Abstract
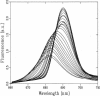
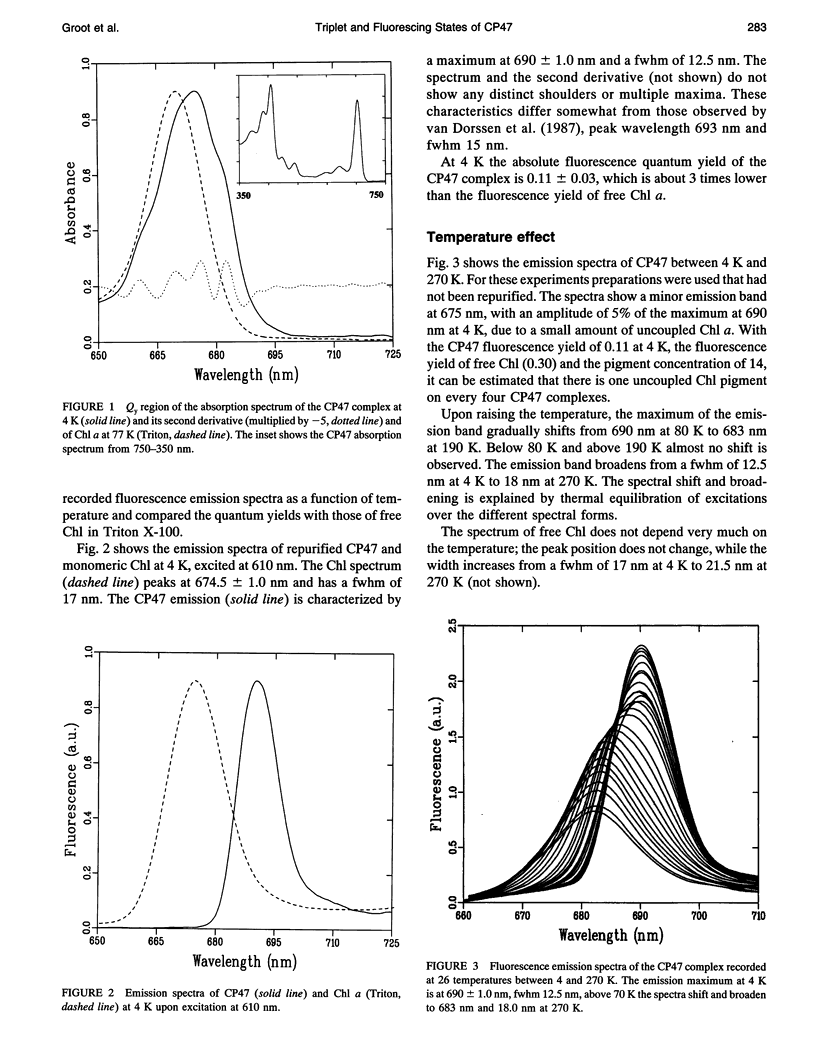
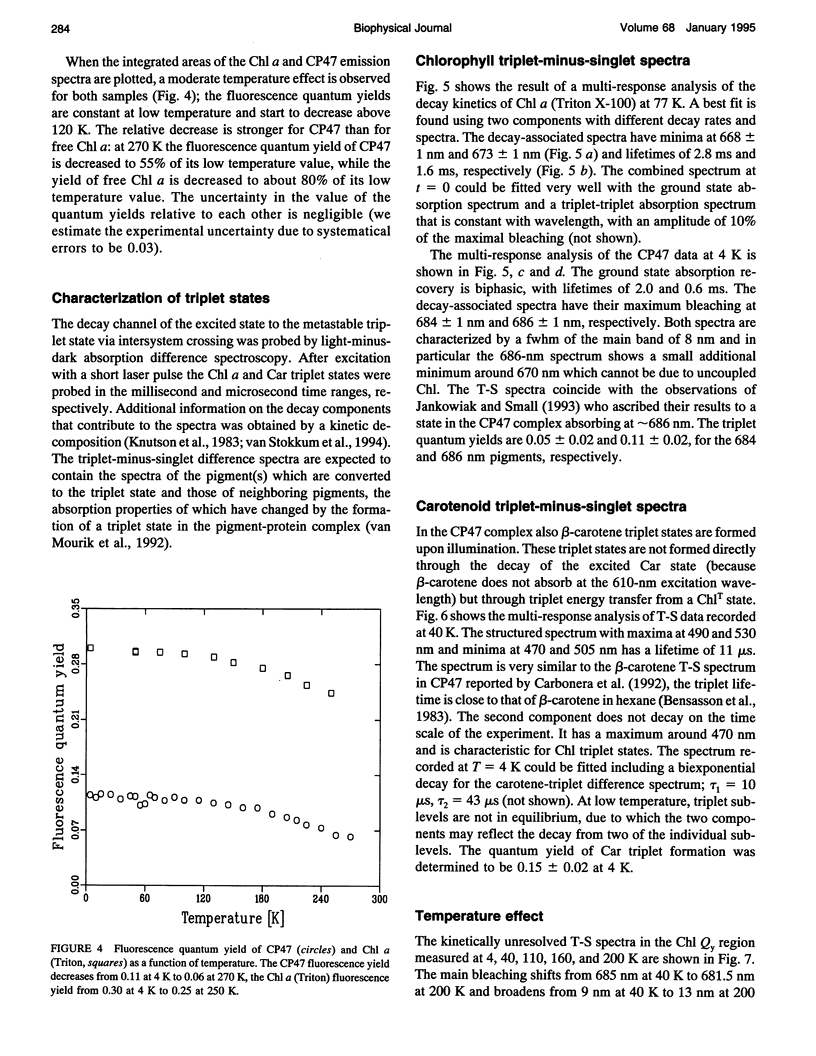
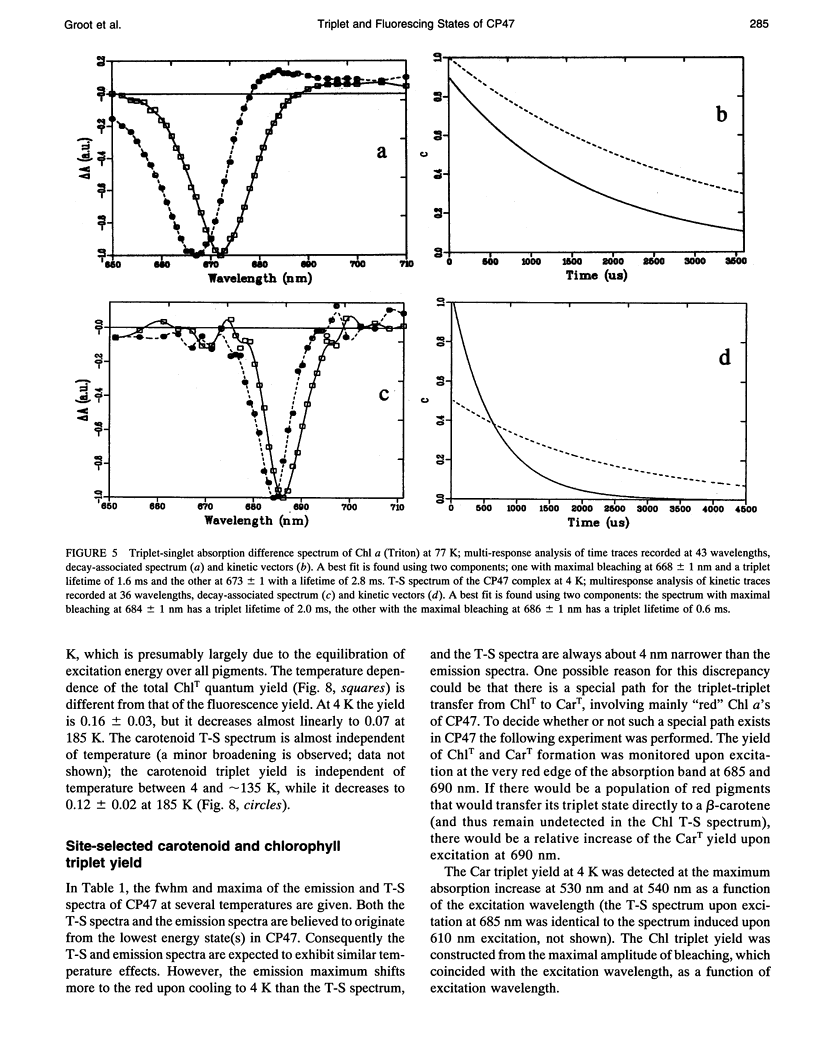
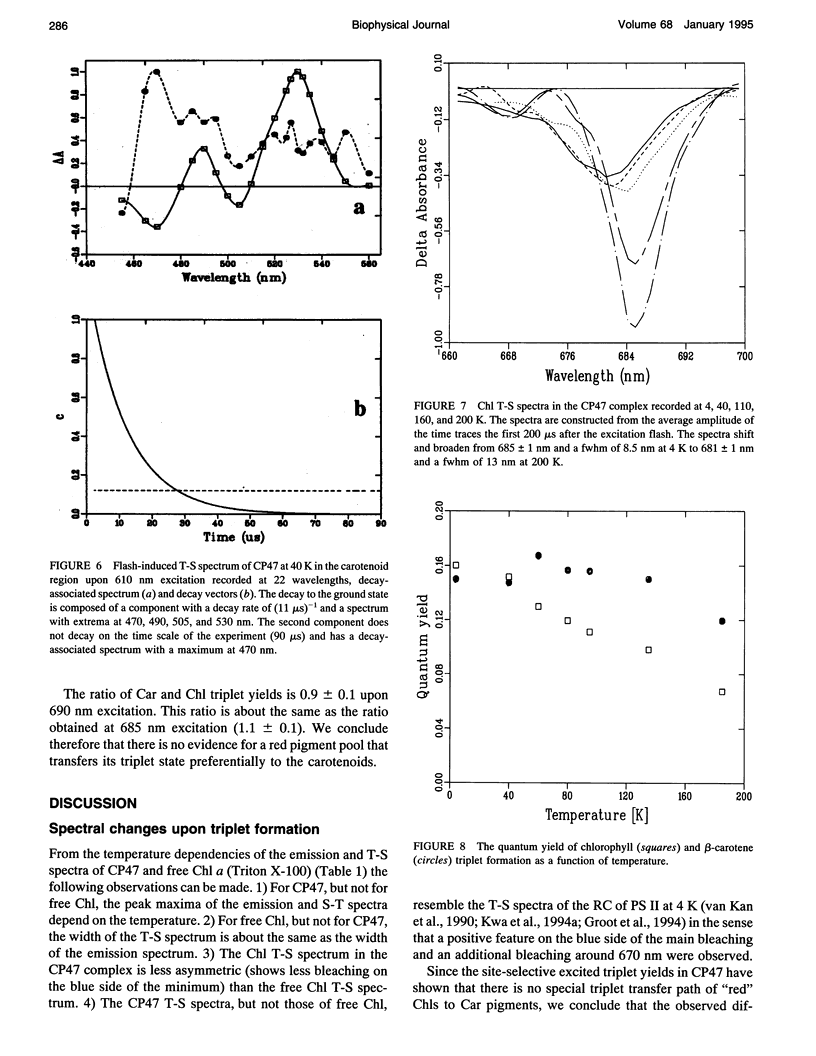
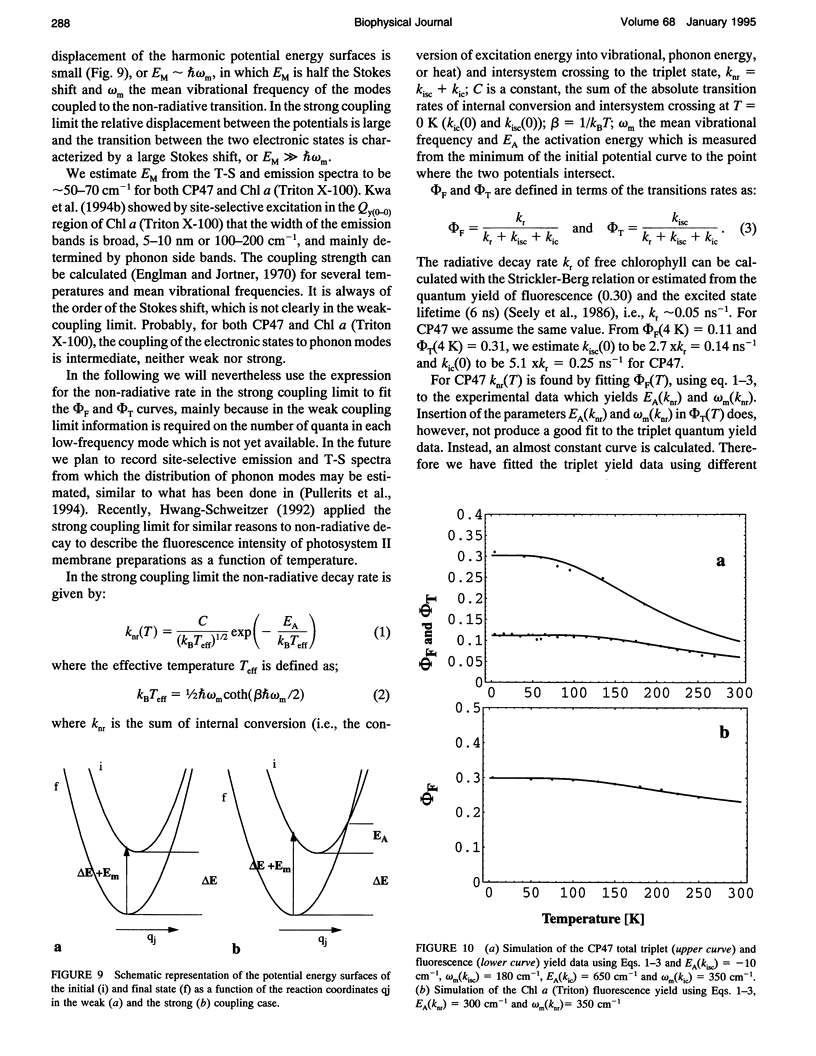
Fluorescence emission and triplet-minus-singlet (T-S) absorption difference spectra of the CP47 core antenna complex of photosystem II were measured as a function of temperature and compared to those of chlorophyll a in Triton X-100. Two spectral species were found in the chlorophyll T-S spectra of CP47, which may arise from a difference in ligation of the pigments or from an additional hydrogen bond, similar to what has been found for Chl molecules in a variety of solvents. The T-S spectra show that the lowest lying state in CP47 is at approximately 685 nm and gives rise to fluorescence at 690 nm at 4 K. The fluorescence quantum yield is 0.11 +/- 0.03 at 4 K, the chlorophyll triplet yield is 0.16 +/- 0.03. Carotenoid triplets are formed efficiently at 4 K through triplet transfer from chlorophyll with a yield of 0.15 +/- 0.02. The major decay channel of the lowest excited state in CP47 is internal conversion, with a quantum yield of about 0.58. Increase of the temperature results in a broadening and blue shift of the spectra due to the equilibration of the excitation over the antenna pigments. Upon increasing the temperature, a decrease of the fluorescence and triplet yields is observed to, at 270 K, a value of about 55% of the low temperature value. This decrease is significantly larger than of chlorophyll a in Triton X-100. Although the coupling to low-frequency phonon or vibration modes of the pigments is probably intermediate in CP47, the temperature dependence of the triplet and fluorescence quantum yield can be modeled using the energy gap law in the strong coupling limit of Englman and Jortner (1970. J. Mol. Phys. 18:145-164) for non-radiative decays. This yields for CP47 an average frequency of the promoting/accepting modes of 350 cm-1 with an activation energy of 650 cm-1 for internal conversion and activationless intersystem crossing to the triplet state through a promoting mode with a frequency of 180 cm-1. For chlorophyll a in Triton X-100 the average frequency of the promoting modes for non-radiative decay is very similar, but the activation energy (300 cm-1) is significantly smaller.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbato R., Race H. L., Friso G., Barber J. Chlorophyll levels in the pigment-binding proteins of photosystem II. A study based on the chlorophyll to cytochrome ratio in different photosystem II preparations. FEBS Lett. 1991 Jul 29;286(1-2):86–90. doi: 10.1016/0014-5793(91)80947-2. [DOI] [PubMed] [Google Scholar]
- Dekker J. P., Betts S. D., Yocum C. F., Boekema E. J. Characterization by electron microscopy of isolated particles and two-dimensional crystals of the CP47-D1-D2-cytochrome b-559 complex of photosystem II. Biochemistry. 1990 Apr 3;29(13):3220–3225. doi: 10.1021/bi00465a011. [DOI] [PubMed] [Google Scholar]
- Ghanotakis D. F., de Paula J. C., Demetriou D. M., Bowlby N. R., Petersen J., Babcock G. T., Yocum C. F. Isolation and characterization of the 47 kDa protein and the D1-D2-cytochrome b-559 complex. Biochim Biophys Acta. 1989 Apr 17;974(1):44–53. doi: 10.1016/s0005-2728(89)80164-1. [DOI] [PubMed] [Google Scholar]
- Groot M. L., Peterman E. J., van Kan P. J., van Stokkum I. H., Dekker J. P., van Grondelle R. Temperature-dependent triplet and fluorescence quantum yields of the photosystem II reaction center described in a thermodynamic model. Biophys J. 1994 Jul;67(1):318–330. doi: 10.1016/S0006-3495(94)80483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan V., Parson W. W., Davis D., Schenck C. C. Kinetics and free energy gaps of electron-transfer reactions in Rhodobacter sphaeroides reaction centers. Biochemistry. 1993 Nov 23;32(46):12324–12336. doi: 10.1021/bi00097a008. [DOI] [PubMed] [Google Scholar]
- Petersen J., Dekker J. P., Bowlby N. R., Ghanotakis D. F., Yocum C. F., Babcock G. T. EPR characterization of the CP47-D1-D2-cytochrome b-559 complex of photosystem II. Biochemistry. 1990 Apr 3;29(13):3226–3231. doi: 10.1021/bi00465a012. [DOI] [PubMed] [Google Scholar]
- Shreve A. P., Cherepy N. J., Franzen S., Boxer S. G., Mathies R. A. Rapid-flow resonance Raman spectroscopy of bacterial photosynthetic reaction centers. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11207–11211. doi: 10.1073/pnas.88.24.11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paula J. C., Liefshitz A., Hinsley S., Lin W., Chopra V., Long K., Williams S. A., Belts S., Yocum C. F. Structure-function relationships in the 47-kDa antenna protein and its complex with the photosystem II reaction center core: insights from picosecond fluorescence decay kinetics and resonance Raman spectroscopy. Biochemistry. 1994 Feb 15;33(6):1455–1466. doi: 10.1021/bi00172a023. [DOI] [PubMed] [Google Scholar]