Abstract
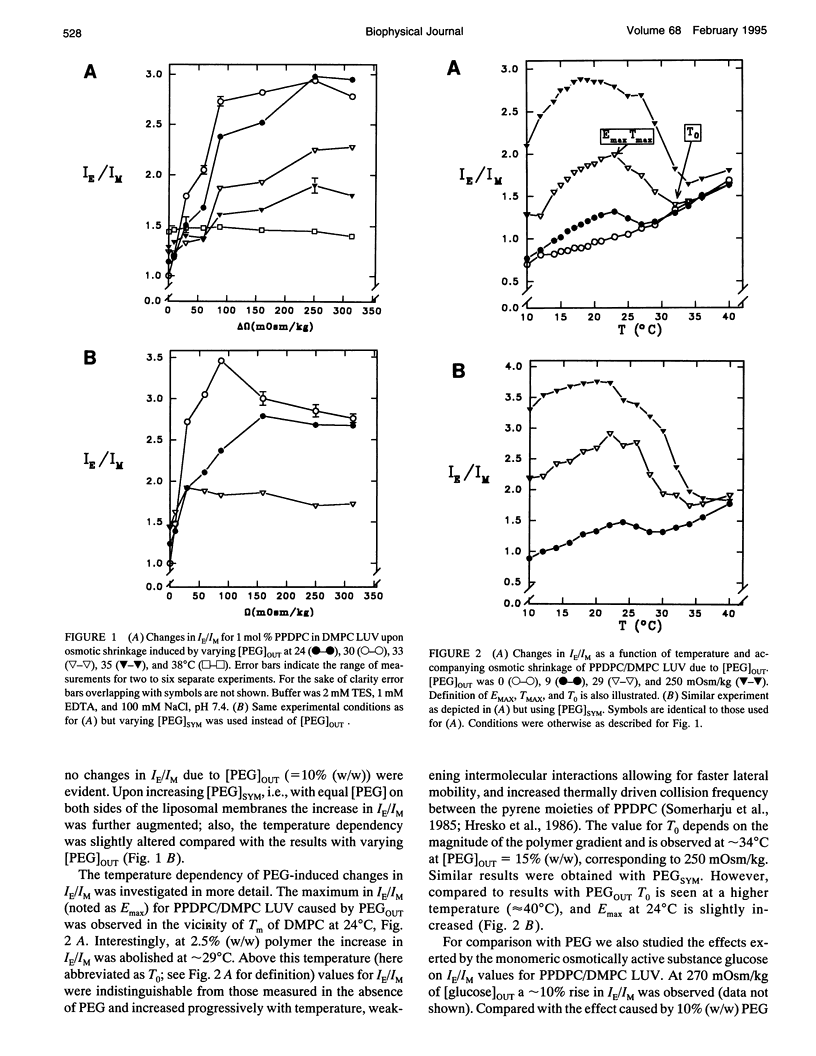
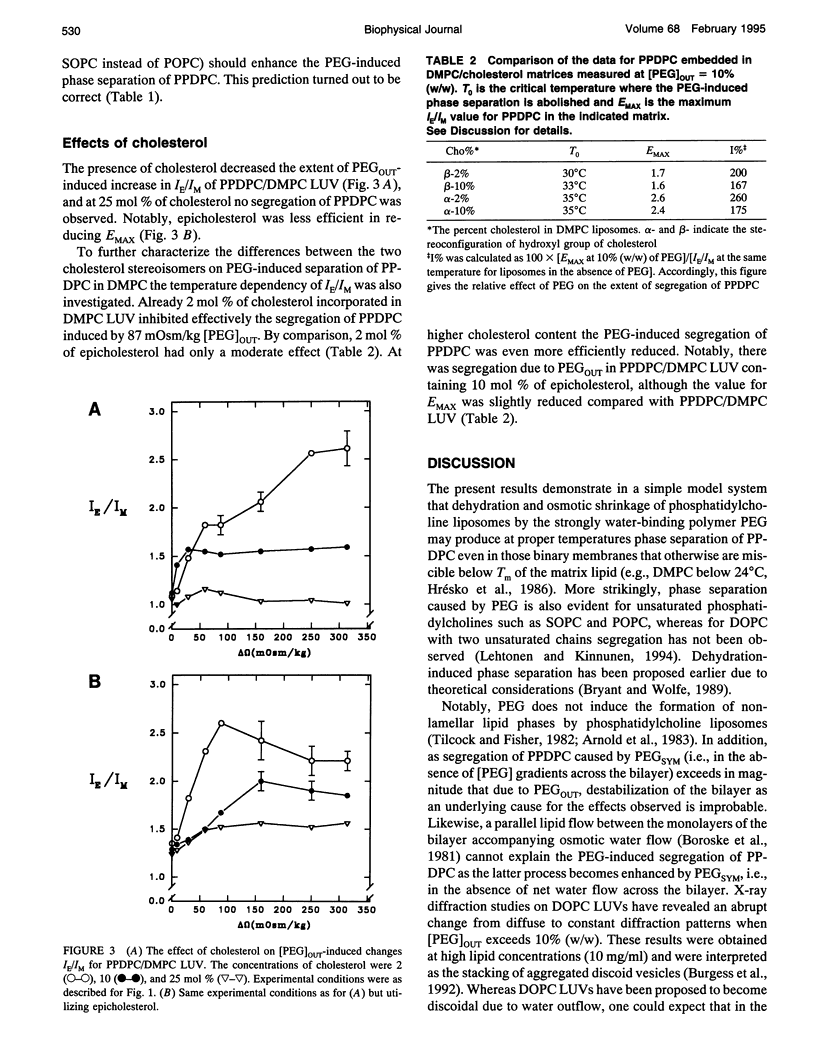
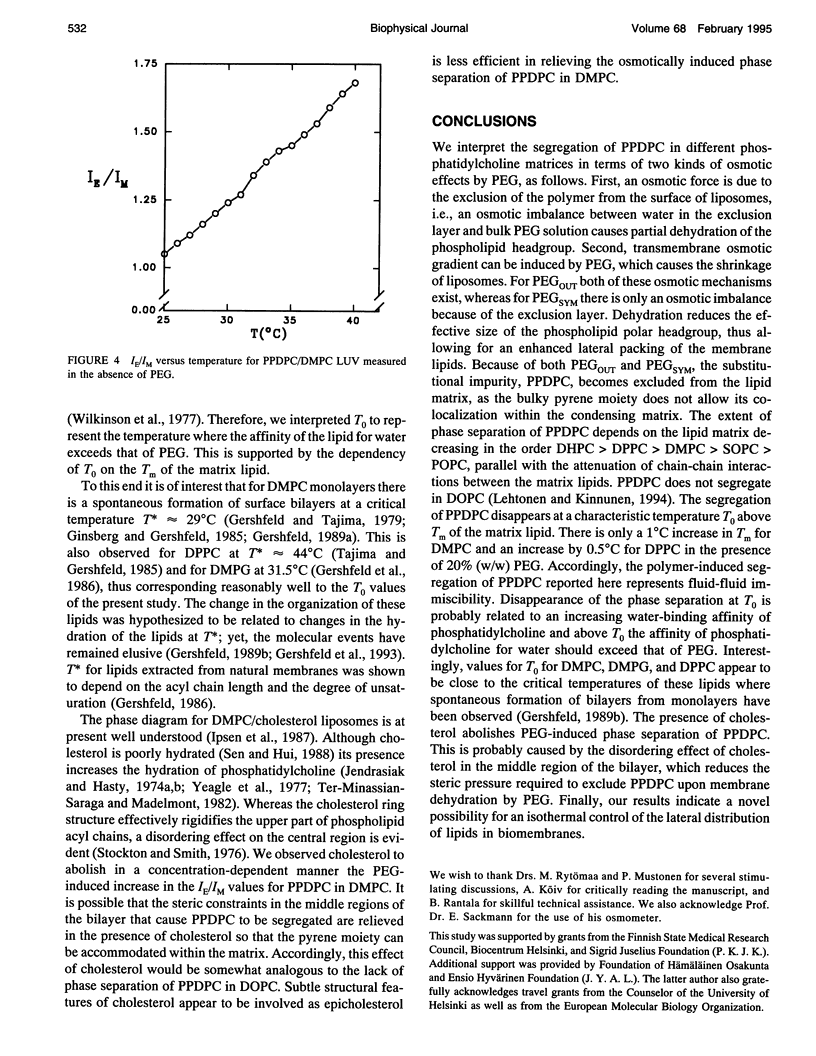
Exclusion of the strongly hygroscopic polymer, poly(ethylene glycol) (PEG), from the surface of phosphatidylcholine liposomes results in an osmotic imbalance between the hydration layer of the liposome surface and the bulk polymer solution, thus causing a partial dehydration of the phospholipid polar headgroups. PEG (average molecular weight of 6000 and in concentrations ranging from 5 to 20%, w/w) was added to the outside of large unilamellar liposomes (LUVs). This leads to, in addition to the dehydration of the outer monolayer, an osmotically driven water outflow and shrinkage of liposomes. Under these conditions phase separation of the fluorescent lipid 1-palmitoyl-2[6-(pyren-1-yl)]decanoyl-sn-glycero-3-phosphocholine (PPDPC) embedded in various phosphatidylcholine matrices was observed, evident as an increase in the excimer-to-monomer fluorescence intensity ratio (IE/IM). Enhanced segregation of the fluorescent lipid was seen upon increasing and equal concentrations of PEG both inside and outside of the LUVs, revealing that osmotic gradient across the membrane is not required, and phase separation results from the dehydration of the lipid. Importantly, phase separation of PPDPC could be induced by PEG also in binary mixtures with 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), 1-stearoyl-2-oleoyl-sn-glycero-3-phosphocholine (SOPC), and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), for which temperature-induced phase segregation of the fluorescent lipid below Tm was otherwise not achieved. In the different lipid matrices the segregation of PPDPC caused by PEG was abolished above characteristic temperatures T0 well above their respective main phase transition temperatures Tm. For 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), DMPC, SOPC, and POPC, T0 was observed at approximately 50, 32, 24, and 20 degrees C, respectively. Notably, the observed phase separation of PPDPC cannot be accounted for the 1 degree C increase in Tm for DMPC or for the increase by 0.5 degrees C for DPPC observed in the presence of 20% (w/w) PEG. At a given PEG concentration maximal increase in IE/IM (correlating to the extent of segregation of PPDPC in the different lipid matrices) decreased in the sequence 1,2-dihexadecyl-sn-glycero-3-phosphocholine (DHPC) > DPPC > DMPC > SOPC > POPC, whereas no evidence for phase separation in 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) LUV was observed (Lehtonen and Kinnunen, 1994, Biophys. J. 66: 1981-1990). Our results indicate that PEG-induced dehydration of liposomal membranes provides the driving force for the segregation of the pyrene lipid. In brief, phase separation of PPDPC from the matrix lipid could be attributed to the diminishing effective size of the phosphatidylcholine polar headgroup resulting from its partial dehydration by PEG. This in turn would allow for enhanced van der Waals interactions between the acyl chains of the matrix lipid, which then caused the exclusion of PPDPC due to the perturbing bulky pyrene moiety. Phase separation in DMPC/PPDPC liposomes was abolished by the inclusion of 25 mol % cholesterol and to a lesser extent by epicholesterol.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold K., Pratsch L., Gawrisch K. Effect of poly(ethylene glycol) on phospholipid hydration and polarity of the external phase. Biochim Biophys Acta. 1983 Feb 9;728(1):121–128. doi: 10.1016/0005-2736(83)90444-3. [DOI] [PubMed] [Google Scholar]
- Arnold K., Zschoernig O., Barthel D., Herold W. Exclusion of poly(ethylene glycol) from liposome surfaces. Biochim Biophys Acta. 1990 Mar;1022(3):303–310. doi: 10.1016/0005-2736(90)90278-v. [DOI] [PubMed] [Google Scholar]
- Ashley R. H., Brammer M. J. A fluorescence polarization study of calcium and phase behaviour in synaptosomal lipids. Biochim Biophys Acta. 1984 Jan 25;769(2):363–369. doi: 10.1016/0005-2736(84)90318-3. [DOI] [PubMed] [Google Scholar]
- Asturias F. J., Pascolini D., Blasie J. K. Evidence that lipid lateral phase separation induces functionally significant structural changes in the Ca+2ATPase of the sarcoplasmic reticulum. Biophys J. 1990 Jul;58(1):205–217. doi: 10.1016/S0006-3495(90)82366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bittman R., Blau L. The phospholipid-cholesterol interaction. Kinetics of water permeability in liposomes. Biochemistry. 1972 Dec 5;11(25):4831–4839. doi: 10.1021/bi00775a029. [DOI] [PubMed] [Google Scholar]
- Boroske E., Elwenspoek M., Helfrich W. Osmotic shrinkage of giant egg-lecithin vesicles. Biophys J. 1981 Apr;34(1):95–109. doi: 10.1016/S0006-3495(81)84839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster J. L., de Valoir T., Dwyer N. D., Winter E., Gustin M. C. An osmosensing signal transduction pathway in yeast. Science. 1993 Mar 19;259(5102):1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Bryant G., Wolfe J. Can hydration forces induce lateral phase separations in lamellar phases? Eur Biophys J. 1989;16(6):369–374. doi: 10.1007/BF00257886. [DOI] [PubMed] [Google Scholar]
- Burack W. R., Yuan Q., Biltonen R. L. Role of lateral phase separation in the modulation of phospholipase A2 activity. Biochemistry. 1993 Jan 19;32(2):583–589. doi: 10.1021/bi00053a025. [DOI] [PubMed] [Google Scholar]
- Burgess S. W., Massenburg D., Yates J., Lentz B. R. Poly(ethylene glycol)-induced lipid mixing but not fusion between synthetic phosphatidylcholine large unilamellar vesicles. Biochemistry. 1991 Apr 30;30(17):4193–4200. doi: 10.1021/bi00231a013. [DOI] [PubMed] [Google Scholar]
- Burgess S. W., McIntosh T. J., Lentz B. R. Modulation of poly(ethylene glycol)-induced fusion by membrane hydration: importance of interbilayer separation. Biochemistry. 1992 Mar 17;31(10):2653–2661. doi: 10.1021/bi00125a004. [DOI] [PubMed] [Google Scholar]
- Cevc G., Marsh D. Hydration of noncharged lipid bilayer membranes. Theory and experiments with phosphatidylethanolamines. Biophys J. 1985 Jan;47(1):21–31. doi: 10.1016/S0006-3495(85)83872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen F. S., Akabas M. H., Finkelstein A. Osmotic swelling of phospholipid vesicles causes them to fuse with a planar phospholipid bilayer membrane. Science. 1982 Jul 30;217(4558):458–460. doi: 10.1126/science.6283637. [DOI] [PubMed] [Google Scholar]
- Cowley A. C., Fuller N. L., Rand R. P., Parsegian V. A. Measurement of repulsive forces between charged phospholipid bilayers. Biochemistry. 1978 Jul 25;17(15):3163–3168. doi: 10.1021/bi00608a034. [DOI] [PubMed] [Google Scholar]
- Crowe J. H., Crowe L. M., Carpenter J. F., Rudolph A. S., Wistrom C. A., Spargo B. J., Anchordoguy T. J. Interactions of sugars with membranes. Biochim Biophys Acta. 1988 Jun 9;947(2):367–384. doi: 10.1016/0304-4157(88)90015-9. [DOI] [PubMed] [Google Scholar]
- Crowe J. H., Whittam M. A., Chapman D., Crowe L. M. Interactions of phospholipid monolayers with carbohydrates. Biochim Biophys Acta. 1984 Jan 11;769(1):151–159. doi: 10.1016/0005-2736(84)90018-x. [DOI] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deamer D. W., Bramhall J. Permeability of lipid bilayers to water and ionic solutes. Chem Phys Lipids. 1986 Jun-Jul;40(2-4):167–188. doi: 10.1016/0009-3084(86)90069-1. [DOI] [PubMed] [Google Scholar]
- Demiel R. A., Guerts van Kessel W. S., van Deenen L. L. The properties of polyunsaturated lecithins in monolayers and liposomes and the interactions of these lecithins with cholesterol. Biochim Biophys Acta. 1972 Apr 14;266(1):26–40. doi: 10.1016/0005-2736(72)90116-2. [DOI] [PubMed] [Google Scholar]
- Einspahr K. J., Maeda M., Thompson G. A., Jr Concurrent changes in Dunaliella salina ultrastructure and membrane phospholipid metabolism after hyperosmotic shock. J Cell Biol. 1988 Aug;107(2):529–538. doi: 10.1083/jcb.107.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund K. K., Vuorinen J., Mikkola J., Virtanen J. A., Kinnunen P. K. Ca2+-induced lateral phase separation in phosphatidic acid/phosphatidylcholine monolayers as revealed by fluorescence microscopy. Biochemistry. 1988 May 3;27(9):3433–3437. doi: 10.1021/bi00409a046. [DOI] [PubMed] [Google Scholar]
- Finer E. G., Darke A. Phospholipid hydration studied by deuteron magnetic resonace spectroscopy. Chem Phys Lipids. 1974 Feb;12(1):1–16. doi: 10.1016/0009-3084(74)90064-4. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W. Excimer-forming lipids in membrane research. Chem Phys Lipids. 1980 Oct;27(3):199–219. doi: 10.1016/0009-3084(80)90036-5. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W., Theilen U., Sackmann E. On two-dimensional passive random walk in lipid bilayers and fluid pathways in biomembranes. J Membr Biol. 1979 Jul 31;48(3):215–236. doi: 10.1007/BF01872892. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Sackmann E. Lateral diffusion in the hydrophobic region of membranes: use of pyrene excimers as optical probes. Biochim Biophys Acta. 1974 Feb 26;339(1):103–115. doi: 10.1016/0005-2736(74)90336-8. [DOI] [PubMed] [Google Scholar]
- Gershfeld N. L., Mudd C. P., Tajima K., Berger R. L. Critical temperature for unilamellar vesicle formation in dimyristoylphosphatidylcholine dispersions from specific heat measurements. Biophys J. 1993 Sep;65(3):1174–1179. doi: 10.1016/S0006-3495(93)81157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershfeld N. L. Phospholipid surface bilayers at the air-water interface. III. Relation between surface bilayer formation and lipid bilayer assembly in cell membranes. Biophys J. 1986 Sep;50(3):457–461. doi: 10.1016/S0006-3495(86)83482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershfeld N. L., Stevens W. F., Jr, Nossal R. J. Equilibrium studies of phospholipid bilayer assembly. Coexistence of surface bilayers and unilamellar vesicles. Faraday Discuss Chem Soc. 1986;(81):19–28. doi: 10.1039/dc9868100019. [DOI] [PubMed] [Google Scholar]
- Gershfeld N. L., Tajima K. Spontaneous formation of lecithin bilayers at the air-water surface. Nature. 1979 Jun 21;279(5715):708–709. doi: 10.1038/279708a0. [DOI] [PubMed] [Google Scholar]
- Gershfeld N. L. The critical unilamellar lipid state: a perspective for membrane bilayer assembly. Biochim Biophys Acta. 1989 Dec 6;988(3):335–350. doi: 10.1016/0304-4157(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Ginsberg L., Gershfeld N. L. Phospholipid surface bilayers at the air-water interface. II. Water permeability of dimyristoylphosphatidylcholine surface bilayers. Biophys J. 1985 Feb;47(2 Pt 1):211–215. doi: 10.1016/s0006-3495(85)83893-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich R. P., Crowe J. H., Crowe L. M., Baldeschwieler J. D. Alterations in membrane surfaces induced by attachment of carbohydrates. Biochemistry. 1991 May 28;30(21):5313–5318. doi: 10.1021/bi00235a026. [DOI] [PubMed] [Google Scholar]
- Gordon-Kamm W. J., Steponkus P. L. Lamellar-to-hexagonalII phase transitions in the plasma membrane of isolated protoplasts after freeze-induced dehydration. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6373–6377. doi: 10.1073/pnas.81.20.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger D. W., Reichert A., Ringsdorf H., Salesse C. Hydrolytic action of phospholipase A2 in monolayers in the phase transition region: direct observation of enzyme domain formation using fluorescence microscopy. Biochim Biophys Acta. 1990 Apr 30;1023(3):365–379. doi: 10.1016/0005-2736(90)90128-b. [DOI] [PubMed] [Google Scholar]
- Haverstick D. M., Glaser M. Visualization of domain formation in the inner and outer leaflets of a phospholipid bilayer. J Cell Biol. 1988 Jun;106(6):1885–1892. doi: 10.1083/jcb.106.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N. B., Hui S. W. Electron microscopic observation of domain movement in reconstituted erythrocyte membranes. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7304–7308. doi: 10.1073/pnas.82.21.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra D. Fluorescence method for measuring the kinetics of Ca2+-induced phase separations in phosphatidylserine-containing lipid vesicles. Biochemistry. 1982 Mar 2;21(5):1055–1061. doi: 10.1021/bi00534a036. [DOI] [PubMed] [Google Scholar]
- Hresko R. C., Sugár I. P., Barenholz Y., Thompson T. E. Lateral distribution of a pyrene-labeled phosphatidylcholine in phosphatidylcholine bilayers: fluorescence phase and modulation study. Biochemistry. 1986 Jul 1;25(13):3813–3823. doi: 10.1021/bi00361a012. [DOI] [PubMed] [Google Scholar]
- Hui S. W. Geometry of phase-separated domains in phospholipid bilayers by diffraction-contrast electron microscopy. Biophys J. 1981 Jun;34(3):383–395. doi: 10.1016/S0006-3495(81)84857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui S. W., Isac T., Boni L. T., Sen A. Action of polyethylene glycol on the fusion of human erythrocyte membranes. J Membr Biol. 1985;84(2):137–146. doi: 10.1007/BF01872211. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Lang F. Cell volume in the regulation of hepatic function: a mechanism for metabolic control. Biochim Biophys Acta. 1991 Dec 12;1071(4):331–350. doi: 10.1016/0304-4157(91)90001-d. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Yamaguchi H., Tazuke S. Phase separation in phospholipid bilayers induced by biologically active polycations. Biochim Biophys Acta. 1990 Jul 9;1026(1):105–112. doi: 10.1016/0005-2736(90)90339-p. [DOI] [PubMed] [Google Scholar]
- Ipsen J. H., Karlström G., Mouritsen O. G., Wennerström H., Zuckermann M. J. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim Biophys Acta. 1987 Nov 27;905(1):162–172. doi: 10.1016/0005-2736(87)90020-4. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Papahadjopoulos D. Phase transitions and phase separations in phospholipid membranes induced by changes in temperature, pH, and concentration of bivalent cations. Biochemistry. 1975 Jan 14;14(1):152–161. doi: 10.1021/bi00672a026. [DOI] [PubMed] [Google Scholar]
- Jendrasiak G. L., Hasty J. H. The electrical conductivity of hydrated phospholipids. Biochim Biophys Acta. 1974 Apr 26;348(1):45–54. doi: 10.1016/0005-2760(74)90091-5. [DOI] [PubMed] [Google Scholar]
- Jendrasiak G. L., Hasty J. H. The hydration of phospholipids. Biochim Biophys Acta. 1974 Jan 23;337(1):79–91. doi: 10.1016/0005-2760(74)90042-3. [DOI] [PubMed] [Google Scholar]
- Junker M., Creutz C. E. Endonexin (annexin IV)-mediated lateral segregation of phosphatidylglycerol in phosphatidylglycerol/phosphatidylcholine membranes. Biochemistry. 1993 Sep 28;32(38):9968–9974. doi: 10.1021/bi00089a012. [DOI] [PubMed] [Google Scholar]
- Keith A. D., Snipes W., Chapman D. Spin-label studies on the aqueous regions of phospholipid multilayers. Biochemistry. 1977 Feb 22;16(4):634–641. doi: 10.1021/bi00623a013. [DOI] [PubMed] [Google Scholar]
- Kim J. T., Mattai J., Shipley G. G. Gel phase polymorphism in ether-linked dihexadecylphosphatidylcholine bilayers. Biochemistry. 1987 Oct 20;26(21):6592–6598. doi: 10.1021/bi00395a005. [DOI] [PubMed] [Google Scholar]
- Kinnunen P. K. On the principles of functional ordering in biological membranes. Chem Phys Lipids. 1991 Mar;57(2-3):375–399. doi: 10.1016/0009-3084(91)90087-r. [DOI] [PubMed] [Google Scholar]
- Klose G., Stelzner F. NMR investigations of the interaction of water with lecithin in benzene solutions. Biochim Biophys Acta. 1974 Aug 21;363(1):1–8. doi: 10.1016/0005-2736(74)90002-9. [DOI] [PubMed] [Google Scholar]
- Knoll W., Schmidt G., Rötzer H., Henkel T., Pfeiffer W., Sackmann E., Mittler-Neher S., Spinke J. Lateral order in binary lipid alloys and its coupling to membrane functions. Chem Phys Lipids. 1991 Mar;57(2-3):363–374. doi: 10.1016/0009-3084(91)90086-q. [DOI] [PubMed] [Google Scholar]
- Lehtonen J. Y., Kinnunen P. K. Changes in the lipid dynamics of liposomal membranes induced by poly(ethylene glycol): free volume alterations revealed by inter- and intramolecular excimer-forming phospholipid analogs. Biophys J. 1994 Jun;66(6):1981–1990. doi: 10.1016/S0006-3495(94)80991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz B. R., Hoechli M., Barenholz Y. Acyl chain order and lateral domain formation in mixed phosphatidylcholine--sphingomyelin multilamellar and unilamellar vesicles. Biochemistry. 1981 Nov 24;20(24):6803–6809. doi: 10.1021/bi00527a010. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., McIntyre G. F., Parks D. J., Yates J. C., Massenburg D. Bilayer curvature and certain amphipaths promote poly(ethylene glycol)-induced fusion of dipalmitoylphosphatidylcholine unilamellar vesicles. Biochemistry. 1992 Mar 17;31(10):2643–2653. doi: 10.1021/bi00125a003. [DOI] [PubMed] [Google Scholar]
- Lerebours B., Wehrli E., Hauser H. Thermodynamic stability and osmotic sensitivity of small unilamellar phosphatidylcholine vesicles. Biochim Biophys Acta. 1993 Oct 10;1152(1):49–60. doi: 10.1016/0005-2736(93)90230-w. [DOI] [PubMed] [Google Scholar]
- Lichtenberg D., Felgner P. L., Thompson T. E. Transition of a liquid crystalline phosphatidylcholine bilayer to the gel phase in a vesicle reduces the internal aqueous volume. Biochim Biophys Acta. 1982 Jan 22;684(2):277–281. doi: 10.1016/0005-2736(82)90017-7. [DOI] [PubMed] [Google Scholar]
- Lis L. J., McAlister M., Fuller N., Rand R. P., Parsegian V. A. Measurement of the lateral compressibility of several phospholipid bilayers. Biophys J. 1982 Mar;37(3):667–672. [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. C., MacDonald R. I., Menco B. P., Takeshita K., Subbarao N. K., Hu L. R. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim Biophys Acta. 1991 Jan 30;1061(2):297–303. doi: 10.1016/0005-2736(91)90295-j. [DOI] [PubMed] [Google Scholar]
- Maeda M., Thompson G. A., Jr On the mechanism of rapid plasma membrane and chloroplast envelope expansion in Dunaliella salina exposed to hypoosmotic shock. J Cell Biol. 1986 Jan;102(1):289–297. doi: 10.1083/jcb.102.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio B., Lucy J. A. Interactions of water-soluble fusogens with phospholipids in monolayers. FEBS Lett. 1978 Oct 15;94(2):301–304. doi: 10.1016/0014-5793(78)80962-4. [DOI] [PubMed] [Google Scholar]
- Maloney K. M., Grainger D. W. Phase separated anionic domains in ternary mixed lipid monolayers at the air-water interface. Chem Phys Lipids. 1993 Apr;65(1):31–42. doi: 10.1016/0009-3084(93)90079-i. [DOI] [PubMed] [Google Scholar]
- Marra J., Israelachvili J. Direct measurements of forces between phosphatidylcholine and phosphatidylethanolamine bilayers in aqueous electrolyte solutions. Biochemistry. 1985 Aug 13;24(17):4608–4618. doi: 10.1021/bi00338a020. [DOI] [PubMed] [Google Scholar]
- Marsh D. Water adsorption isotherms and hydration forces for lysolipids and diacyl phospholipids. Biophys J. 1989 Jun;55(6):1093–1100. doi: 10.1016/S0006-3495(89)82906-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinac B., Adler J., Kung C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature. 1990 Nov 15;348(6298):261–263. doi: 10.1038/348261a0. [DOI] [PubMed] [Google Scholar]
- Martinac B., Buechner M., Delcour A. H., Adler J., Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massenburg D., Lentz B. R. Poly(ethylene glycol)-induced fusion and rupture of dipalmitoylphosphatidylcholine large, unilamellar extruded vesicles. Biochemistry. 1993 Sep 7;32(35):9172–9180. doi: 10.1021/bi00086a024. [DOI] [PubMed] [Google Scholar]
- Mayer L. D., Nelsestuen G. L. Calcium and prothrombin-induced lateral phase separation in membranes. Biochemistry. 1981 Apr 28;20(9):2457–2463. doi: 10.1021/bi00512a015. [DOI] [PubMed] [Google Scholar]
- McConnell H. M., Tamm L. K., Weis R. M. Periodic structures in lipid monolayer phase transitions. Proc Natl Acad Sci U S A. 1984 May;81(10):3249–3253. doi: 10.1073/pnas.81.10.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn R., Dluhy R., Taraschi T., Cameron D. G., Mantsch H. H. Raman and Fourier transform infrared spectroscopic studies of the interaction between glycophorin and dimyristoylphosphatidylcholine. Biochemistry. 1981 Nov 10;20(23):6699–6706. doi: 10.1021/bi00526a027. [DOI] [PubMed] [Google Scholar]
- Miller A., Schmidt G., Eibl H., Knoll W. Ca2+-induced phase separation in black lipid membranes and its effect on the transport of a hydrophobic ion. Biochim Biophys Acta. 1985 Mar 14;813(2):221–229. doi: 10.1016/0005-2736(85)90237-8. [DOI] [PubMed] [Google Scholar]
- Mittler-Neher S., Knoll W. pH-control of the miscibility properties of a binary lipid alloy and its influence on the ion transport by gramicidin. Biochim Biophys Acta. 1990 Jul 24;1026(2):167–170. doi: 10.1016/0005-2736(90)90060-2. [DOI] [PubMed] [Google Scholar]
- Morris C. E., Sigurdson W. J. Stretch-inactivated ion channels coexist with stretch-activated ion channels. Science. 1989 Feb 10;243(4892):807–809. doi: 10.1126/science.2536958. [DOI] [PubMed] [Google Scholar]
- Mustonen P., Kinnunen P. K. On the reversal by deoxyribonucleic acid of the binding of adriamycin to cardiolipin-containing liposomes. J Biol Chem. 1993 Jan 15;268(2):1074–1080. [PubMed] [Google Scholar]
- Mustonen P., Lehtonen J., Kõiv A., Kinnunen P. K. Effects of sphingosine on peripheral membrane interactions: comparison of adriamycin, cytochrome c, and phospholipase A2. Biochemistry. 1993 May 25;32(20):5373–5380. doi: 10.1021/bi00071a012. [DOI] [PubMed] [Google Scholar]
- Mustonen P., Virtanen J. A., Somerharju P. J., Kinnunen P. K. Binding of cytochrome c to liposomes as revealed by the quenching of fluorescence from pyrene-labeled phospholipids. Biochemistry. 1987 Jun 2;26(11):2991–2997. doi: 10.1021/bi00385a006. [DOI] [PubMed] [Google Scholar]
- Oliet S. H., Bourque C. W. Mechanosensitive channels transduce osmosensitivity in supraoptic neurons. Nature. 1993 Jul 22;364(6435):341–343. doi: 10.1038/364341a0. [DOI] [PubMed] [Google Scholar]
- Op den Kamp J. A., Kauerz M. T., van Deenen L. L. Action of pancreatic phospholipase A2 on phosphatidylcholine bilayers in different physical states. Biochim Biophys Acta. 1975 Oct 6;406(2):169–177. doi: 10.1016/0005-2736(75)90001-2. [DOI] [PubMed] [Google Scholar]
- Orci L., Thorens B., Ravazzola M., Lodish H. F. Localization of the pancreatic beta cell glucose transporter to specific plasma membrane domains. Science. 1989 Jul 21;245(4915):295–297. doi: 10.1126/science.2665080. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Cherry R. J., Chapman D. Phase transitions and heterogeneity in lipid bilayers. Science. 1973 Aug 10;181(4099):557–559. doi: 10.1126/science.181.4099.557. [DOI] [PubMed] [Google Scholar]
- Paltauf F., Hauser H., Phillips M. C. Monolayer characteristics of some 1,2-diacyl, I-alkyl-2-acyl and 1,2-dialkyl phospholipids at the air-water interface. Biochim Biophys Acta. 1971 Dec 3;249(2):539–547. doi: 10.1016/0005-2736(71)90129-5. [DOI] [PubMed] [Google Scholar]
- Parente R. A., Lentz B. R. Fusion and phase separation monitored by lifetime changes of a fluorescent phospholipid probe. Biochemistry. 1986 Mar 11;25(5):1021–1026. doi: 10.1021/bi00353a011. [DOI] [PubMed] [Google Scholar]
- Peters R., Beck K. Translational diffusion in phospholipid monolayers measured by fluorescence microphotolysis. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7183–7187. doi: 10.1073/pnas.80.23.7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard H. B., Pazoles C. J., Creutz C. E., Scott J. H., Zinder O., Hotchkiss A. An osmotic mechanism for exocytosis from dissociated chromaffin cells. J Biol Chem. 1984 Jan 25;259(2):1114–1121. [PubMed] [Google Scholar]
- Poulin R., Pegg A. E. Regulation of ornithine decarboxylase expression by anisosmotic shock in alpha-difluoromethylornithine-resistant L1210 cells. J Biol Chem. 1990 Mar 5;265(7):4025–4032. [PubMed] [Google Scholar]
- Rand R. P., Fuller N. L., Butko P., Francis G., Nicholls P. Measured change in protein solvation with substrate binding and turnover. Biochemistry. 1993 Jun 15;32(23):5925–5929. doi: 10.1021/bi00074a001. [DOI] [PubMed] [Google Scholar]
- Rand R. P. Raising water to new heights. Science. 1992 May 1;256(5057):618–618. doi: 10.1126/science.256.5057.618. [DOI] [PubMed] [Google Scholar]
- Rayner M. D., Starkus J. G., Ruben P. C., Alicata D. A. Voltage-sensitive and solvent-sensitive processes in ion channel gating. Kinetic effects of hyperosmolar media on activation and deactivation of sodium channels. Biophys J. 1992 Jan;61(1):96–108. doi: 10.1016/S0006-3495(92)81819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers W., Glaser M. Characterization of lipid domains in erythrocyte membranes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1364–1368. doi: 10.1073/pnas.88.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D. S., Choppin P. W. Biochemical studies on cell fusion. I. Lipid composition of fusion-resistant cells. J Cell Biol. 1985 Oct;101(4):1578–1590. doi: 10.1083/jcb.101.4.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D. S., Robinson J. M., Davidson R. L. Cell fusion and intramembrane particle distribution in polyethylene glycol-resistant cells. J Cell Biol. 1983 Sep;97(3):909–917. doi: 10.1083/jcb.97.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe E. S. Induction of lateral phase separations in binary lipid mixtures by alcohol. Biochemistry. 1987 Jan 13;26(1):46–51. doi: 10.1021/bi00375a007. [DOI] [PubMed] [Google Scholar]
- Ruocco M. J., Siminovitch D. J., Griffin R. G. Comparative study of the gel phases of ether- and ester-linked phosphatidylcholines. Biochemistry. 1985 May 7;24(10):2406–2411. doi: 10.1021/bi00331a003. [DOI] [PubMed] [Google Scholar]
- Sankaram M. B., Marsh D., Thompson T. E. Determination of fluid and gel domain sizes in two-component, two-phase lipid bilayers. An electron spin resonance spin label study. Biophys J. 1992 Aug;63(2):340–349. doi: 10.1016/S0006-3495(92)81619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinsky B. S., Yeagle P. L. Effects of potassium on lipid-protein interactions in light sarcoplasmic reticulum. Biochemistry. 1990 Jan 16;29(2):415–421. doi: 10.1021/bi00454a016. [DOI] [PubMed] [Google Scholar]
- Sen A., Hui S. W. Direct measurement of headgroup hydration of polar lipids in inverted micelles. Chem Phys Lipids. 1988 Dec;49(3):179–184. doi: 10.1016/0009-3084(88)90005-9. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Silvius J. R. Calcium-induced lipid phase separations and interactions of phosphatidylcholine/anionic phospholipid vesicles. Fluorescence studies using carbazole-labeled and brominated phospholipids. Biochemistry. 1990 Mar 27;29(12):2930–2938. doi: 10.1021/bi00464a007. [DOI] [PubMed] [Google Scholar]
- Smaby J. M., Muderhwa J. M., Brockman H. L. Is lateral phase separation required for fatty acid to stimulate lipases in a phosphatidylcholine interface? Biochemistry. 1994 Feb 22;33(7):1915–1922. doi: 10.1021/bi00173a039. [DOI] [PubMed] [Google Scholar]
- Somerharju P. J., Virtanen J. A., Eklund K. K., Vainio P., Kinnunen P. K. 1-Palmitoyl-2-pyrenedecanoyl glycerophospholipids as membrane probes: evidence for regular distribution in liquid-crystalline phosphatidylcholine bilayers. Biochemistry. 1985 May 21;24(11):2773–2781. doi: 10.1021/bi00332a027. [DOI] [PubMed] [Google Scholar]
- Stockton G. W., Smith I. C. A deuterium nuclear magnetic resonance study of the condensing effect of cholesterol on egg phosphatidylcholine bilayer membranes. I. Perdeuterated fatty acid probes. Chem Phys Lipids. 1976 Oct;17(2-3):251–263. doi: 10.1016/0009-3084(76)90070-0. [DOI] [PubMed] [Google Scholar]
- Tajima K., Gershfeld N. L. Phospholipid surface bilayers at the air-water interface. I. Thermodynamic properties. Biophys J. 1985 Feb;47(2 Pt 1):203–209. doi: 10.1016/s0006-3495(85)83892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D., Chong P. L. E/M dips. Evidence for lipids regularly distributed into hexagonal super-lattices in pyrene-PC/DMPC binary mixtures at specific concentrations. Biophys J. 1992 Oct;63(4):903–910. doi: 10.1016/S0006-3495(92)81672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter-Minassian-Saraga L., Madelmont G. Cholesterol-induced modulation of membrane hydration studies by thermal analysis. FEBS Lett. 1982 Jan 11;137(1):137–140. doi: 10.1016/0014-5793(82)80332-3. [DOI] [PubMed] [Google Scholar]
- Tilcock C. P., Cullis P. R. The polymorphic phase behaviour of mixed phosphatidylserine-phosphatidylethanolamine model systems as detected by 31P-NMR. Biochim Biophys Acta. 1981 Feb 20;641(1):189–201. doi: 10.1016/0005-2736(81)90583-6. [DOI] [PubMed] [Google Scholar]
- Tilcock C. P., Fisher D. The interaction of phospholipid membranes with poly(ethylene glycol). Vesicle aggregation and lipid exchange. Biochim Biophys Acta. 1982 Jun 14;688(2):645–652. doi: 10.1016/0005-2736(82)90375-3. [DOI] [PubMed] [Google Scholar]
- Tilly B. C., van den Berghe N., Tertoolen L. G., Edixhoven M. J., de Jonge H. R. Protein tyrosine phosphorylation is involved in osmoregulation of ionic conductances. J Biol Chem. 1993 Sep 25;268(27):19919–19922. [PubMed] [Google Scholar]
- Watson P. A. Direct stimulation of adenylate cyclase by mechanical forces in S49 mouse lymphoma cells during hyposmotic swelling. J Biol Chem. 1990 Apr 25;265(12):6569–6575. [PubMed] [Google Scholar]
- Wattiaux-De Coninck S., Dubois F., Wattiaux R. Lateral phase separations and structural integrity of the inner membrane of rat-liver mitochondria. Effect of compression. Implications in the centrifugation of these organelles. Biochim Biophys Acta. 1977 Dec 15;471(3):421–435. doi: 10.1016/0005-2736(77)90047-5. [DOI] [PubMed] [Google Scholar]
- Webb M. S., Hui S. W., Steponkus P. L. Dehydration-induced lamellar-to-hexagonal-II phase transitions in DOPE/DOPC mixtures. Biochim Biophys Acta. 1993 Jan 18;1145(1):93–104. doi: 10.1016/0005-2736(93)90385-d. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. A., Morowitz H. J., Prestegard J. H. Hydration of phosphatidylocholine. Adsorption isotherm and proton nuclear magnetic resonance studies. Biophys J. 1977 Nov;20(2):169–179. doi: 10.1016/S0006-3495(77)85542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M., Ohnishi S., Ito T. Osmoelastic coupling in biological structures: decrease in membrane fluidity and osmophobic association of phospholipid vesicles in response to osmotic stress. Biochemistry. 1989 May 2;28(9):3710–3715. doi: 10.1021/bi00435a013. [DOI] [PubMed] [Google Scholar]
- Yamazaki M., Ohshika M., Kashiwagi N., Asano T. Phase transitions of phospholipid vesicles under osmotic stress and in the presence of ethylene glycol. Biophys Chem. 1992 May;43(1):29–37. doi: 10.1016/0301-4622(92)80039-8. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L., Hutton W. C., Huang C., Martin R. B. Phospholipid head-group conformations; intermolecular interactions and cholesterol effects. Biochemistry. 1977 Oct 4;16(20):4344–4349. doi: 10.1021/bi00639a003. [DOI] [PubMed] [Google Scholar]


