Abstract
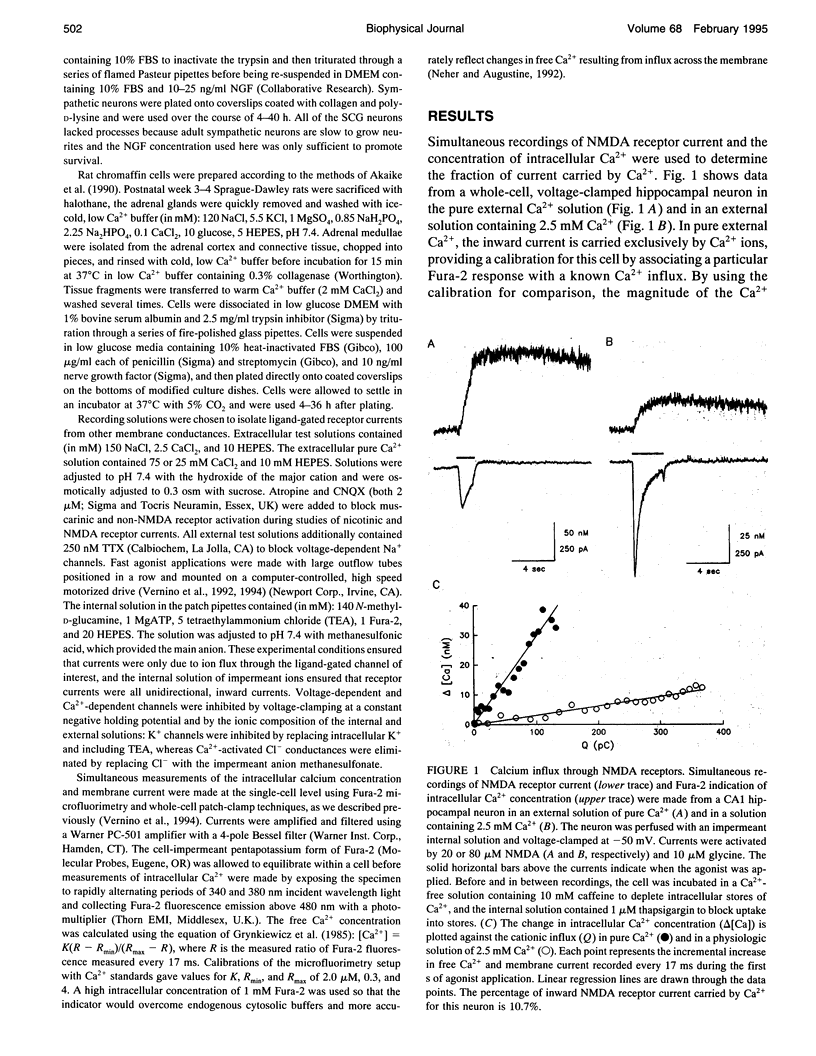
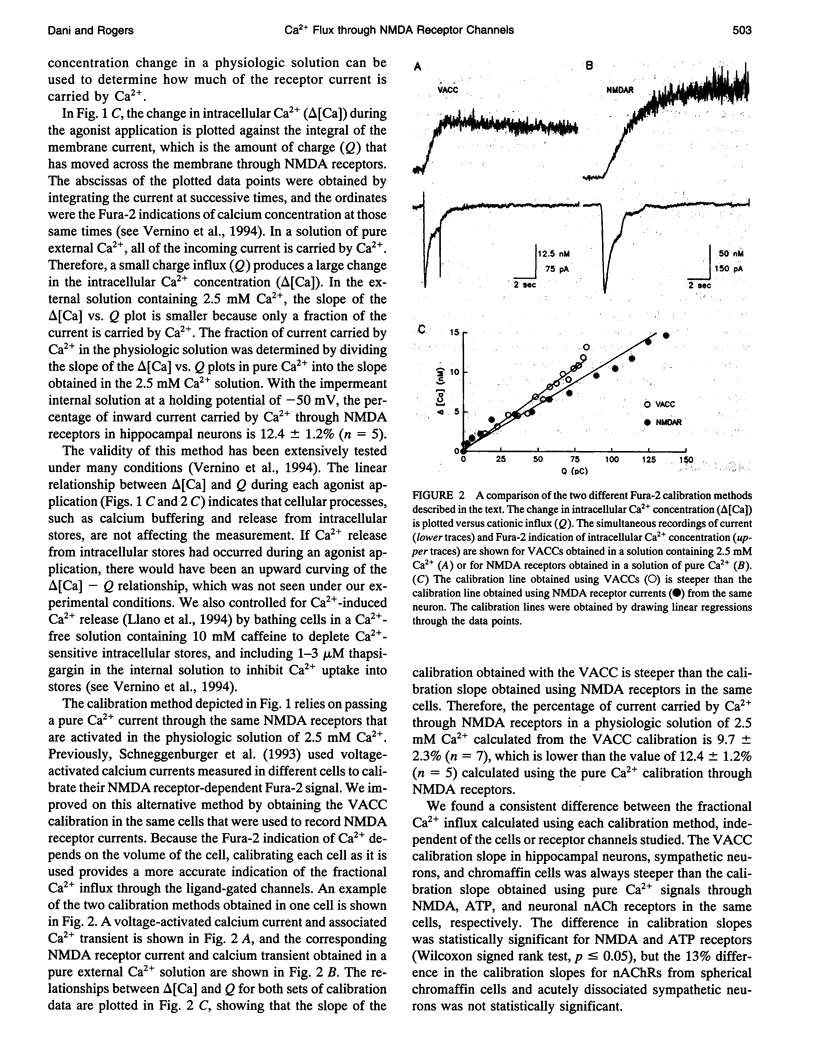
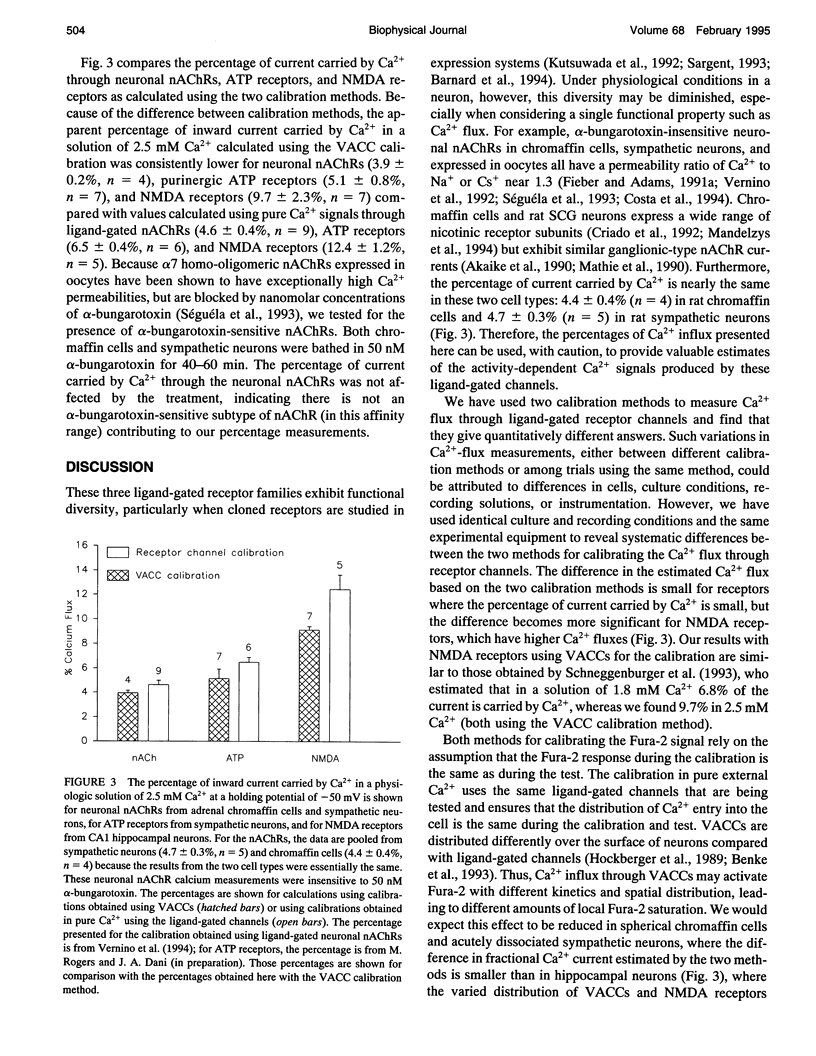
NMDA receptors, ATP receptors, and nicotinic ACh receptors respond to agonist by undergoing conformational changes that open weakly selective cationic channels that are permeable to calcium. We determined the fraction of the current carried by calcium by simultaneously measuring membrane current using whole-cell patch-clamp techniques and intracellular Ca2+ using the fluorescent indicator Fura-2. The Fura-2 response to free Ca2+ was calibrated individually for each cell. Two different calibration methods are compared: one uses voltage-activated Ca2+ channels, and the other uses the same ligand-gated channels that are being tested but in a pure Ca2+ solution. The two methods give quantitatively different results. The method using pure Ca2+ currents through ligand-gated channels calibrates the Fura-2 signal through the same influx pathway that generates the test response, thus controlling for the distribution of channels and ensuring a similar interaction between the incoming Ca2+ and Fura-2. In a physiologic solution containing 2.5 mM Ca2+ at a holding potential of -50 mV, the percentage of inward current carried by Ca2+ through NMDA receptors in hippocampal neurons is 12.4%. By comparison, in sympathetic neurons the percentage of current carried by Ca2+ through neuronal nAChRs is 4.7%, and through ATP-activated purinergic receptors it is 6.5%. These percentages can be used to estimate the amount of Ca2+ entry through these receptors during synaptic activation, but care must be exercised in considering the many subtypes of each receptor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike A., Mine Y., Sasa M., Takaori S. Voltage and current clamp studies of muscarinic and nicotinic excitation of the rat adrenal chromaffin cells. J Pharmacol Exp Ther. 1990 Oct;255(1):333–339. [PubMed] [Google Scholar]
- Barnard E. A., Burnstock G., Webb T. E. G protein-coupled receptors for ATP and other nucleotides: a new receptor family. Trends Pharmacol Sci. 1994 Mar;15(3):67–70. doi: 10.1016/0165-6147(94)90280-1. [DOI] [PubMed] [Google Scholar]
- Benke T. A., Jones O. T., Collingridge G. L., Angelides K. J. N-Methyl-D-aspartate receptors are clustered and immobilized on dendrites of living cortical neurons. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7819–7823. doi: 10.1073/pnas.90.16.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Costa A. C., Patrick J. W., Dani J. A. Improved technique for studying ion channels expressed in Xenopus oocytes, including fast superfusion. Biophys J. 1994 Jul;67(1):395–401. doi: 10.1016/S0006-3495(94)80494-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado M., Alamo L., Navarro A. Primary structure of an agonist binding subunit of the nicotinic acetylcholine receptor from bovine adrenal chromaffin cells. Neurochem Res. 1992 Mar;17(3):281–287. doi: 10.1007/BF00966671. [DOI] [PubMed] [Google Scholar]
- Decker E. R., Dani J. A. Calcium permeability of the nicotinic acetylcholine receptor: the single-channel calcium influx is significant. J Neurosci. 1990 Oct;10(10):3413–3420. doi: 10.1523/JNEUROSCI.10-10-03413.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Gibb A. J., Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992 Sep 10;359(6391):144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Evans R. J., Derkach V., Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992 Jun 11;357(6378):503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Fieber L. A., Adams D. J. Acetylcholine-evoked currents in cultured neurones dissociated from rat parasympathetic cardiac ganglia. J Physiol. 1991 Mar;434:215–237. doi: 10.1113/jphysiol.1991.sp018466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieber L. A., Adams D. J. Adenosine triphosphate-evoked currents in cultured neurones dissociated from rat parasympathetic cardiac ganglia. J Physiol. 1991 Mar;434:239–256. doi: 10.1113/jphysiol.1991.sp018467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hockberger P. E., Tseng H. Y., Connor J. A. Fura-2 measurements of cultured rat Purkinje neurons show dendritic localization of Ca2+ influx. J Neurosci. 1989 Jul;9(7):2272–2284. doi: 10.1523/JNEUROSCI.09-07-02272.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Calcium permeability of the N-methyl-D-aspartate receptor channel in hippocampal neurons in culture. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11573–11577. doi: 10.1073/pnas.90.24.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuwada T., Kashiwabuchi N., Mori H., Sakimura K., Kushiya E., Araki K., Meguro H., Masaki H., Kumanishi T., Arakawa M. Molecular diversity of the NMDA receptor channel. Nature. 1992 Jul 2;358(6381):36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Lieberman D. N., Mody I. Regulation of NMDA channel function by endogenous Ca(2+)-dependent phosphatase. Nature. 1994 May 19;369(6477):235–239. doi: 10.1038/369235a0. [DOI] [PubMed] [Google Scholar]
- Llano I., DiPolo R., Marty A. Calcium-induced calcium release in cerebellar Purkinje cells. Neuron. 1994 Mar;12(3):663–673. doi: 10.1016/0896-6273(94)90221-6. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Malenka R. C., Nicoll R. A. Mechanisms underlying long-term potentiation of synaptic transmission. Annu Rev Neurosci. 1991;14:379–397. doi: 10.1146/annurev.ne.14.030191.002115. [DOI] [PubMed] [Google Scholar]
- Mandelzys A., Pié B., Deneris E. S., Cooper E. The developmental increase in ACh current densities on rat sympathetic neurons correlates with changes in nicotinic ACh receptor alpha-subunit gene expression and occurs independent of innervation. J Neurosci. 1994 Apr;14(4):2357–2364. doi: 10.1523/JNEUROSCI.14-04-02357.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A., Colquhoun D., Cull-Candy S. G. Rectification of currents activated by nicotinic acetylcholine receptors in rat sympathetic ganglion neurones. J Physiol. 1990 Aug;427:625–655. doi: 10.1113/jphysiol.1990.sp018191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987 Dec;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles K., Audigier S. S., Greengard P., Huganir R. L. Autoregulation of phosphorylation of the nicotinic acetylcholine receptor. J Neurosci. 1994 May;14(5 Pt 2):3271–3279. doi: 10.1523/JNEUROSCI.14-05-03271.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle C., Choquet D., Korn H., Changeux J. P. Calcium influx through nicotinic receptor in rat central neurons: its relevance to cellular regulation. Neuron. 1992 Jan;8(1):135–143. doi: 10.1016/0896-6273(92)90115-t. [DOI] [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond L. A., Blackstone C. D., Huganir R. L. Phosphorylation of amino acid neurotransmitter receptors in synaptic plasticity. Trends Neurosci. 1993 Apr;16(4):147–153. doi: 10.1016/0166-2236(93)90123-4. [DOI] [PubMed] [Google Scholar]
- Sargent P. B. The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R., Zhou Z., Konnerth A., Neher E. Fractional contribution of calcium to the cation current through glutamate receptor channels. Neuron. 1993 Jul;11(1):133–143. doi: 10.1016/0896-6273(93)90277-x. [DOI] [PubMed] [Google Scholar]
- Séguéla P., Wadiche J., Dineley-Miller K., Dani J. A., Patrick J. W. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993 Feb;13(2):596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimasa T., North R. A. Calcium entry through acetylcholine-channels can activate potassium conductance in bullfrog sympathetic neurons. Brain Res. 1984 Mar 19;295(2):364–367. doi: 10.1016/0006-8993(84)90987-9. [DOI] [PubMed] [Google Scholar]
- Trouslard J., Marsh S. J., Brown D. A. Calcium entry through nicotinic receptor channels and calcium channels in cultured rat superior cervical ganglion cells. J Physiol. 1993 Aug;468:53–71. doi: 10.1113/jphysiol.1993.sp019759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernino S., Amador M., Luetje C. W., Patrick J., Dani J. A. Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron. 1992 Jan;8(1):127–134. doi: 10.1016/0896-6273(92)90114-s. [DOI] [PubMed] [Google Scholar]
- Vernino S., Rogers M., Radcliffe K. A., Dani J. A. Quantitative measurement of calcium flux through muscle and neuronal nicotinic acetylcholine receptors. J Neurosci. 1994 Sep;14(9):5514–5524. doi: 10.1523/JNEUROSCI.14-09-05514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M. M., Dani J. A. Ionic permeability characteristics of the N-methyl-D-aspartate receptor channel. J Gen Physiol. 1994 Feb;103(2):231–248. doi: 10.1085/jgp.103.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Neher E. Calcium permeability of nicotinic acetylcholine receptor channels in bovine adrenal chromaffin cells. Pflugers Arch. 1993 Dec;425(5-6):511–517. doi: 10.1007/BF00374879. [DOI] [PubMed] [Google Scholar]
- Zorumski C. F., Thio L. L., Clark G. D., Clifford D. B. Calcium influx through N-methyl-D-aspartate channels activates a potassium current in postnatal rat hippocampal neurons. Neurosci Lett. 1989 May 8;99(3):293–299. doi: 10.1016/0304-3940(89)90462-x. [DOI] [PubMed] [Google Scholar]


