Abstract
The establishment of Borrelia burgdorferi infection involves numerous interactions between the bacteria and a variety of vertebrate host and arthropod vector tissues. This complex process requires regulated synthesis of many bacterial proteins. We now demonstrate that these spirochetes utilize a LuxS/autoinducer-2 (AI-2)-based quorum-sensing mechanism to regulate protein expression, the first system of cell-cell communication to be described in a spirochete. The luxS gene of B. burgdorferi was identified and demonstrated to encode a functional enzyme by complementation of an Escherichia coli luxS mutant. Cultured B. burgdorferi responded to AI-2 by altering the expression levels of a large number of proteins, including the complement regulator factor H-binding Erp proteins. Through this mechanism, a population of Lyme disease spirochetes may synchronize production of specific proteins needed for infection processes.
Borrelia burgdorferi persists in nature through an infectious cycle involving warm-blooded vertebrates and ticks of the genus Ixodes (55). To complete this cycle, B. burgdorferi must interact with many different tissues of the vertebrate host and the arthropod vector. In an unfed tick, bacterial infection is generally restricted to the midgut. While the tick feeds, bacteria migrate from the tick's midgut into the hemolymph, target and penetrate the salivary glands, and are transmitted into the bite wound with the saliva. Spirochetes then disseminate throughout the mammal and reside extracellularly in a variety of tissues (55). Feeding by a second tick presumably attracts bacteria, since ticks acquire large numbers of B. burgdorferi when engorging on infected mammals (10, 56).
The many different vector and host tissues encountered by B. burgdorferi during the transmission process likely requires that the bacteria synthesize proteins specific for interactions with each tissue type. In support of this hypothesis, many bacterial proteins have been observed to be differentially synthesized during the B. burgdorferi infection cycle (34, 58). Among the proteins produced by B. burgdorferi during infection are those that facilitate interactions between the bacteria and specific host cells or extracellular components (19, 28, 42). Others presumably function in nutrient acquisition in diverse host tissues (8, 44). Additionally, B. burgdorferi are also exposed to innate and acquired immune system responses from the vertebrate host (31, 69, 72), and a number of bacterial proteins are synthesized during mammalian infection to help protect against these host responses (29, 40, 41, 65).
In order for B. burgdorferi to differentially synthesize proteins during its infectious cycle, the bacteria must possess regulatory networks to sense its environment, transmit this information to cellular targets, and regulate the expression of appropriate genes and proteins. Such regulatory mechanisms may serve to control gene expression at the levels of both the individual bacterium and the population as a whole. Evidence of the importance of precise gene regulation was provided by a recent study which found that mutant B. burgdorferi defective in gene regulation were unable to disseminate and cause disease in mammals (3). The mechanisms by which B. burgdorferi regulates gene expression in vivo are poorly understood, although recent in vitro studies have provided some clues. A number of proteins are differentially expressed by Lyme disease spirochetes in response to changing culture conditions, such as temperature, pH, and certain chemicals (1, 2, 4, 13, 32, 51, 57, 66, 74). Yet while some environmental cues have been identified, the mechanisms by which B. burgdorferi senses any of these stimuli or transmits such information to regulate protein synthesis have remained largely unknown.
Many bacterial species use quorum-sensing mechanisms to regulate gene expression at the level of the population. Such bacteria secrete a specific compound (an autoinducer) into their surroundings, while simultaneously sampling the environment for its presence. When low quantities of autoinducer are produced by a population, each bacterium can detect little or no autoinducer. But when the autoinducer concentration increases in the culture, the high levels sensed by all of the bacteria serve as a signal for them to alter gene expression. In this manner, bacteria can coordinate a behavior, such as production of virulence factors during host infection (21, 37, 53).
Two general types of autoinducers have been identified in bacteria: those that are specific for the species producing it (for example, homoserine lactones or certain polypeptides), and a second type, autoinducer-2 (AI-2), that is well conserved across species. AI-2 is produced from methionine and ATP through a five-step process catalyzed by S-adenosylmethionine synthetase (MetK), a methyltransferase, S-adenosylhomocysteine/5-methylthioribose nucleosidase (Pfs), and LuxS (17, 54). The final step involves an apparently spontaneous cyclization of the LuxS product (4,5-dihydroxy-2,3-pentanedione) with borate to produce AI-2 (17) The first three enzymes in this pathway appear to be essential for bacterial survival (68). LuxS is apparently also an essential enzyme of many bacteria in nature, since AI-2-mediated quorum sensing is implicated in the regulation of virulence properties in a wide variety of pathogenic bacteria (18, 20, 23, 25, 27, 43, 59, 60, 62, 71).
We now demonstrated that B. burgdorferi encodes a functional LuxS enzyme, enabling it to synthesize AI-2. Furthermore, addition of this autoinducer to cultured B. burgdorferi had profound effects on expression levels of many bacterial proteins, indications of the importance of this quorum-sensing system in the regulation of borrelial protein expression.
MATERIALS AND METHODS
Bacteria and plasmids.
All three strains of B. burgdorferi used in these studies are infectious to both mammals and ticks and have low in vitro passage histories. Strain B31 was originally isolated from an infected tick collected on Shelter Island, N.Y. (9). The genome of a subculture of B31 was recently sequenced (14, 24). Strain N40 was originally isolated from an infected tick collected in Westchester County, N.Y. (5). Strain 297 was isolated from the cerebrospinal fluid of a human Lyme disease patient, with the infection reportedly acquired in Westchester County, N.Y. (61). B. burgdorferi was cultured in modified Barbour-Stoener-Kelly medium (BSK-H) containing 6% rabbit serum (Sigma, St. Louis, Mo.).
Vibrio harveyi BB120 is wild type. Strain BB170 is a luxN mutant of BB120 and is sensitive to AI-2 but not to AI-1 (6). V. harveyi were cultured in autoinducer bioassay (AB) medium (26).
Purified chromosomal DNA from B. burgdorferi B31 was used as a template to PCR amplify ORF BB0377. The oligonucleotides used were LUXS-7: (TAAAATACAAACAGGAGGAAAAAAATG; 5′ of the gene to the start codon) and LUXS-6 (CTATTTTGTAAATTTTATGAGCTAAGG; 3′ of the gene). The putative B. burgdorferi luxS gene contains a weak Shine-Delgarno sequence (24). To increase translational efficiency of this gene, oligonucleotide LUXS-7 was designed to introduce a consensus Shine-Delgarno sequence, AGGAGG, 5′ of the start codon. The resulting amplicon was cloned into vector pCR2.1 to produce pBLS563, containing a complete open reading frame (ORF) BB0377 oriented such that the inserted gene is under the transcriptional control of the pCR2.1 lac promoter. The pBLS563 insert was completely sequenced, confirming that no nucleotide errors were introduced during the PCR and cloning processes.
Recombinant plasmid pBLS521 contains a fragment of the B. burgdorferi B31 plasmid cp32-7 cloned into pCR2.1 (64). No gene in this plasmid bears any homology with any gene known to function in quorum sensing, and it served as a negative control in autoinducer analyses.
Escherichia coli DH5α contains a defective luxS and is thus unable to synthesize AI-2 (67, 68). Both pBLS521 and pBLS563 were introduced into DH5α, and transformants selected by plating on Luria-Bertani (LB) medium containing kanamycin (50 μg/ml).
AI-2 analyses.
E. coli DH5α, or DH5α transformed with either pBLS563 or pBLS521, was grown overnight in LB broth at 37°C with aeration. For bacteria containing either plasmid, kanamycin was added to a concentration of 50 μg/ml. Overnight cultures were diluted 50-fold into LB broth lacking antibiotic and grown with aeration for 2 h at 37°C. IPTG (isopropyl-β-d-thiogalactopyranoside) was added to each culture to a concentration of 4 mg/ml, followed by incubation for an additional 3 h. Bacteria were pelleted by centrifugation, and supernatants were sterilized by passage through a 0.22-μm (pore-size) filter.
For analysis of AI-2 production by B. burgdorferi, cultures were grown under a variety of different conditions: at temperatures of 23 or 34°C or shifted from 23 to 34°C (66), at pH 6.5 and 8, and at low and high cell densities (ca. 105 or 108 bacteria per ml, respectively).
The marine bacterium V. harveyi utilizes two distinct quorum-sensing mechanisms to regulate bioluminescence, AI-2 and AI-1. V. harveyi BB170 is defective in response to AI-1 and can thus be used to assay AI-2 alone. Since the structure of AI-2 is conserved among bacteria, autoinducer produced by unrelated bacteria causes V. harveyi to produce light, a characteristic that can be exploited to assay AI-2 produced by any species of bacterium (6). This bioassay appears to be specific for AI-2 (6, 54). Autoinducer bioassays of cell-free culture media were performed essentially as previously described (26, 67). Briefly, an overnight culture of V. harveyi BB170 was diluted 1:5,000 into fresh AB medium. To aliquots of the diluted BB170 culture were added 1/10 volumes of the sterile culture supernatant being examined. As a positive control, filter-sterilized supernatant from an overnight culture of V. harveyi BB120 was also assayed. As negative controls, fresh AB, LB, or BSK-H media were assayed. Bioassay aliquots were incubated at 30°C with aeration. At 1-h intervals, aliquots were removed from each bioassay tube and analyzed by using a TopCount luminescence counter with 96-well format (Packard, Meriden, Conn.).
Cultivation of B. burgdorferi with AI-2.
B. burgdorferi B31 were cultured at either 23 or 34°C to mid-exponential phase (ca. 106 bacteria/ml) and then divided into two equal aliquots. Sterile supernatants of E. coli DH5α containing either pBLS563 or pBLS521 were prepared as described above, and each was added to one of the B. burgdorferi aliquots at a dilution of 1:100. At the same time, [35S]methionine and [35S]cysteine were added to a final concentration of 100 μCi/ml to metabolically label newly synthesized proteins. The B. burgdorferi were cultured for either 2 days at 34°C or 5 days at 23°C (approximately 2 to 3 doublings at each temperature) (66). Prior to processing, cultures were examined microscopically at a ×400 magnification to ensure that there were no contaminating organisms. Borreliae were then pelleted by centrifugation, washed twice with phosphate-buffered saline (PBS), and lysed by heating in a boiling water bath. Then, 1-mg aliquots of each lysate were subjected to isoelectric focusing at between pH 3 to 10 by using precast IPG strips (Bio-Rad, Hercules, Calif.), followed by reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; i.e., two-dimensional PAGE). Labeled proteins were detected by autoradiography.
To examine the effects of AI-2 on specific proteins, B. burgdorferi were cultured as described above, with or without added AI-2, but without the addition of radiolabeled amino acids. Bacteria were harvested, washed with PBS, and lysed. Equal amounts of total protein from each lysate were separated by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were immunoblot analyzed (46) with one of two monoclonal antibodies, specific for either ErpA/I/N or OspC (22, 45).
RESULTS
B. burgdorferi LuxS enzyme.
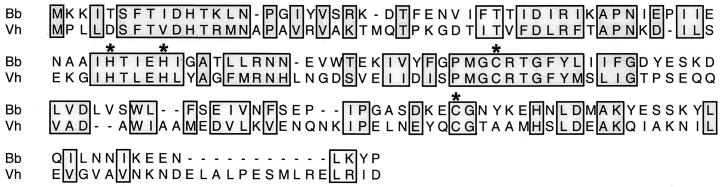
The complete genome sequence of the B. burgdorferi type strain B31 has been determined (14, 24). We noted that the chromosomal ORF given the reference number BB0377 encodes a protein similar to the LuxS proteins of other bacteria (Fig. 1). Most significantly, the B. burgdorferi protein contains all of the conserved amino acids predicted to be involved in enzymatic function of other bacterial LuxS proteins (30, 52). Additionally, the putative B. burgdorferi luxS gene appears to be located in an operon with genes homologous to metK and pfs, which encode two other enzymes essential for AI-2 synthesis (24, 54).
FIG. 1.
Alignment of the predicted amino acid sequences of the LuxS proteins of B. burgdorferi (Bb) and V. harveyi (Vh). Residues predicted to be involved in LuxS function are indicated by asterisks (30, 54).
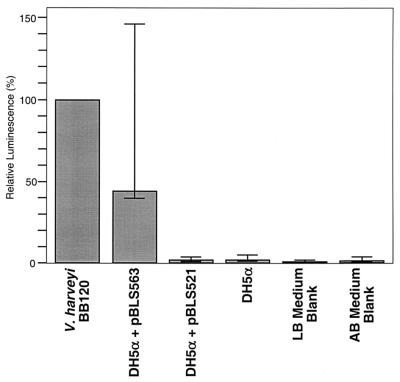
We first sought to establish whether B. burgdorferi ORF BB0377 encodes a functional LuxS, by examining its ability to complement a luxS-defective E. coli. For this study, we used the laboratory E. coli DH5α, which contains a mutation in luxS, and is thus incapable of producing AI-2 (67). pBLS563, which contains ORF BB0377 under the control of the E. coli lac promoter, was introduced into DH5α and induced with IPTG, and the culture supernatant was assayed for AI-2 activity. The pBLS563-containing DH5α supernatant significantly induced bioluminescence by the V. harveyi reporter strain, reaching levels comparable to those induced by culture supernatant of wild-type V. harveyi (Fig. 2). In contrast, culture supernatants from both untransformed E. coli DH5α and DH5α carrying control plasmid pBLS521 failed to induce V. harveyi luminescence, being comparable to culture medium blanks. We conclude that B. burgdorferi ORF BB0377 is indeed a homolog of luxS, indicating that these bacteria can produce AI-2.
FIG. 2.
Results of bioassays of AI-2 activity, reported as the percentage of V. harveyi BB120 control supernatant after 2 h of incubation. Averages of five assays are reported, with range bars indicating high and low extremes. Plasmid pBLS563 encodes the B. burgdorferi LuxS enzyme.
Effects of AI-2 on B. burgdorferi protein synthesis.
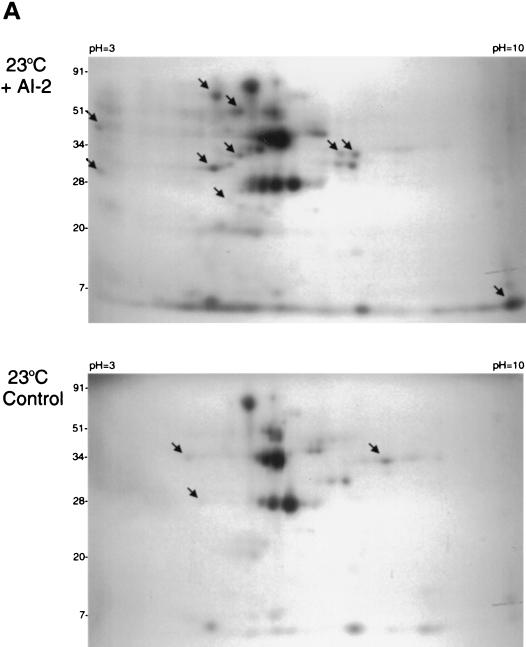
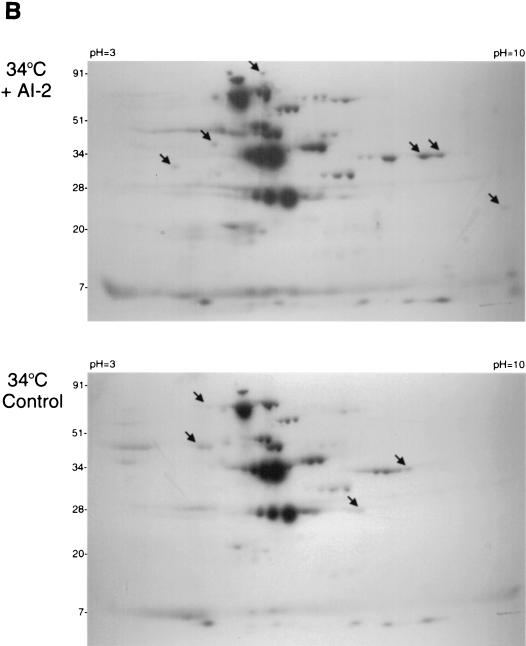
Having established that the Lyme disease spirochete encodes a functional LuxS enzyme, we next examined the effect of AI-2 on B. burgdorferi protein expression. Cell-free supernatants were produced from E. coli DH5α either expressing the B. burgdorferi LuxS enzyme or containing the control plasmid. Bioassays confirmed that the luxS+ bacterial supernatant contained AI-2, whereas the control supernatant did not. Newly synthesized proteins were metabolically labeled by the addition of 35S-labeled amino acids. The addition of the AI-2-containing supernatant to cultured B. burgdorferi had striking effects on the expression levels of a considerable number of labeled proteins (Fig. 3). For bacteria cultured at 23°C, the presence of AI-2 resulted in the increased expression of 23 detectable proteins and the decreased expression of 7 others. At least 18 proteins increased in expression level upon addition of AI-2, whereas 7 decreased in expression for bacteria grown at 34°C. We also noted that B. burgdorferi responded differently to AI-2 depending upon the culture temperature, with the majority of proteins affected by AI-2 in 23°C-cultivated bacteria being different from those affected in bacteria incubated at 34°C (Fig. 3). These data indicate that Lyme disease spirochetes possess the ability to recognize AI-2 and to respond to its presence by modulating expression of specific proteins, apparently in a temperature-dependent manner.
FIG. 3.
Effects of AI-2 on B. burgdorferi protein expression. Arrowheads indicate representative proteins whose expression was either increased or decreased in response to AI-2-containing supernatant. Note that the levels of a large number of proteins were altered by AI-2; at least 30 35S-labeled proteins were affected by AI-2 in bacteria cultured at 23°C, and 25 35S-labeled proteins were affected in bacteria grown at 34°C.
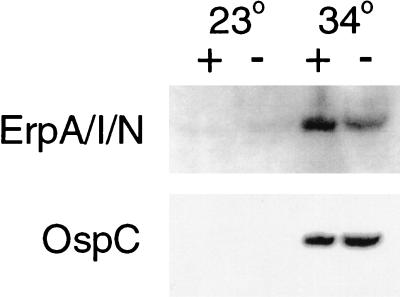
Earlier studies performed in our laboratory and by others identified several B. burgdorferi proteins whose expression is altered by various environmental parameters. Synthesis of the Erp and OspC surface proteins are all influenced by culture temperature, with significantly greater amounts of these proteins being produced by bacteria grown at 34°C than by those grown at 23°C (57, 63, 66). Since culture temperature also influenced the responses of B. burgdorferi to AI-2, we explored the effects of autoinducer on those proteins. Addition of AI-2-containing supernatant to B. burgdorferi cultivated at 34°C resulted in an approximately twofold increase in ErpA/I/N expression relative to those bacteria incubated in supernatant that lacked AI-2 (Fig. 4). The autoinducer had no perceptible effect on the levels of OspC. For bacteria grown at 23°C, the presence of the autoinducer had no detectable effect on either protein. These data are consistent with results of previous studies indicating that B. burgdorferi controls the expression of Erp and OspC proteins through distinct mechanisms (4).
FIG. 4.
Effects of added AI-2 on the expression of specific proteins by B. burgdorferi. Bacteria were cultivated in BSK-H medium containing either control cell-free E. coli supernatant (−) or BSK-H containing supernatant with AI-2 (+) and then lysed and analyzed by immunoblot with monoclonal antibodies specific for ErpA/I/N or OspC.
AI-2 bioassays of cultured B. burgdorferi.
We next examined cultured B. burgdorferi for the production of AI-2 by using V. harveyi bioluminescence assays. Culture supernatants were assayed from bacteria grown at either a constant 23 or 34°C or shifted from 23 to 34°C. The pH of the culture medium can also affect B. burgdorferi protein synthesis, so supernatants were assayed from cultures having either acidic or basic pH. In the event that culture density might influence AI-2 levels, supernatants were assayed from cultures having either low or high bacterial densities. The possibility of differences among B. burgdorferi strains was also considered, so three strains, B31, N40, and 297, were examined. However, none of these culture supernatants detectably induced luminescence by V. harveyi (data not shown). Hence, we conclude that cultured B. burgdorferi either do not produce AI-2 or synthesize the autoinducer at levels far too low to be detected by bioassay.
DISCUSSION
Results from these studies demonstrated that B. burgdorferi encodes a functional LuxS protein. Genes homologous to the other enzymes required for AI-2 synthesis are also present in these bacteria (24, 54), likely providing B. burgdorferi with the capability of producing AI-2. B. burgdorferi also possesses the ability to recognize AI-2, and use this signal to alter expression of many specific proteins. Consequently, we hypothesize that B. burgdorferi uses AI-2 during its natural infectious cycle to regulate protein synthesis, potentially enabling a population of bacteria to coordinate the expression of virulence determinants. Earlier studies revealed that the regulation of protein expression by B. burgdorferi involves a complex network that is responsive to many environmental parameters, including temperature, pH, and soluble chemicals (1, 2, 4, 13, 57, 66, 74). The discovery that these bacteria utilize quorum sensing as part of their regulatory machinery indicates how elaborate these networks truly are.
Lyme disease spirochetes regulate expression of both the complement factor H-binding Erp proteins and transmission-associated OspC proteins in response to temperature (57, 63, 65, 66). Our studies indicated that AI-2 also stimulates Erp expression and yet has no apparent effect on OspC. It is possible, however, that OspC expression is affected by AI-2 in vivo, but culture medium either contains or lacks an additional regulatory factor which influences OspC synthesis. Earlier work indicated that B. burgdorferi regulates OspC and Erp production through different mechanisms, since OspC expression is influenced by culture pH, whereas that of Erp proteins is not (4). Additionally, different soluble chemicals affect synthesis of each protein type (4). The different effects of AI-2 on OspC and Erp expression further support the hypothesis of distinct regulatory mechanisms for each protein.
We note that a different set of proteins was affected in the bacteria grown at 23°C than in those cultivated at 34°C, indicating a connection between temperature- and AI-2-dependent gene regulation. Previous studies have noted a correlation between temperature and protein expression, with some proteins associated with vertebrate infection being synthesized at temperatures near that of mammalian blood (such as 34°C), while other proteins involved in tick infection are more highly expressed at ambient temperature (15, 39, 47, 57, 66, 73). Hence, the results from our studies suggest that AI-2-mediated quorum sensing may function in both the vertebrate host and the arthropod vector, perhaps regulating expression of different sets of proteins in each environment.
In the present studies, we were unable to detect AI-2 synthesis by cultivated B. burgdorferi. However, this lack of detection in vitro was not altogether surprising, since several B. burgdorferi substances known to be synthesized in vivo are not detectably produced by the bacterium during growth in the artificial environment of culture medium (34, 58; J. C. Miller and B. Stevenson, unpublished results). The composition of BSK-H medium apparently provides a mixture of messages to the bacteria, since both mammal- and arthropod-specific proteins are synthesized during in vitro cultivation. We propose that, while B. burgdorferi is most likely capable of synthesizing AI-2, culture medium does not provide a signal(s) necessary for autoinducer production. In support of our hypothesis, a growing list of diverse bacterial species have been identified that regulate autoinducer synthesis, producing the signal only when certain conditions are met. Some bacteria synthesize high levels of autoinducer during the mid-exponential growth phase in culture but significantly less autoinducer during the late exponential and stationary phases, presumably using bacterial growth rate and nutrient supply as signals to regulate autoinducer synthesis (7, 18, 23, 36, 67). Depletion of glucose levels in culture medium reduces autoinducer synthesis by E. coli (67), and Streptococcus pyogenes produces autoinducer in response to amino acid starvation (62). Autoinducer production by Pseudomonas species is also regulated by a variety of environmental factors (11, 16, 38, 48, 70).
There have been several previous reports suggesting the existence of a cell density-dependent quorum-sensing mechanism for gene regulation in B. burgdorferi (33, 35, 49). In those earlier studies, it was observed that cultured bacteria in late exponential phase produced greater quantities of certain proteins than did bacteria in early exponential phase cultures. However, further experimentation indicated that the observed effects on protein expression were actually due to changes in the pH of the culture medium, which acidifies during bacterial growth (12, 50). No changes in protein profile were detected when bacteria were grown to high densities in media buffered to remain at a basic pH. Furthermore, proteins previously reported as being induced at high cell density were produced by bacteria at low culture density when grown in preacidified media (12, 50). AI-2 does not appear to play a role in this pH-dependent regulatory mechanism, since our studies found that AI-2 was undetectable in cultured bacteria grown in either acidic or basic medium, or at either low or high cell densities.
The spirochetes comprise an ancient bacterial lineage. The B. burgdorferi LuxS/AI-2 system is the first example of quorum sensing to be reported in a spirochete; this finding raises the possibility that such regulatory mechanisms may be widespread among other members of this phylum. Further characterization of the B. burgdorferi system might well have important consequences in understanding the pathogenic properties of many other infectious spirochetes.
First described in 1982 (9), B. burgdorferi has since been the focus of extensive research, yet very little is known about how these bacteria are responsible for the many aspects of Lyme disease. Transmission from a tick to a human or other mammal requires bacterial interactions with a large number of vector and host tissues, with precise regulation of protein expression being necessary for efficient bacterial progress through the infectious cycle. The profound effects of AI-2 on cultured bacteria indicate that the LuxS/AI-2-mediated quorum-sensing system is an important facet of B. burgdorferi gene regulation. Defining the intricacies of this system will greatly advance our understanding of these complex bacteria and their interactions with the mammals and ticks they infect. In addition, understanding the mechanisms regulating bacterial protein synthesis will indicate novel targets for improved therapies to prevent and treat Lyme disease.
Acknowledgments
This work was supported by National Institute of Allergy and Infectious Diseases grant AI44254.
We thank Alex Bobrov, Ken Parrish, and Robert Perry for bacterial strains and advice on AI-2 bioassays; Bonnie Bassler for calling luxS to our attention; Nazira El-Hage, Melissa Hines, Natalie Mickelsen, and Jennifer Miller for technical assistance; and Patti Rosa, Tony Sinai, Jennifer Miller, and Wolf Zückert for helpful discussions on these studies and critical reading of the manuscript.
Editor: D. L. Burns
REFERENCES
- 1.Akins, D. R., K. W. Bourell, M. J. Caimano, M. V. Norgard, and J. D. Radolf. 1998. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J. Clin. Investig. 101:2240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alban, P. S., P. W. Johnson, and D. R. Nelson. 2000. Serum-starvation-induced changes in protein synthesis and morphology on Borrelia burgdorferi. Microbiology 146:119-127. [DOI] [PubMed] [Google Scholar]
- 3.Anguita, J., S. Samanta, B. Revilla, K. Suk, S. Das, S. W. Barthold, and E. Fikrig. 2000. Borrelia burgdorferi gene expression in vivo and spirochete pathogenicity. Infect. Immun. 68:1222-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babb, K., N. El-Hage, J. C. Miller, J. A. Carroll, and B. Stevenson. 2001. Distinct regulatory pathways control the synthesis of Borrelia burgdorferi infection-associated OspC and Erp surface proteins. Infect. Immun. 69:4146-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthold, S. W., M. S. de Souza, J. L. Janotka, A. L. Smith, and D. H. Persing. 1993. Chronic Lyme borreliosis in the laboratory mouse. Am. J. Pathol. 143:959-972. [PMC free article] [PubMed] [Google Scholar]
- 6.Bassler, B. L., E. P. Greenberg, and A. M. Stevens. 1997. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J. Bacteriol. 179:4043-4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blosser-Middleton, R. S., and K. M. Gray. 2001. Multiple N-acyl homoserine lactone signals in Rhizobium leguminosarum are synthesized in a distinct temporal pattern. J. Bacteriol. 183:6771-6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bono, J. L., K. Tilly, B. Stevenson, D. Hogan, and P. Rosa. 1998. Oligopeptide permease in Borrelia burgdorferi: putative peptide-binding components encoded by both chromosomal and plasmid loci. Microbiology 144:1033-1044. [DOI] [PubMed] [Google Scholar]
- 9.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease -a tick-borne spirochetosis? Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 10.Burkot, T. R., J. Piesman, and R. A. Wirtz. 1994. Quantitation of the Borrelia burgdorferi outer surface protein A in Ixodes scapularis: fluctuations during the tick life cycle, doubling times and loss while feeding. J. Infect. Dis. 170:883-889. [DOI] [PubMed] [Google Scholar]
- 11.Cao, H., G. Krishnan, B. Goumnerov, J. Tsongalis, R. Tompkins, and L. G. Rahme. 2001. A quorum sensing-associated virulence gene of Pseudomonas aeruginosa encodes a LysR-like transcription regulator with a unique self-regulatory mechanism. Proc. Natl. Acad. Sci. USA 98:14613-14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll, J. A., R. M. Cordova, and C. F. Garon. 2000. Identification of eleven pH-regulated genes in Borrelia burgdorferi localized to linear plasmids. Infect. Immun. 68:6677-6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carroll, J. A., C. F. Garon, and T. G. Schwan. 1999. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect. Immun. 67:3181-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs of an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 15.Cassatt, D. R., N. K. Patel, N. D. Ulbrandt, and M. S. Hanson. 1998. DbpA, but not OspA, is expressed by Borrelia burgdorferi during spirochetemia and is a target for protective antibodies. Infect. Immun. 66:5379-5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chancey, S. T., D. W. Wood, and L. S. Pierson. 1999. Two-component transcriptional regulation of N-acyl-homoserine lactone production in Pseudomonas aureofaciens. Appl. Environ. Microbiol. 65:2294-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, X., S. Schauder, N. Potier, A. Van Dorsselaer, I. Pelczer, B. L. Bassler, and F. M. Hughson. 2002. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415:545-549. [DOI] [PubMed] [Google Scholar]
- 18.Chung, W. O., Y. Park, R. J. Lamont, R. McNab, B. Barbieri, and D. R. Demuth. 2001. Signaling system in Porphyromonas gingivalis based on a LuxS protein. J. Bacteriol. 183:3903-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coleman, J. L., J. A. Gebbia, J. Piesman, J. L. Degen, T. H. Bugge, and J. L. Benach. 1997. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell 89:1111-1119. [DOI] [PubMed] [Google Scholar]
- 20.Day, W. A., and A. T. Maurelli. 2001. Shigella flexneri LuxS quorum-sensing system modulates virB expression but is not essential for virulence. Infect. Immun. 69:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Kievit, T. R., and B. H. Iglewski. 2000. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 68:4839-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Hage, N., K. Babb, J. A. Carroll, N. Lindstrom, E. R. Fischer, J. C. Miller, R. D. Gilmore, Jr., M. L. Mbow, and B. Stevenson. 2001. Surface exposure and protease insensitivity of Borrelia burgdorferi Erp (OspEF-related) lipoproteins. Microbiology 147:821-830. [DOI] [PubMed] [Google Scholar]
- 23.Fong, K. P., W. O. Chung, R. J. Lamont, and D. R. Demuth. 2001. Intra- and interspecies regulation of gene expression by Actinobacillus actinomycetemcomitans LuxS. Infect. Immun. 69:7625-7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J.-F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidmann, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete: Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 25.Frias, J., E. Olle, and M. Alsina. 2001. Periodontal pathogens produce quorum sensing signal molecules. Infect. Immun. 69:3431-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberg, E. P., J. W. Hastings, and S. Ulitzur. 1979. Induction of luciferase synthesis in Beneckea harveyi by other marine bacteria. Arch. Microbiol. 120:87-91. [Google Scholar]
- 27.Gruenheid, S., and B. B. Finlay. 2000. Crowd control: quorum sensing in pathogenic E. coli. Trends Microbiol. 8:442-443. [DOI] [PubMed] [Google Scholar]
- 28.Guo, B. P., S. J. Norris, L. C. Rosenberg, and M. Höök. 1995. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect. Immun. 63:3467-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hellwage, J., T. Meri, T. Heikkilä, A. Alitalo, J. Panelius, P. Lahdenne, I. J. T. Seppäl, and S. Meri. 2001. The complement regulatory factor H binds to the surface protein OspE of Borrelia burgdorferi. J. Biol. Chem. 276:8427-8435. [DOI] [PubMed] [Google Scholar]
- 30.Hilgers, M. T., and M. L. Ludwig. 2001. Crystal structure of the quorum-sensing protein LuxS reveals a catalytic metal site. Proc. Natl. Acad. Sci. USA 98:11169-11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu, L. T., and M. S. Klempner. 1997. Host-pathogen interactions in the immunopathogenesis of Lyme disease. J. Clin. Immunol. 17:354-365. [DOI] [PubMed] [Google Scholar]
- 32.Hübner, A., X. Yang, D. M. Nolen, T. G. Popova, P. C. Cabello, and M. V. Norgard. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. USA 98:12724-12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Indest, K. J., and M. T. Philipp. 2000. DNA-binding proteins possibly involved in regulation of the post-logarithmic-phase expression of lipoprotein P35 in Borrelia burgdorferi. J. Bacteriol. 182:522-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Indest, K. J., R. Ramamoorthy, and M. T. Philipp. 2001. Transcriptional regulation in spirochetes of medical importance, p. 159-170. In M. H. Saier and J. García-Lara (ed.), The spirochetes: molecular and cellular biology. Horizon Press, Oxford, England.
- 35.Indest, K. J., R. Ramamoorthy, M. Sole, R. D. Gilmore, Jr., B. J. B. Johnson, and M. T. Philipp. 1997. Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro. Infect. Immun. 65:1165-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joyce, E. A., B. L. Bassler, and A. Wright. 2000. Evidence for a signaling system in Helicobacter pylori: detection of a luxS-encoded system. J. Bacteriol. 182:3638-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaiser, D. 1996. Bacteria also vote. Science 272:1598-1599. [DOI] [PubMed] [Google Scholar]
- 38.Köhler, T., C. van Delden, L. K. Curty, M. M. Hamzehpour, and J. C. Pechere. 2001. Overexpression of the MexEF-OprN multidrug efflux system affects cell-to-cell signalling in Pseudomonas aeruginosa. J. Bacteriol. 183:5213-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konkel, M. E., and K. Tilly. 2000. Temperature-regulated expression of bacterial virulence genes. Microbes Infect. 2:157-166. [DOI] [PubMed] [Google Scholar]
- 40.Kraiczy, P., C. Skerka, V. Brade, and P. F. Zipfel. 2001. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect. Immun. 69:7800-7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurtenbach, K., S. DeMichelis, S. Etti, S. M. Schäfer, H.-S. Sewell, V. Brade, and P. Kraiczy. 2002. Host association of Borrelia burgdorferi sensu lato -the key role of host complement. Trends Microbiol. 10:74-79. [DOI] [PubMed] [Google Scholar]
- 42.Leong, J. M., H. Wang, L. Magoun, J. A. Field, P. E. Morrissey, D. Robbins, J. B. Tatro, J. Coburn, and N. Parveen. 1998. Different classes of proteoglycans contribute to the attachment of Borrelia burgdorferi to cultured endothelial and brain cells. Infect. Immun. 66:994-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyon, W. R., J. C. Madden, J. C. Levin, J. L. Stein, and M. G. Caparon. 2001. Mutation of luxS affects growth and virulence factor expression in Streptococcus pyogenes. Mol. Microbiol. 42:145-157. [DOI] [PubMed] [Google Scholar]
- 44.Margolis, N., D. Hogan, K. Tilly, and P. A. Rosa. 1994. Plasmid location of Borrelia purine biosynthesis gene homologs. J. Bacteriol. 176:6427-6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mbow, M. L., R. D. Gilmore, Jr., and R. G. Titus. 1999. An OspC-specific monoclonal antibody passively protects mice from tick-transmitted infection by Borrelia burgdorferi B31. Infect. Immun. 67:5470-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller, J. C., N. El-Hage, K. Babb, and B. Stevenson. 2000. Borrelia burgdorferi B31 Erp proteins that are dominant immunoblot antigens of animals infected with isolate B31 are recognized by only a subset of human Lyme disease patient sera. J. Clin. Microbiol. 38:1569-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pal, U., A. M. deSilva, R. R. Montgomery, D. Fish, J. Anguita, J. F. Anderson, Y. Lobet, and E. Fikrig. 2000. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J. Clin. Investig. 106:561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pessi, G., F. Williams, Z. Hindle, K. Heurlier, M. T. G. Holden, M. Cámara, D. Haas, and P. Williams. 2001. The global posttranscriptional regulator RmsA modulates production of virulence determinants and N-acylhomoserine lactones in Pseudomonas aeruginosa. J. Bacteriol. 183:6676-6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramamoorthy, R., and M. T. Philipp. 1998. Differential expression of Borrelia burgdorferi proteins during growth in vitro. Infect. Immun. 66:5119-5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramamoorthy, R., and D. Scholl-Meeker. 2001. Borrelia burgdorferi proteins whose expression is similarly affected by culture temperature and pH. Infect. Immun. 69:2739-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 99:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruzheinikov, S. N., S. K. Das, S. E. Sedelnikova, A. Hartley, S. J. Foster, M. J. Horsburg, A. G. Cox, C. W. McCleod, A. Mekhalfia, G. M. Blackburn, D. W. Rice, and P. J. Baker. 2001. The 1.2 Å structure of a novel quorum-sensing protein: Bacillus subtilis LuxS. J. Mol. Biol. 313:111-122. [DOI] [PubMed] [Google Scholar]
- 53.Schauder, S., and B. L. Bassler. 2001. The languages of bacteria. Genes Dev. 15:1468-1480. [DOI] [PubMed] [Google Scholar]
- 54.Schauder, S., S. Shokat, M. G. Surette, and B. L. Bassler. 2001. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol. Microbiol. 41:463-476. [DOI] [PubMed] [Google Scholar]
- 55.Schwan, T. G., W. Burgdorfer, and P. A. Rosa. 1999. Borrelia, p. 746-758. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 56.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete. Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seshu, J., and J. T. Skare. 2001. The many faces of Borrelia burgdorferi, p. 147-158. In M. H. Saier and J. García-Lara (ed.), The spirochetes: molecular and cellular biology. Horizon Press, Oxford, England.
- 59.Sperandio, V., J. L. Mellies, W. Nguyen, S. Shin, and J. B. Kaper. 1999. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorragic and enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 96:15196-15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sperandio, V., A. G. Torres, J. A. Girón, and J. B. Kaper. 2001. Quorum sensing is a global regulatory mechanism in enterohemorragic Escherichia coli O157:H7. J. Bacteriol. 183:5187-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steere, A. C., R. L. Grodzicki, A. N. Kornblatt, J. E. Craft, A. G. Barbour, W. Burgdorfer, G. P. Schmid, E. Johnson, and S. E. Malawista. 1983. The spirochetal etiology of Lyme disease. N. Engl. J. Med. 308:733-740. [DOI] [PubMed] [Google Scholar]
- 62.Steiner, K., and H. Malke. 2001. relA-independent amino acid starvation response network of Streptococcus pyogenes. J. Bacteriol. 183:7354-7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stevenson, B., J. L. Bono, T. G. Schwan, and P. Rosa. 1998. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect. Immun. 66:2648-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stevenson, B., S. Casjens, and P. Rosa. 1998. Evidence of past recombination events among the genes encoding the Erp antigens of Borrelia burgdorferi. Microbiology 144:1869-1879. [DOI] [PubMed] [Google Scholar]
- 65.Stevenson, B., N. El-Hage, M. A. Hines, J. C. Miller, and K. Babb. 2002. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect. Immun. 70:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stevenson, B., T. G. Schwan, and P. A. Rosa. 1995. Temperature-related differential expression of antigens in the Lyme disease spirochete. Borrelia burgdorferi. Infect. Immun. 63:4535-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Surette, M. G., and B. L. Bassler. 1998. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 95:7046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Surette, M. G., M. B. Miller, and B. L. Bassler. 1999. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc. Natl. Acad. Sci. USA 96:1639-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Szczepanski, A., and J. L. Benach. 1991. Lyme borreliosis: host responses to Borrelia burgdorferi. Microbiol. Rev. 55:21-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Delden, C., R. Comte, and M. Bally. 2001. Stringent response activates quorum sensing and modulates cell density-dependent gene expression in Pseudomonas aeruginosa. J. Bacteriol. 183:5376-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Winzer, K., Y. Sun, A. Green, M. Delory, D. Blackley, K. R. Hardie, T. J. Baldwin, and C. M. Tang. 2002. Role of Neisseria meningitidis luxS in cell-to-cell signalling and bacteremic infection. Infect. Immun. 70:2245-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wooten, R. M., and J. J. Weis. 2001. Host-pathogen interactions promoting inflammatory Lyme arthritis: use of mouse models for dissection of disease processes. Curr. Opin. Microbiol. 4:274-279. [DOI] [PubMed] [Google Scholar]
- 73.Yang, X., M. S. Goldberg, T. G. Popova, G. B. Schoeler, S. K. Wikel, K. E. Hagman, and M. V. Norgard. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 37:1470-1479. [DOI] [PubMed] [Google Scholar]
- 74.Yang, X., T. G. Popova, M. S. Goldberg, and M. V. Norgard. 2001. Influence of cultivation media on genetic regulatory patterns in Borrelia burgdorferi. Infect. Immun. 69:4159-4163. [DOI] [PMC free article] [PubMed] [Google Scholar]