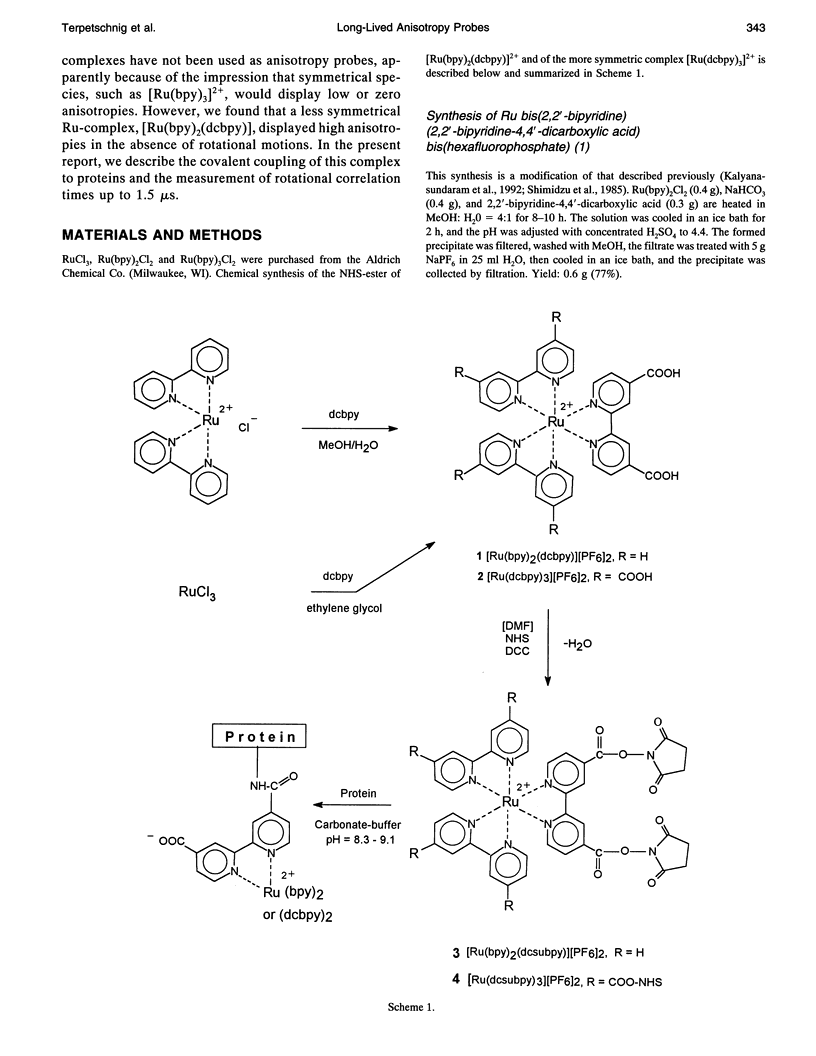
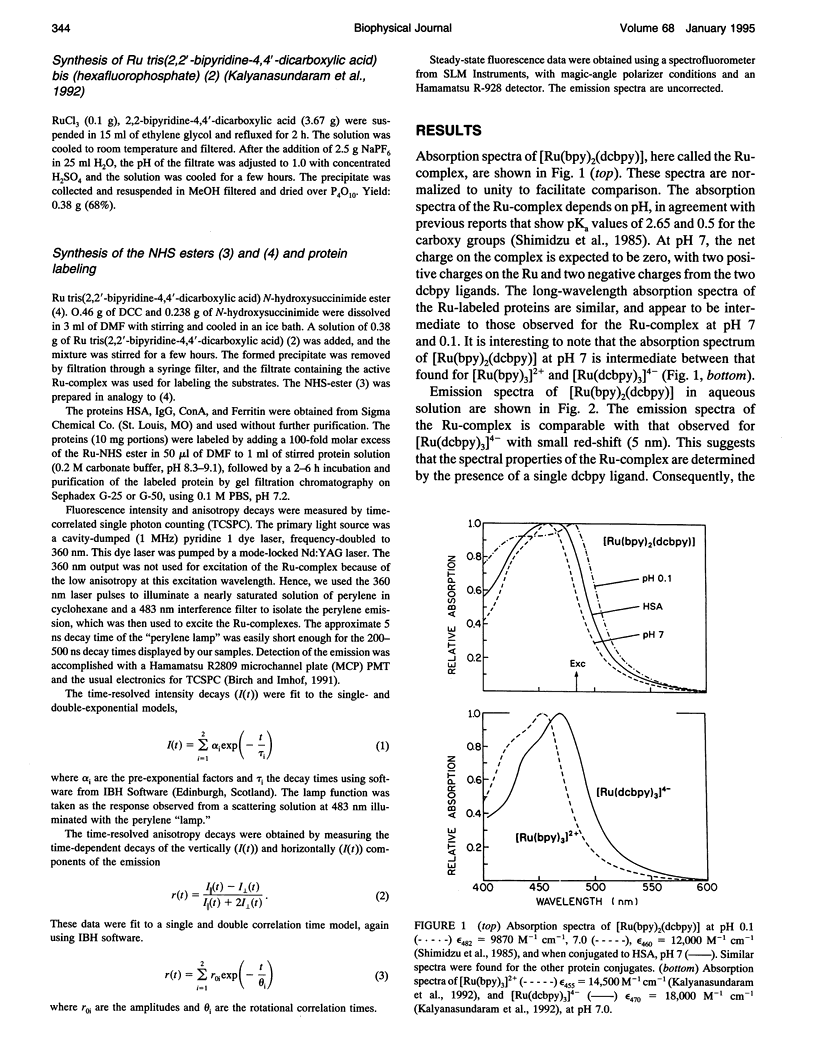
Abstract
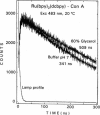
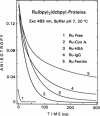
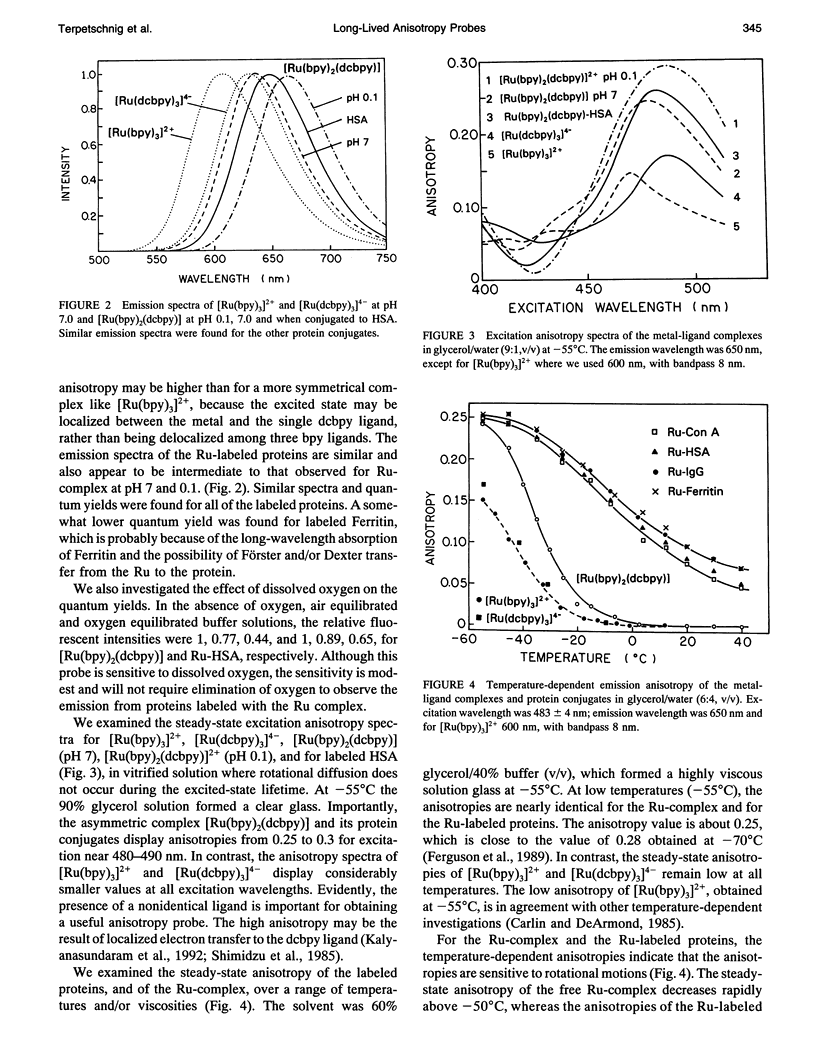
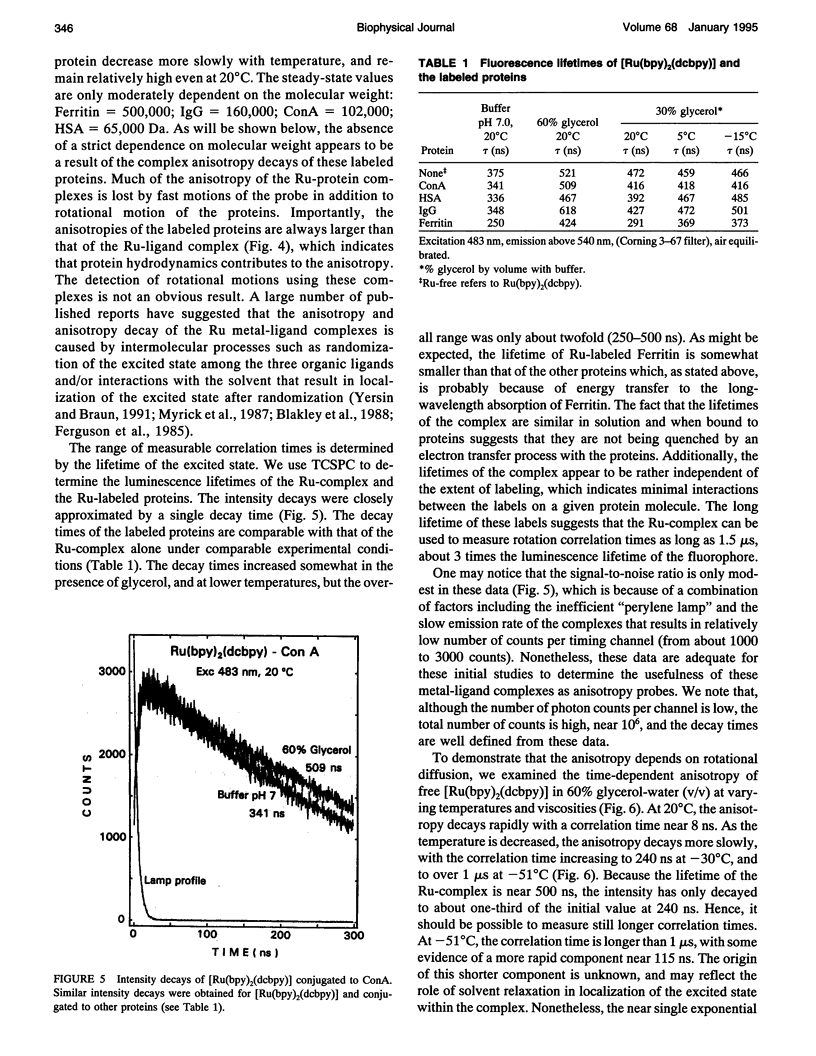
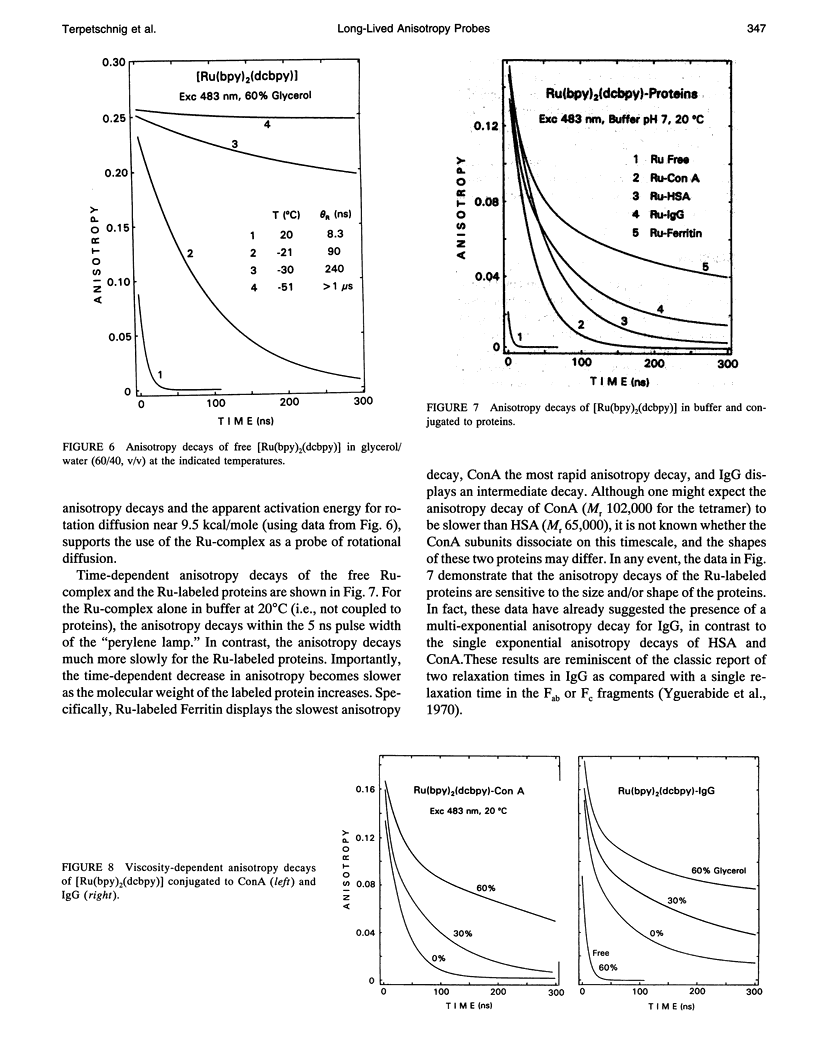
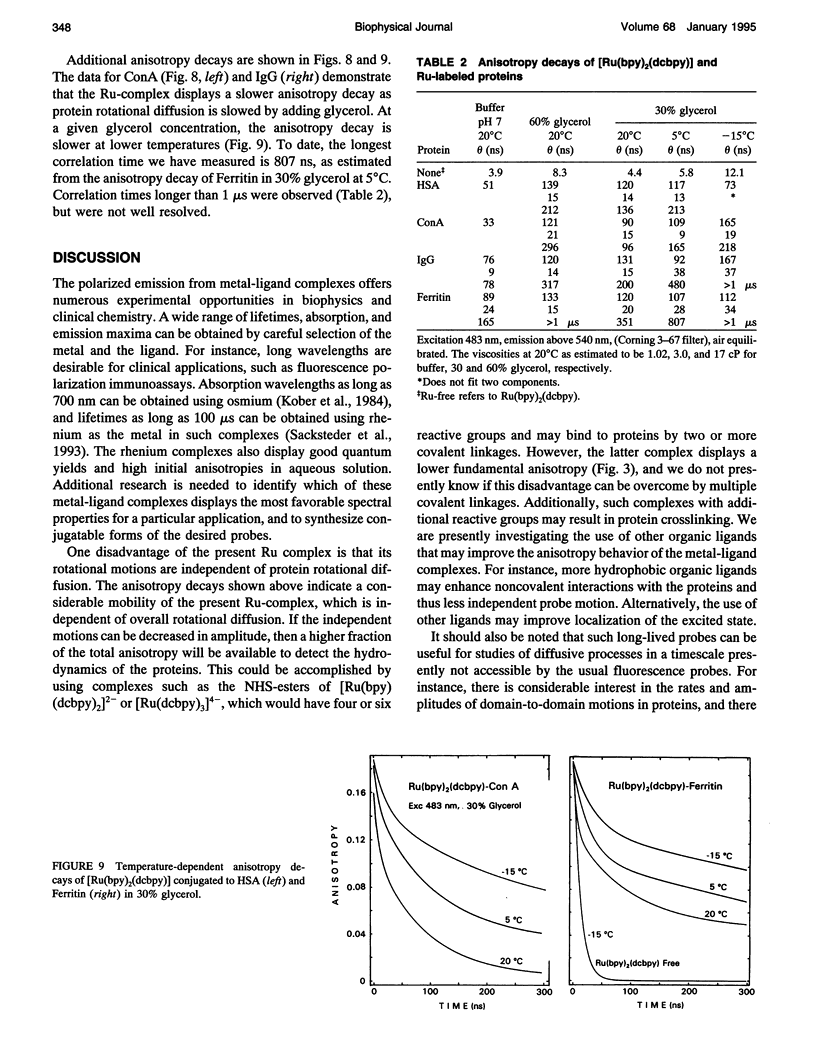
We describe the use of asymmetric Ru-ligand complexes as a new class of luminescent probes that can be used to measure rotational motions of proteins. These complexes are known to display luminescent lifetimes ranging from 10 to 4000 ns. In this report, we show that the asymmetric complex Ru(bpy)2(dcbpy) (PF6)2 displays a high anisotropy value when excited in the long wavelength absorption band. For covalent linkage to proteins, we synthesized the N-hydroxy succinimide ester of this metal-ligand complex. To illustrate the usefulness of these probes, we describe the intensity and anisotropy decays of [Ru(bpy)2(dcbpy)] when covalently linked to human serum albumin, concanavalin A (ConA), human immunoglobulin G (IgG), and Ferritin, and measured in solutions of increased viscosity. These data demonstrate that the probes can be used to measure rotational motions on the 10 ns to 1.5 microseconds timescale, which so far has been inaccessible using luminescence methods. The present probe [Ru(bpy)2(dcbpy)] can be regarded as the first of a class of metal-ligand complexes, each with different chemical reactivity and spectral properties, for studies of macromolecular dynamics.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholdi M., Barrantes F. J., Jovin T. M. Rotational molecular dynamics of the membrane-bound acetylcholine receptor revealed by phosphorescence spectroscopy. Eur J Biochem. 1981 Nov;120(2):389–397. doi: 10.1111/j.1432-1033.1981.tb05716.x. [DOI] [PubMed] [Google Scholar]
- Birmachu W., Voss J. C., Louis C. F., Thomas D. D. Protein and lipid rotational dynamics in cardiac and skeletal sarcoplasmic reticulum detected by EPR and phosphorescence anisotropy. Biochemistry. 1993 Sep 14;32(36):9445–9453. doi: 10.1021/bi00087a024. [DOI] [PubMed] [Google Scholar]
- Cobb C. E., Hustedt E. J., Beechem J. M., Beth A. H. Protein rotational dynamics investigated with a dual EPR/optical molecular probe. Spin-labeled eosin. Biophys J. 1993 Mar;64(3):605–613. doi: 10.1016/S0006-3495(93)81419-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haran G., Haas E., Szpikowska B. K., Mas M. T. Domain motions in phosphoglycerate kinase: determination of interdomain distance distributions by site-specific labeling and time-resolved fluorescence energy transfer. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11764–11768. doi: 10.1073/pnas.89.24.11764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawato S., Sigel E., Carafoli E., Cherry R. J. Rotation of cytochrome oxidase in phospholipid vesicles. Investigations of interactions between cytochrome oxidases and between cytochrome oxidase and cytochrome bc1 complex. J Biol Chem. 1981 Jul 25;256(14):7518–7527. [PubMed] [Google Scholar]
- Lakowicz J. R., Gryczynski I., Kuśba J., Wiczk W., Szmacinski H., Johnson M. L. Site-to-site diffusion in proteins as observed by energy transfer and frequency-domain fluorometry. Photochem Photobiol. 1994 Jan;59(1):16–29. doi: 10.1111/j.1751-1097.1994.tb04996.x. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Maliwal B. P., Cherek H., Balter A. Rotational freedom of tryptophan residues in proteins and peptides. Biochemistry. 1983 Apr 12;22(8):1741–1752. doi: 10.1021/bi00277a001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz J. R., Prendergast F. G., Hogen D. Fluorescence anisotropy measurements under oxygen quenching conditions as a method to quantify the depolarizing rotations of fluorophores. Application to diphenylhexatriene in isotropic solvents and in lipid bilayers. Biochemistry. 1979 Feb 6;18(3):520–527. doi: 10.1021/bi00570a022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlebach T., Cherry R. J. Rotational diffusion and self-association of band 3 in reconstituted lipid vesicles. Biochemistry. 1985 Feb 12;24(4):975–983. doi: 10.1021/bi00325a025. [DOI] [PubMed] [Google Scholar]
- Müller M., Krebs J. J., Cherry R. J., Kawato S. Rotational diffusion of the ADP/ATP translocator in the inner membrane of mitochondria and in proteoliposomes. J Biol Chem. 1984 Mar 10;259(5):3037–3043. [PubMed] [Google Scholar]
- Thomas D. D., Carlsen W. F., Stryer L. Fluorescence energy transfer in the rapid-diffusion limit. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5746–5750. doi: 10.1073/pnas.75.12.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensel T. G., Meares C. F., Vlachy V., Matthew J. B. Distribution of ions around DNA, probed by energy transfer. Proc Natl Acad Sci U S A. 1986 May;83(10):3267–3271. doi: 10.1073/pnas.83.10.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yguerabide J., Epstein H. F., Stryer L. Segmental flexibility in an antibody molecule. J Mol Biol. 1970 Aug;51(3):573–590. doi: 10.1016/0022-2836(70)90009-4. [DOI] [PubMed] [Google Scholar]