Abstract
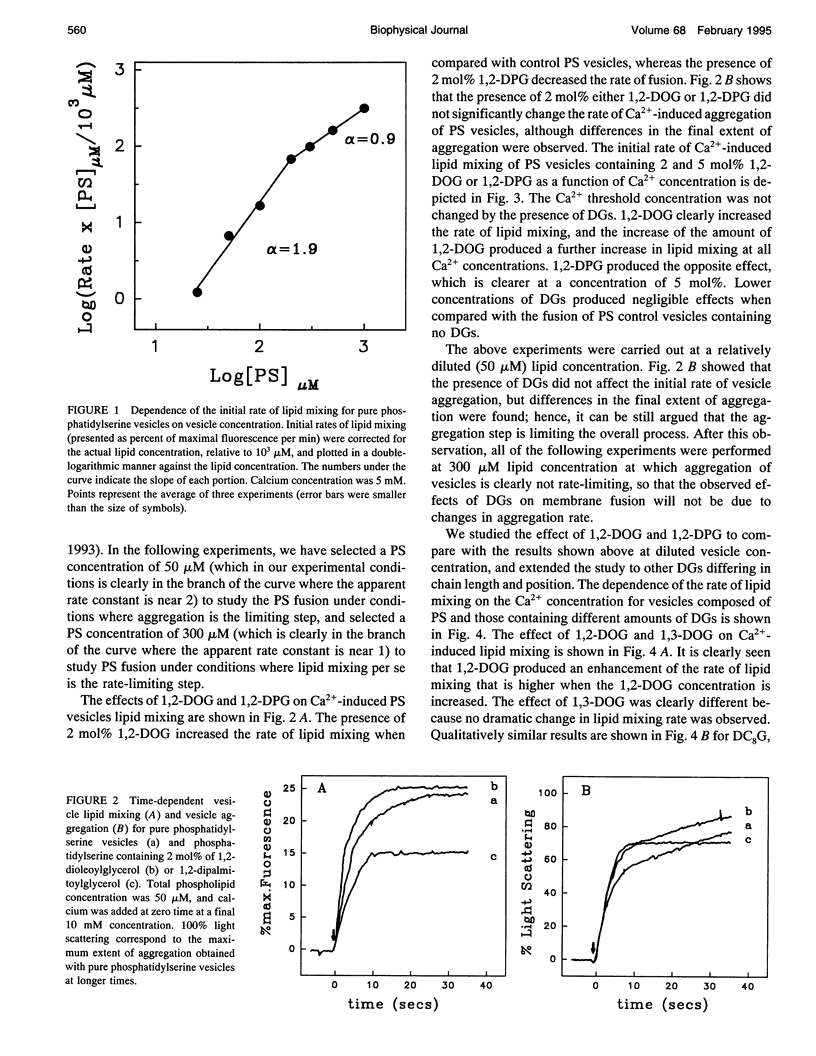
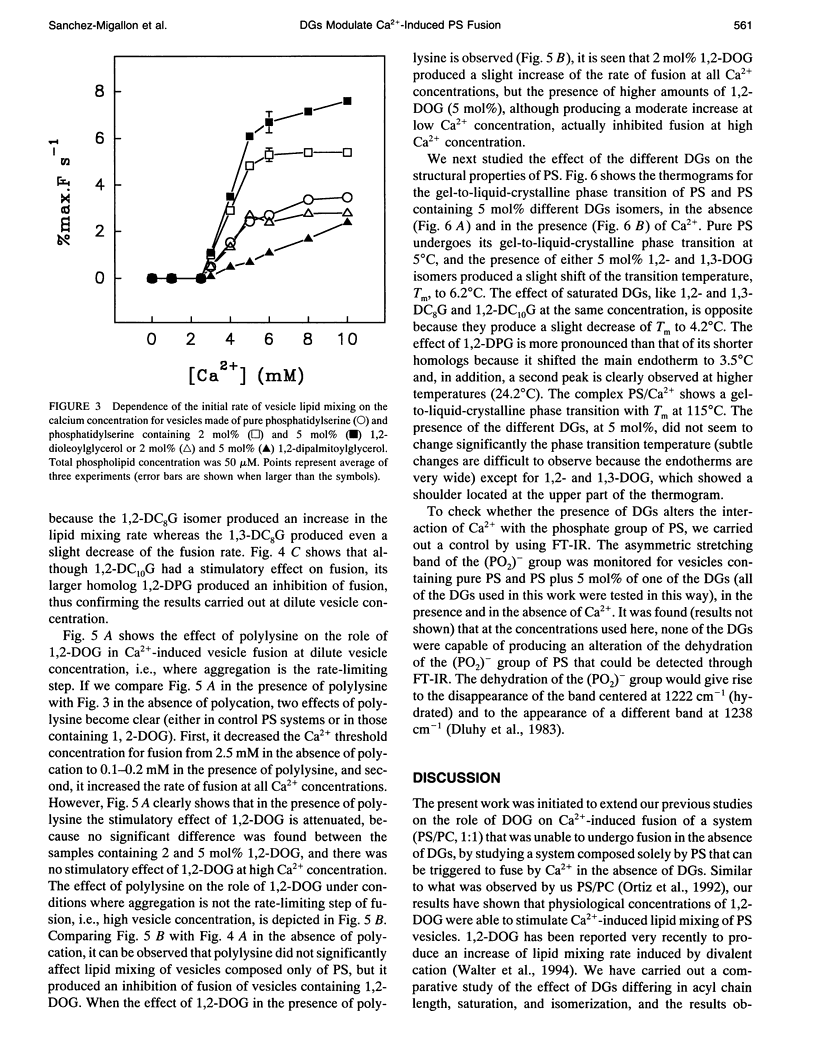
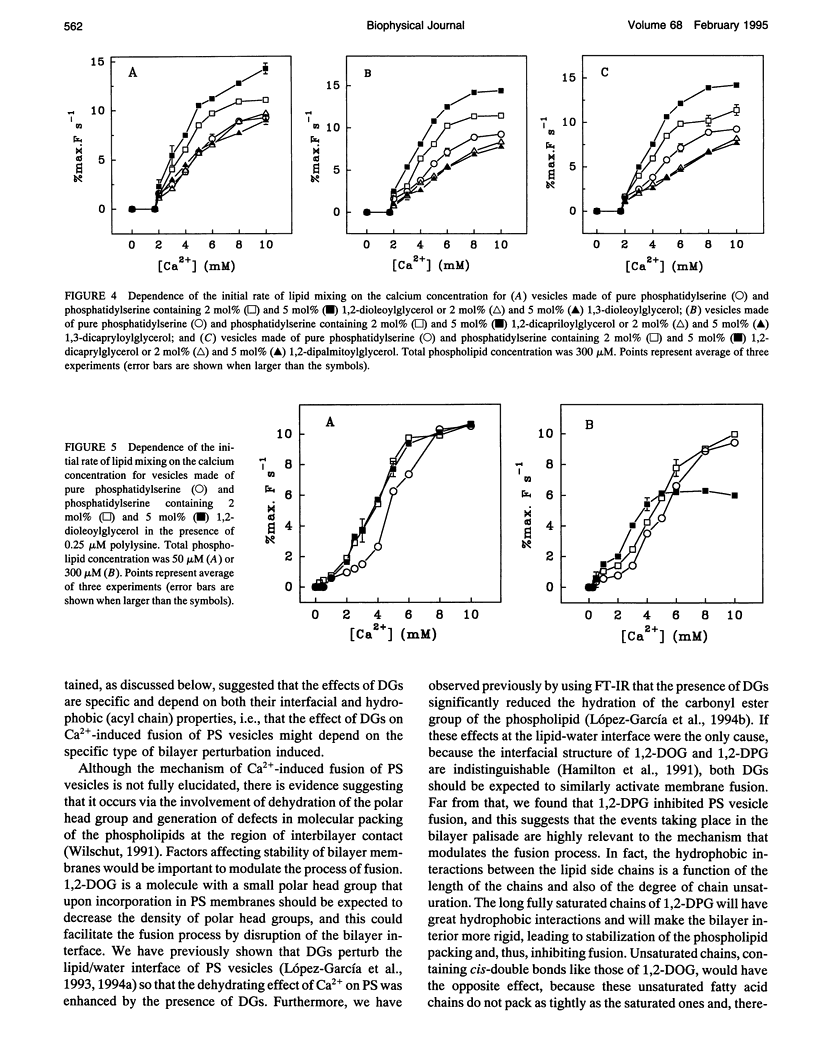
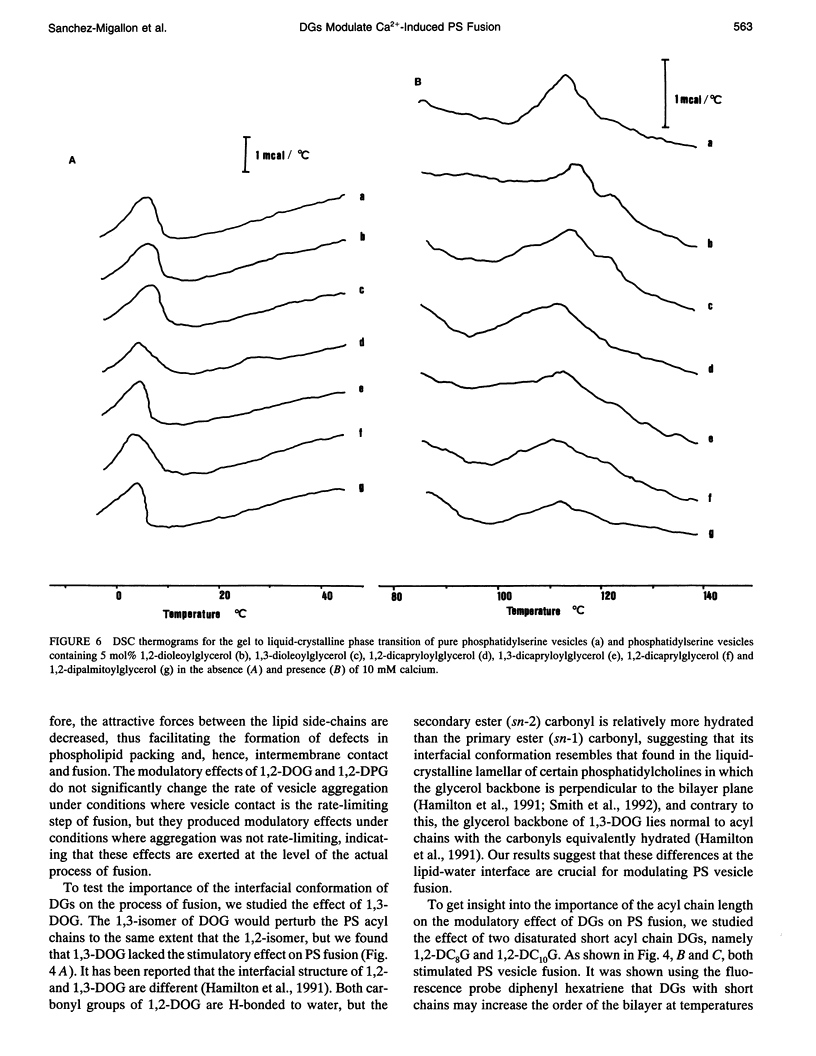
We have studied the effect of physiological concentrations of different diacylglycerols on Ca(2+)-induced fusion between phosphatidylserine vesicles. We monitored vesicle fusion as mixing of membrane lipids under conditions where the limiting factor was the aggregation and also in conditions where this aggregation was not the limiting factor. We found that diacylglycerols have a different modulating effect on the Ca(2+)-induced fusion: i) depending on their interfacial conformation, so that 1,2-isomers of diacylglycerols containing unsaturated or short saturated acyl chains stimulated fusion and their 1,3-isomers did not, and ii) depending on their specific type of bilayer interior perturbation, so that diacylglycerols containing unsaturated or short chain saturated acyl chains stimulated fusion but those containing long-chain saturated acyl chains did not. These requirements resembled those required for the diacylglycerol activation of protein kinase C, suggesting that diacylglycerol acts in both the specific activation of this enzyme and the induction of membrane fusion through the same perturbation of lipid structure. We found that polylysine affected the stimulatory role of 1,2-dioleoylglycerol differently, depending on whether aggregation was the limiting factor of fusion. When we studied the effect of very low concentrations of diacylglycerols on the bulk structural properties of phosphatidylserine, we found that they neither significantly perturbed the thermotropic transitions of phosphatidylserine nor affected the interaction of Ca2+ with the phosphate group of phosphatidylserine. The underlying mechanism of fusion between phosphatidylserine vesicles is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahkong Q. F., Fisher D., Tampion W., Lucy J. A. The fusion of erythrocytes by fatty acids, esters, retinol and alpha-tocopherol. Biochem J. 1973 Sep;136(1):147–155. doi: 10.1042/bj1360147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bolen E. J., Sando J. J. Effect of phospholipid unsaturation on protein kinase C activation. Biochemistry. 1992 Jun 30;31(25):5945–5951. doi: 10.1021/bi00140a034. [DOI] [PubMed] [Google Scholar]
- Carrier D., Pézolet M. Investigation of polylysine-dipalmitoylphosphatidylglycerol interactions in model membranes. Biochemistry. 1986 Jul 15;25(14):4167–4174. doi: 10.1021/bi00362a027. [DOI] [PubMed] [Google Scholar]
- Das S., Rand R. P. Diacylglycerol causes major structural transitions in phospholipid bilayer membranes. Biochem Biophys Res Commun. 1984 Oct 30;124(2):491–496. doi: 10.1016/0006-291x(84)91580-8. [DOI] [PubMed] [Google Scholar]
- Das S., Rand R. P. Modification by diacylglycerol of the structure and interaction of various phospholipid bilayer membranes. Biochemistry. 1986 May 20;25(10):2882–2889. doi: 10.1021/bi00358a022. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Irvine R. F., Bray J., Quinn P. J. Long-chain unsaturated diacylglycerols cause a perturbation in the structure of phospholipid bilayers rendering them susceptible to phospholipase attack. Biochem Biophys Res Commun. 1984 Dec 14;125(2):836–842. doi: 10.1016/0006-291x(84)90615-6. [DOI] [PubMed] [Google Scholar]
- De Boeck H., Zidovetzki R. Effects of diacylglycerols on the structure of phosphatidylcholine bilayers: a 2H and 31P NMR study. Biochemistry. 1989 Sep 5;28(18):7439–7446. doi: 10.1021/bi00444a043. [DOI] [PubMed] [Google Scholar]
- Epand R. M. Diacylglycerols, lysolecithin, or hydrocarbons markedly alter the bilayer to hexagonal phase transition temperature of phosphatidylethanolamines. Biochemistry. 1985 Dec 3;24(25):7092–7095. doi: 10.1021/bi00346a011. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Stafford A. R., Lester D. S. Lipid vesicles which can bind to protein kinase C and activate the enzyme in the presence of EGTA. Eur J Biochem. 1992 Sep 1;208(2):327–332. doi: 10.1111/j.1432-1033.1992.tb17190.x. [DOI] [PubMed] [Google Scholar]
- Gad A. E., Bental M., Elyashiv G., Weinberg H., Nir S. Promotion and inhibition of vesicle fusion by polylysine. Biochemistry. 1985 Oct 22;24(22):6277–6282. doi: 10.1021/bi00343a035. [DOI] [PubMed] [Google Scholar]
- Gad A. E., Silver B. L., Eytan G. D. Polycation-induced fusion of negatively-charged vesicles. Biochim Biophys Acta. 1982 Aug 25;690(1):124–132. doi: 10.1016/0005-2736(82)90246-2. [DOI] [PubMed] [Google Scholar]
- Goldberg E. M., Lester D. S., Borchardt D. B., Zidovetzki R. Effects of diacylglycerols and Ca2+ on structure of phosphatidylcholine/phosphatidylserine bilayers. Biophys J. 1994 Feb;66(2 Pt 1):382–393. doi: 10.1016/s0006-3495(94)80788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Fernández J. C., Aranda F. J., Micol V., Villalaín J., Ortiz A. Effect of diacylglycerols on calcium-induced fusion of phosphatidylserine/phosphatidylcholine vesicles. Biochem Soc Trans. 1989 Dec;17(6):957–960. doi: 10.1042/bst0170957. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Bhamidipati S. P., Kodali D. R., Small D. M. The interfacial conformation and transbilayer movement of diacylglycerols in phospholipid bilayers. J Biol Chem. 1991 Jan 15;266(2):1177–1186. [PubMed] [Google Scholar]
- Hammes G. G., Schullery S. E. Structure of macromolecular aggregates. II. Construction of model membranes from phospholipids and polypeptides. Biochemistry. 1970 Jun 23;9(13):2555–2563. doi: 10.1021/bi00815a001. [DOI] [PubMed] [Google Scholar]
- Hartmann W., Galla H. J. Binding of polylysine to charged bilayer membranes: molecular organization of a lipid.peptide complex. Biochim Biophys Acta. 1978 Jun 2;509(3):474–490. doi: 10.1016/0005-2736(78)90241-9. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Lapetina E. G., Reep B., Ganong B. R., Bell R. M. Exogenous sn-1,2-diacylglycerols containing saturated fatty acids function as bioregulators of protein kinase C in human platelets. J Biol Chem. 1985 Feb 10;260(3):1358–1361. [PubMed] [Google Scholar]
- López-García F., Micol V., Villalaín J., Gómez-Fernández J. C. Infrared spectroscopic study of the interaction of diacylglycerol with phosphatidylserine in the presence of calcium. Biochim Biophys Acta. 1993 Sep 8;1169(3):264–272. doi: 10.1016/0005-2760(93)90250-d. [DOI] [PubMed] [Google Scholar]
- López-García F., Villalaín J., Gómez-Fernández J. C. Diacylglycerol, phosphatidylserine and Ca2+: a phase behavior study. Biochim Biophys Acta. 1994 Mar 23;1190(2):264–272. doi: 10.1016/0005-2736(94)90083-3. [DOI] [PubMed] [Google Scholar]
- López-García F., Villalaín J., Gómez-Fernández J. C., Quinn P. J. The phase behavior of mixed aqueous dispersions of dipalmitoyl derivatives of phosphatidylcholine and diacylglycerol. Biophys J. 1994 Jun;66(6):1991–2004. doi: 10.1016/S0006-3495(94)80992-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieva J. L., Goñi F. M., Alonso A. Phospholipase C-promoted membrane fusion. Retroinhibition by the end-product diacylglycerol. Biochemistry. 1993 Feb 2;32(4):1054–1058. doi: 10.1021/bi00055a009. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Nomura H., Ase K., Sekiguchi K., Kikkawa U., Nishizuka Y., Nakano Y., Satoh T. Stereospecificity of diacylglycerol for stimulus-response coupling in platelets. Biochem Biophys Res Commun. 1986 Nov 14;140(3):1143–1151. doi: 10.1016/0006-291x(86)90754-0. [DOI] [PubMed] [Google Scholar]
- Ogita K., Ono Y., Kikkawa U., Nishizuka Y. Expression, separation, and assay of protein kinase C subspecies. Methods Enzymol. 1991;200:228–234. doi: 10.1016/0076-6879(91)00142-j. [DOI] [PubMed] [Google Scholar]
- Ohki K., Sekiya T., Yamauchi T., Nozawa Y. Effect of phosphatidylinositol replacement by diacylglycerol on various physical properties of artificial membranes with respect to the role of phosphatidylinositol response. Biochim Biophys Acta. 1982 Dec 22;693(2):341–350. doi: 10.1016/0005-2736(82)90441-2. [DOI] [PubMed] [Google Scholar]
- Ortiz A., Aranda F. J., Villalaín J., San Martín C., Micol V., Gómez-Fernandez J. C. 1,2-Dioleoylglycerol promotes calcium-induced fusion in phospholipid vesicles. Chem Phys Lipids. 1992 Oct;62(3):215–224. doi: 10.1016/0009-3084(92)90058-w. [DOI] [PubMed] [Google Scholar]
- Ortiz A., Villalaín J., Gómez-Fernández J. C. Interaction of diacylglycerols with phosphatidylcholine vesicles as studied by differential scanning calorimetry and fluorescence probe depolarization. Biochemistry. 1988 Dec 13;27(25):9030–9036. doi: 10.1021/bi00425a022. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Vail W. J., Jacobson K., Poste G. Cochleate lipid cylinders: formation by fusion of unilamellar lipid vesicles. Biochim Biophys Acta. 1975 Jul 3;394(3):483–491. doi: 10.1016/0005-2736(75)90299-0. [DOI] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Rando R. R., Young N. The stereospecific activation of protein kinase C. Biochem Biophys Res Commun. 1984 Jul 31;122(2):818–823. doi: 10.1016/s0006-291x(84)80107-2. [DOI] [PubMed] [Google Scholar]
- Siegel D. P., Banschbach J., Alford D., Ellens H., Lis L. J., Quinn P. J., Yeagle P. L., Bentz J. Physiological levels of diacylglycerols in phospholipid membranes induce membrane fusion and stabilize inverted phases. Biochemistry. 1989 May 2;28(9):3703–3709. doi: 10.1021/bi00435a012. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Kustanovich I., Bhamidipati S., Salmon A., Hamilton J. A. Interfacial conformation of dipalmitoylglycerol and dipalmitoylphosphatidylcholine in phospholipid bilayers. Biochemistry. 1992 Nov 24;31(46):11660–11664. doi: 10.1021/bi00161a054. [DOI] [PubMed] [Google Scholar]
- Struck D. K., Hoekstra D., Pagano R. E. Use of resonance energy transfer to monitor membrane fusion. Biochemistry. 1981 Jul 7;20(14):4093–4099. doi: 10.1021/bi00517a023. [DOI] [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Rasmussen H. A tumour promoter, 12-O-tetradecanoylphorbol 13-acetate, increases cellular 1,2-diacylglycerol content through a mechanism other than phosphoinositide hydrolysis in Swiss-mouse 3T3 fibroblasts. Biochem J. 1987 May 1;243(3):647–653. doi: 10.1042/bj2430647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A., Siegel D. P. Divalent cation-induced lipid mixing between phosphatidylserine liposomes studied by stopped-flow fluorescence measurements: effects of temperature, comparison of barium and calcium, and perturbation by DPX. Biochemistry. 1993 Apr 6;32(13):3271–3281. doi: 10.1021/bi00064a009. [DOI] [PubMed] [Google Scholar]
- Walter A., Steer C. J., Blumenthal R. Polylysine induces pH-dependent fusion of acidic phospholipid vesicles: a model for polycation-induced fusion. Biochim Biophys Acta. 1986 Oct 9;861(2):319–330. doi: 10.1016/0005-2736(86)90434-7. [DOI] [PubMed] [Google Scholar]
- Walter A., Yeagle P. L., Siegel D. P. Diacylglycerol and hexadecane increase divalent cation-induced lipid mixing rates between phosphatidylserine large unilamellar vesicles. Biophys J. 1994 Feb;66(2 Pt 1):366–376. doi: 10.1016/s0006-3495(94)80786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilschut J., Düzgüneş N., Fraley R., Papahadjopoulos D. Studies on the mechanism of membrane fusion: kinetics of calcium ion induced fusion of phosphatidylserine vesicles followed by a new assay for mixing of aqueous vesicle contents. Biochemistry. 1980 Dec 23;19(26):6011–6021. doi: 10.1021/bi00567a011. [DOI] [PubMed] [Google Scholar]
- Wooten M. W., Vandenplas M., Nel A. E. Rapid purification of protein kinase C from rat brain. A novel method employing protamine-agarose affinity column chromatography. Eur J Biochem. 1987 Apr 15;164(2):461–467. doi: 10.1111/j.1432-1033.1987.tb11079.x. [DOI] [PubMed] [Google Scholar]
- Zidovetzki R., Lester D. S. The mechanism of activation of protein kinase C: a biophysical perspective. Biochim Biophys Acta. 1992 Apr 7;1134(3):261–272. doi: 10.1016/0167-4889(92)90185-e. [DOI] [PubMed] [Google Scholar]
- van Gorkom L. C., Nie S. Q., Epand R. M. Hydrophobic lipid additives affect membrane stability and phase behavior of N-monomethyldioleoylphosphatidylethanolamine. Biochemistry. 1992 Jan 28;31(3):671–677. doi: 10.1021/bi00118a006. [DOI] [PubMed] [Google Scholar]


