at the onset of a twitch (duration, 15 ms; amplitude, approximately 3% of muscle length) caused a faster and larger development of contractile force than in an isometric twitch. The stretch accelerated the decrease of the 1.0 reflection intensity (I1,0). The magnitude of increase of the 1,1 reflection intensity (I1,1) was reduced by the stretch, but its time course was also accelerated. A release applied at the peak of a twitch or later (duration, 5 ms; amplitude, approximately 1.5%) caused only a partial redevelopment of tension. The release produced clear reciprocal changes of reflections toward their relaxed levels, i.e., the I1,0 increased and the I1,1 decreased. A release applied earlier than the twitch peak had smaller effects on the reflection intensities. The results suggest that a strength applied at the onset of a twitch causes a faster radial movement of the myosin heads toward actin, whereas a release applied at or later than the peak of a twitch accelerates their return to the thick filament backbone. The results are discussed in the context of the regulation of the myosin head attachment by calcium.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amemiya Y., Iwamoto H., Kobayashi T., Sugi H., Tanaka H., Wakabayashi K. Time-resolved X-ray diffraction studies on the effect of slow length changes on tetanized frog skeletal muscle. J Physiol. 1988 Dec;407:231–241. doi: 10.1113/jphysiol.1988.sp017412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Briden K. L., Alpert N. R. The effect of shortening on the time-course of active state decay. J Gen Physiol. 1972 Aug;60(2):202–220. doi: 10.1085/jgp.60.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi G., Griffiths P. J., Bagni M. A., Ashley C. C., Maeda Y. Time-resolved changes in equatorial x-ray diffraction and stiffness during rise of tetanic tension in intact length-clamped single muscle fibers. Biophys J. 1991 Jun;59(6):1273–1283. doi: 10.1016/S0006-3495(91)82342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi G., Griffiths P. J., Taylor S. Muscular contraction: kinetics of crossbridge attachment studied by high-frequency stiffness measurements. Science. 1982 Jul 2;217(4554):70–72. doi: 10.1126/science.6979780. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Edman K. A. Depression of mechanical performance by active shortening during twitch and tetanus of vertebrate muscle fibres. Acta Physiol Scand. 1980 May;109(1):15–26. doi: 10.1111/j.1748-1716.1980.tb06559.x. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Flitney F. W. Laser diffraction studies of sarcomere dynamics during 'isometric' relaxation in isolated muscle fibres of the frog. J Physiol. 1982 Aug;329:1–20. doi: 10.1113/jphysiol.1982.sp014287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension transients during the rise of tetanic tension in frog muscle fibres. J Physiol. 1986 Mar;372:595–609. doi: 10.1113/jphysiol.1986.sp016027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Ridgway E. B. Extra calcium on shortening in barnacle muscle. Is the decrease in calcium binding related to decreased cross-bridge attachment, force, or length? J Gen Physiol. 1987 Sep;90(3):321–340. doi: 10.1085/jgp.90.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Ridgway E. B., Yates L. D., Allen T. Muscle cross-bridge attachment: effects on calcium binding and calcium activation. Adv Exp Med Biol. 1988;226:89–99. [PubMed] [Google Scholar]
- HILL A. V. The abrupt transition from rest to activity in muscle. Proc R Soc Lond B Biol Sci. 1949 Oct;136(884):399–420. doi: 10.1098/rspb.1949.0033. [DOI] [PubMed] [Google Scholar]
- Harford J. J., Squire J. M. Evidence for structurally different attached states of myosin cross-bridges on actin during contraction of fish muscle. Biophys J. 1992 Aug;63(2):387–396. doi: 10.1016/S0006-3495(92)81613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselgrove J. C., Huxley H. E. X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J Mol Biol. 1973 Jul 15;77(4):549–568. doi: 10.1016/0022-2836(73)90222-2. [DOI] [PubMed] [Google Scholar]
- Haselgrove J. C., Stewart M., Huxley H. E. Cross-bridge movement during muscle contraction. Nature. 1976 Jun 17;261(5561):606–608. doi: 10.1038/261606a0. [DOI] [PubMed] [Google Scholar]
- Hatta I., Sugi H., Tamura Y. Stiffness changes in frog skeletal muscle during contraction recorded using ultrasonic waves. J Physiol. 1988 Sep;403:193–209. doi: 10.1113/jphysiol.1988.sp017245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen P. Calcium transients in skeletal muscle fibres under isometric conditions and during and after a quick stretch. J Muscle Res Cell Motil. 1991 Dec;12(6):566–578. doi: 10.1007/BF01738445. [DOI] [PubMed] [Google Scholar]
- Haugen P., Sten-Knudsen O. The time course of the contractile force measured during a twitch under fixed sarcomere length. J Muscle Res Cell Motil. 1987 Apr;8(2):173–187. doi: 10.1007/BF01753993. [DOI] [PubMed] [Google Scholar]
- Hill T. L., Eisenberg E., Greene L. Theoretical model for the cooperative equilibrium binding of myosin subfragment 1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3186–3190. doi: 10.1073/pnas.77.6.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley H. E., Simmons R. M., Faruqi A. R., Kress M., Bordas J., Koch M. H. Changes in the X-ray reflections from contracting muscle during rapid mechanical transients and their structural implications. J Mol Biol. 1983 Sep 15;169(2):469–506. doi: 10.1016/s0022-2836(83)80062-x. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Simmons R. M., Faruqi A. R., Kress M., Bordas J., Koch M. H. Millisecond time-resolved changes in x-ray reflections from contracting muscle during rapid mechanical transients, recorded using synchrotron radiation. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2297–2301. doi: 10.1073/pnas.78.4.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley H. E. The structural basis of contraction and regulation in skeletal muscle. Kaibogaku Zasshi. 1975 Dec;50(6):310–325. [PubMed] [Google Scholar]
- Irving M., Lombardi V., Piazzesi G., Ferenczi M. A. Myosin head movements are synchronous with the elementary force-generating process in muscle. Nature. 1992 May 14;357(6374):156–158. doi: 10.1038/357156a0. [DOI] [PubMed] [Google Scholar]
- Iwamoto H., Sugaya R., Sugi H. Force-velocity relation of frog skeletal muscle fibres shortening under continuously changing load. J Physiol. 1990 Mar;422:185–202. doi: 10.1113/jphysiol.1990.sp017979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress M., Huxley H. E., Faruqi A. R., Hendrix J. Structural changes during activation of frog muscle studied by time-resolved X-ray diffraction. J Mol Biol. 1986 Apr 5;188(3):325–342. doi: 10.1016/0022-2836(86)90158-0. [DOI] [PubMed] [Google Scholar]
- Lymn R. W. Myosin subfragment-1 attachment to actin. Expected effect on equatorial reflections. Biophys J. 1978 Jan;21(1):93–98. doi: 10.1016/S0006-3495(78)85510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara I., Yagi N. A time-resolved X-ray diffraction study of muscle during twitch. J Physiol. 1978 May;278:297–307. doi: 10.1113/jphysiol.1978.sp012305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky R. J., St Onge H., Yu L., Lymn R. W. X-ray diffraction of actively shortening muscle. Proc Natl Acad Sci U S A. 1976 Mar;73(3):813–817. doi: 10.1073/pnas.73.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITCHIE J. M. The effect of nitrate on the active state of muscle. J Physiol. 1954 Oct 28;126(1):155–168. doi: 10.1113/jphysiol.1954.sp005200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway E. B., Gordon A. M. Muscle calcium transient. Effect of post-stimulus length changes in single fibers. J Gen Physiol. 1984 Jan;83(1):75–103. doi: 10.1085/jgp.83.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K., Tanaka H., Amemiya Y., Fujishima A., Kobayashi T., Hamanaka T., Sugi H., Mitsui T. Time-resolved x-ray diffraction studies on the intensity changes of the 5.9 and 5.1 nm actin layer lines from frog skeletal muscle during an isometric tetanus using synchrotron radiation. Biophys J. 1985 Jun;47(6):847–850. doi: 10.1016/S0006-3495(85)83989-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K., Tanaka H., Saito H., Moriwaki N., Ueno Y., Amemiya Y. Dynamic X-ray diffraction of skeletal muscle contraction: structural change of actin filaments. Adv Biophys. 1991;27:3–13. doi: 10.1016/0065-227x(91)90004-w. [DOI] [PubMed] [Google Scholar]
- Yu L. C., Steven A. C., Naylor G. R., Gamble R. C., Podolsky R. J. Distribution of mass in relaxed frog skeletal muscle and its redistribution upon activation. Biophys J. 1985 Mar;47(3):311–321. doi: 10.1016/S0006-3495(85)83921-7. [DOI] [PMC free article] [PubMed] [Google Scholar]