Abstract
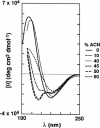
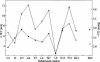
Specific conformational effects have been reported for amphipathic model peptides upon binding of defined hydrophobic domains to nonpolar stationary phases during reversed-phase high performance liquid chromatography (RP-HPLC). Such induced conformations are found to be especially pronounced for peptides that are amphipathic in an alpha-helical conformation. Such induced amphipathic conformations resulted in substantially later elution than predicted using amino acid-based retention coefficients. In the present study, the induced conformational behavior of model peptides observed during RP-HPLC was correlated with their secondary structure as determined by circular dichroism (CD) spectroscopy in both aqueous solution and C18-mimetic environments. The experimental retention times of the peptides studied were found to correlate with their CD spectra in the presence of lipids, whereas a poor correlation was observed with their CD spectra in the presence of trifluoroethanol. A new approach was developed to evaluate the induction of secondary structure in peptides due to interactions at aqueous/lipid interfaces, which involves the measurement of the CD ellipticities of peptides bound to a set of C18-coated quartz plates. An excellent correlation was found in this environment between the RP-HPLC retention times and CD ellipticities of the bound peptides.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blondelle S. E., Büttner K., Houghten R. A. Evaluation of peptide-peptide interactions using reversed-phase high-performance liquid chromatography. J Chromatogr. 1992 Nov 20;625(2):199–206. doi: 10.1016/0021-9673(92)85203-6. [DOI] [PubMed] [Google Scholar]
- Blondelle S. E., Houghten R. A. Design of model amphipathic peptides having potent antimicrobial activities. Biochemistry. 1992 Dec 22;31(50):12688–12694. doi: 10.1021/bi00165a020. [DOI] [PubMed] [Google Scholar]
- Blondelle S. E., Houghten R. A. Hemolytic and antimicrobial activities of the twenty-four individual omission analogues of melittin. Biochemistry. 1991 May 14;30(19):4671–4678. doi: 10.1021/bi00233a006. [DOI] [PubMed] [Google Scholar]
- Blondelle S. E., Simpkins L. R., Pérez-Payá E., Houghten R. A. Influence of tryptophan residues on melittin's hemolytic activity. Biochim Biophys Acta. 1993 Oct 6;1202(2):331–336. doi: 10.1016/0167-4838(93)90024-l. [DOI] [PubMed] [Google Scholar]
- Büttner K., Blondelle S. E., Ostresh J. M., Houghten R. A. Perturbation of peptide conformations induced in anisotropic environments. Biopolymers. 1992 Jun;32(6):575–583. doi: 10.1002/bip.360320602. [DOI] [PubMed] [Google Scholar]
- Büttner K., Pinilla C., Appel J. R., Houghten R. A. Anomalous reversed-phase high-performance liquid chromatographic behavior of synthetic peptides related to antigenic helper T cell sites. J Chromatogr. 1992 Nov 20;625(2):191–198. doi: 10.1016/0021-9673(92)85202-5. [DOI] [PubMed] [Google Scholar]
- Hearn M. T., Aguilar M. I. High-performance liquid chromatography of amino acids, peptides and proteins. LXXIII. Investigations on the relationships between molecular structure, retention and band-broadening properties of polypeptides separated by reversed-phase high-performance liquid chromatography. J Chromatogr. 1987 Jun 26;397:47–70. doi: 10.1016/s0021-9673(01)84989-x. [DOI] [PubMed] [Google Scholar]
- Houghten R. A., Bray M. K., Degraw S. T., Kirby C. J. Simplified procedure for carrying out simultaneous multiple hydrogen fluoride cleavages of protected peptide resins. Int J Pept Protein Res. 1986 Jun;27(6):673–678. doi: 10.1111/j.1399-3011.1986.tb01064.x. [DOI] [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser E. T., Kézdy F. J. Peptides with affinity for membranes. Annu Rev Biophys Biophys Chem. 1987;16:561–581. doi: 10.1146/annurev.bb.16.060187.003021. [DOI] [PubMed] [Google Scholar]
- McLean L. R., Hagaman K. A., Owen T. J., Krstenansky J. L. Minimal peptide length for interaction of amphipathic alpha-helical peptides with phosphatidylcholine liposomes. Biochemistry. 1991 Jan 8;30(1):31–37. doi: 10.1021/bi00215a005. [DOI] [PubMed] [Google Scholar]
- Pérez-Payá E., Houghten R. A., Blondelle S. E. Determination of the secondary structure of selected melittin analogues with different haemolytic activities. Biochem J. 1994 Apr 15;299(Pt 2):587–591. doi: 10.1042/bj2990587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay S. C., Condie C. C. Conformational studies of aqueous melittin: thermodynamic parameters of the monomer-tetramer self-association reaction. Biochemistry. 1983 Feb 1;22(3):695–700. doi: 10.1021/bi00272a026. [DOI] [PubMed] [Google Scholar]
- Regnier F. E. The role of protein structure in chromatographic behavior. Science. 1987 Oct 16;238(4825):319–323. doi: 10.1126/science.3310233. [DOI] [PubMed] [Google Scholar]
- Smith L. J., Clark D. C. Measurement of the secondary structure of adsorbed protein by circular dichroism. 1. Measurements of the helix content of adsorbed melittin. Biochim Biophys Acta. 1992 May 22;1121(1-2):111–118. doi: 10.1016/0167-4838(92)90344-d. [DOI] [PubMed] [Google Scholar]
- Terwilliger T. C., Eisenberg D. The structure of melittin. I. Structure determination and partial refinement. J Biol Chem. 1982 Jun 10;257(11):6010–6015. doi: 10.2210/pdb1mlt/pdb. [DOI] [PubMed] [Google Scholar]
- Terwilliger T. C., Weissman L., Eisenberg D. The structure of melittin in the form I crystals and its implication for melittin's lytic and surface activities. Biophys J. 1982 Jan;37(1):353–361. doi: 10.1016/S0006-3495(82)84683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. S., Ikeda K., Yang J. T. Ordered conformation of polypeptides and proteins in acidic dodecyl sulfate solution. Biochemistry. 1981 Feb 3;20(3):566–570. doi: 10.1021/bi00506a019. [DOI] [PubMed] [Google Scholar]
- Wu C. S., Yang J. T. Sequence-dependent conformations of short polypeptides in a hydrophobic environment. Mol Cell Biochem. 1981 Oct 30;40(2):109–122. doi: 10.1007/BF00224754. [DOI] [PubMed] [Google Scholar]
- Yang J. T., Wu C. S., Martinez H. M. Calculation of protein conformation from circular dichroism. Methods Enzymol. 1986;130:208–269. doi: 10.1016/0076-6879(86)30013-2. [DOI] [PubMed] [Google Scholar]
- Zhong L., Johnson W. C., Jr Environment affects amino acid preference for secondary structure. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4462–4465. doi: 10.1073/pnas.89.10.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N. E., Mant C. T., Hodges R. S. Effect of preferred binding domains on peptide retention behavior in reversed-phase chromatography: amphipathic alpha-helices. Pept Res. 1990 Jan-Feb;3(1):8–20. [PubMed] [Google Scholar]