Abstract
By using mice genomically lacking the mononuclear phagocytic growth factor colony-stimulating factor 1 and thereby deficient in macrophage and dendritic cell populations, we show that these cells play a dual role: they constitute a major defense against systemic infection but also facilitate cerebral bacterial invasion by Listeria monocytogenes.
Listeria monocytogenes is a gram-positive, facultative intracellular bacterium which may cause sepsis as well as severe infections in the nervous system in humans and domestic animals. In humans, these neural infections are manifested as meningitis in premature children and immunosuppressed patients but occasionally, severe necrotizing brainstem encephalitis in otherwise healthy individuals can occur (9). Both retrograde axonal transport in peripheral nerves to the brainstem (1, 13) and blood-borne invasion (2, 15) have been suggested as routes of entry for the bacteria to the brain.
Macrophages have been suggested as serving as vectors in the blood-borne spread of L. monocytogenes bacteria to reach cerebral endothelial cells for further spread into the brain parenchyma (6-8, 10). By using a snout model of listerial murine infection, we have obtained evidence that L. monocytogenes needs to replicate in a nonneuronal cell before it invades the central nervous system (CNS) along the peripheral neural route (12). This is consistent with in vitro data showing that the invasion of cultured neurons is a relatively rare event (5, 14). However, neurons are efficiently invaded in vitro by direct cell-to-cell spread from infected macrophages or microglial cells (5). Since the nature of these cells in vivo is not known, we asked whether macrophages could play a role in facilitating invasion of the bacteria along peripheral nerves.
Macrophages play an important role in the primary host defense response, since depletion of macrophages impairs Listeria resistance (17, 18), and cytokine-stimulated macrophages possess listeriocidal mechanisms. Furthermore, macrophages and dendritic cells act as antigen-presenting cells and trigger Listeria-specific protective immune responses (22).
To analyze the roles of macrophages and dendritic cells in the neural spread of L. monocytogenes, we used recessive osteopetrotic mutation (op−/−) mice, which exhibit depletion of monocytes and of populations of macrophages and dendritic cells in many tissues (16, 21, 25, 27). These mice show a null mutation in the colony-stimulating factor 1 (CSF-1) gene, the product of which is the major regulator of cells of the mononuclear phagocytic lineage (20, 24, 28). However, some macrophage populations are relatively unaffected by the absence of CSF-1, and these mice have been shown to mount humoral and cell-mediated immune responses (26). Nevertheless, an increased susceptibility of op−/− mice to infection by Mycobacterium tuberculosis and L. monocytogenes has recently been reported (11, 21).
We herein present data showing a dual role of CSF-1-dependent cells during L. monocytogenes infection in vivo: they play a detrimental role by facilitating listerial invasion along the peripheral nerve route but are a relevant component of the antilisterial defense in the limiting of systemic spread of the bacteria.
Homozygous (op−/−) and heterozygous (op+/−) mice were used in this study. Densities of tissue macrophages in the op+/− mice are not different from those of control mice (4). The heterozygous (op+/−) breeding pairs were obtained from the Jackson Laboratory (Bar Harbor, Maine). The op−/− mice were distinguished by the absence of incisors and by a characteristic skull deformation. They were maintained after weaning on a diet of wet mesh chow. Phenotypically wild-type (WT) offspring were regularly screened for the presence of op-mutated alleles by PCR followed by restriction analysis as described below.
L. monocytogenes WT strains EGD (serotype 1/2a) and EGD ΔplcB2 (with a defective lecithinase; both obtained from T. Chakraborty, Institut für Medizinische Mikrobiologie, Justus-Liebig-Universität Gieβen, Gieβen, Germany) were grown in brain heart infusion (BHI) broth and BHI agar (both from Difco Laboratories, Detroit, Mich.) at 37°C. For infection, bacteria were grown at 37°C in BHI broth to late exponential phase (optical density at 600 nm, 0.8), washed once with phosphate-buffered saline (PBS), suspended in 0.9% NaCl, and quantified on BHI agar plates.
L. monocytogenes (107 CFU) in 100 μl of PBS was injected subcutaneously into the left side of snouts of mice under Metofane anesthesia. Tissues were dissected at different times after infection, homogenized, and lysed with sterile 0.6% Triton X-100 in PBS. The tissue lysates were diluted in sterile 0.9% NaCl, and 100 μl of the suspension was plated on duplicate BHI agar plates.
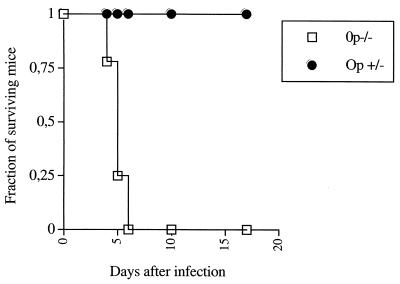
In a first series of experiments, op−/− and op+/− mice were infected in the snout with L. monocytogenes. Whereas all heterozygous mice survived the infection, all op−/− mice died within 7 days of infection (Fig. 1). Thus, CSF-1-dependent cells play a fundamental role in the control of host survival after subcutaneous infection, as also shown in a systemic (intravenous) model of listerial murine infection (11).
FIG. 1.
The survival of 15 op+/− and 17 op−/− mice infected in the snout with 107 CFU of L. monocytogenes was recorded. The difference in survival rates between the two groups is significant (P < 0.05 [Wilcoxon U-test after a Kaplan-Meier survival analysis]).
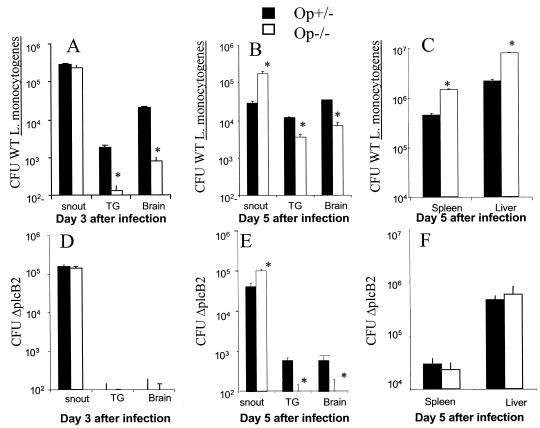
We next quantified L. monocytogenes at the site of infection, the trigeminal ganglia (TG), the brain stem, the liver, and the spleen. op−/− mice showed lower numbers of bacteria in the TG and the brain than the heterozygous controls (Fig. 2A and B). On the other hand, op−/− mice showed higher numbers of L. monocytogenes in the snout, spleen, and liver than those in the heterozygous controls (Fig. 2A, B, and C). Taken together, our data suggest that while CSF-1-dependent macrophages control bacterial levels in the snout, spleen, and liver, they facilitate invasion of the bacterium in the CNS. Accordingly, in vitro-infected macrophages have been suggested to be vectors by which L. monocytogenes gains access to privileged niches such as the CNS (5, 6).
FIG. 2.
CFU were measured in the snouts, TG, brains (panels A, B, D, and E), spleens, and livers (panels C and F) of op−/− and op+/− mice (n = six animals per group) at 3 (panels A and D) and 5 (panels B, C, E, and F) days after snout infection with 107 WT (panels A, B, and C) or ΔplcB2 (panels D, E, and F) L. monocytogenes bacteria. Bacterial load was expressed as the mean CFU per tissue ± the standard error of the mean. Results representative of two independent experiments for each condition are shown. Asterisks indicate results for which differences from values for op+/− mice were significant (P < 0.05 [Student t test]).
We next asked whether CSF-1-dependent macrophages differentially controlled the L. monocytogenes bacteria invading cells from the extracellular milieu versus those spreading from cell to cell. For this purpose, we studied the outcome of infection of op−/− and op+/− mice with the lecithinase-deficient ΔplcB2 mutant, which cannot spread from cell to cell (23). Mice infected with the ΔplcB2 mutant displayed strikingly diminished L. monocytogenes levels in the TG and the brain, compared to those infected with WT bacteria (Fig. 2). Thus, confirming previous reports (7, 12, 19), intracellular spread appears to be important for listerial neuroinvasion. That there is a higher listerial load in the TG and brains of op−/− mice infected with WT bacteria compared with those infected with ΔplcB2 mutants suggests that both CSF-1-dependent and -independent cells are involved in neuroinvasion through cell-to-cell spreading (Fig. 2A, B, D, and E). Mice infected with ΔplcB2 and WT bacteria showed similar listerial loads in the snout, indicating that cell-to-cell spreading was not determining the rate of bacterial proliferation in this tissue. Accordingly, CSF-1-dependent cells also controlled ΔplcB2 bacterial load in this tissue (Fig. 2B and E).
op−/− and op+/− animals showed lower ΔplcB2 L. monocytogenes levels in the spleen and liver than did mice infected with WT bacteria, indicating that cell-to-cell spreading is also important for systemic listerial dissemination (Fig. 2C and F). CSF-1-dependent cells in the spleen and liver are not important in the control of extracellular listerial spread, since op−/− and op+/− mice showed similar ΔplcB2 bacterial levels in these organs (Fig. 2F). Thus, our data suggest that CSF-1-dependent macrophages participate in the control of intracellular spread of L. monocytogenes in these organs (Fig. 2C and F).
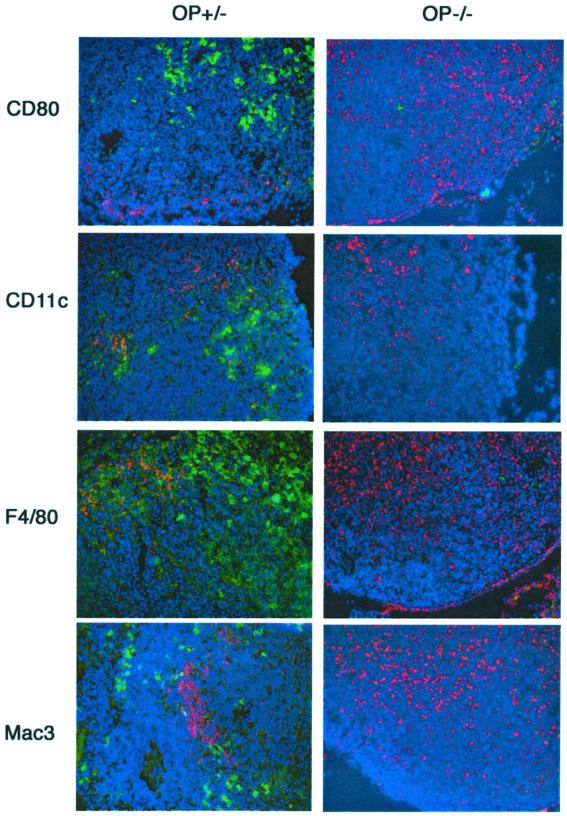
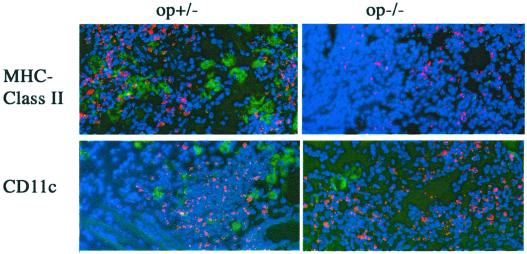
We then asked whether a diminished conveyance of Listeria into the superficial cervical lymph nodes that drain the site of infection, which could result in a deficient induction of the adaptive immune responses, was related to the increased susceptibility of op−/− mice. op−/− mice showed a higher density of immunostaining for L. monocytogenes in the superficial cervical draining lymph node than controls (Fig. 3). However, op−/− lymph nodes showed decreased levels of CD11c, F4/80, Mac3, and CD80 (B7-1) cells (Fig. 3), suggesting a deficient presentation of listerial antigens but not a diminished conveyance of pathogen antigens into the draining lymph nodes, as reported for an experimental infection with M. tuberculosis (21). op−/− mice also showed diminished numbers of CD11c and anti-MHC class II-positive cells in the snout compared to op+/− controls (Fig. 4). Thus, a reduced quantity of CSF-1-dependent cells could account for both the decreased neuroinvasion and the increased bacterial levels at the site of infection of op−/− mice compared to WT controls.
FIG. 3.
Immunostaining of L. monocytogenes in the superficial cervical draining lymph nodes of op−/− and op+/− mice 3 days after infection. Listeria (in red) and CD80 (B7-1), CD11c, F4/80, and Mac3 macrophage and dendritic cell markers (in green) were stained with specific antibodies in the tissue sections, followed by staining with fluorochrome-labeled secondary antibodies as described previously (12). All primary antibodies were purchased from BD-Pharmingen (San Diego, Calif.), except F4/80 (Serotec, Oxford, United Kingdom) and the anti-Listeria polyclonal antibodies (Difco). Nuclei (in blue) were stained with 4′,6′-diamidino-2-phenylindole (DAPI). Representative stained sections from three individual mice are shown (magnification, ×160). Note the increased density of immunostaining for listerial antigens and the decreased levels of CD80-, CD11c-, F4/80- and Mac3-positive cells in the lymph nodes from op−/− mice compared to controls.
FIG. 4.
Immunostaining of L. monocytogenes in the snouts of op−/− and op+/− mice 3 days after infection. Listeria bacteria (in red) and CD11c and MHC class II (BD-Pharmingen) cell markers (in green) were stained in the tissue sections by using specific antibodies as described previously (12). Nuclei (in blue) were stained with DAPI (magnification, ×320). Note the decreased density of cells stained with anti-CD11c and anti-MHC class II in the snouts of op−/− mice compared to controls.
In conclusion, we suggest a dual role for CSF-1-dependent macrophage-dendritic cells in the control of L. monocytogenes. CSF-1-dependent cells are of importance in the control of L. monocytogenes both systemically and at the site of infection. Although these cells are not necessary for conveying Listeria bacteria into the draining lymph node, they are probably involved in antigen presentation at this site. On the other hand, CSF-1-dependent and -independent cells facilitate listerial neuroinvasion.
Acknowledgments
This work was supported by a grant (711,1213/97) from SJFR and by Amgen and the Karolinska Institute.
Editor: J. D. Clements
REFERENCES
- 1.Antal, E. A., E. M. Loberg, P. Bracht, K. K. Melby, and J. Maehlen. 2001. Evidence for intraaxonal spread of Listeria monocytogenes from the periphery to the central nervous system. Brain Pathol. 11:432-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berche, P. 1995. Bacteremia is required for invasion of the murine central nervous system by Listeria monocytogenes. Microb. Pathog. 18:323-336. [DOI] [PubMed] [Google Scholar]
- 3.Blanot, S., M. M. Joly, F. Vilde, F. Jaubert, O. Clement, G. Frija, and P. Berche. 1997. A gerbil model for rhombencephalitis due to Listeria monocytogenes. Microb. Pathog. 23:39-48. [DOI] [PubMed] [Google Scholar]
- 4.Cecchini, M. G., M. G. Dominguez, S. Mocci, A. Wetterwald, R. Felix, H. Fleisch, O. Chisholm, W. Hofstetter, J. W. Pollard, and E. R. Stanley. 1994. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development 120:1357-1372. [DOI] [PubMed] [Google Scholar]
- 5.Dramsi, S., S. Levi, A. Triller, and P. Cossart. 1998. Entry of Listeria monocytogenes into neurons occurs by cell-to-cell spread: an in vitro study. Infect. Immun. 66:4461-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drevets, D. A. 1999. Dissemination of Listeria monocytogenes by infected phagocytes. Infect. Immun. 67:3512-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drevets, D. A., T. A. Jelinek, and N. E. Freitag. 2001. Listeria monocytogenes-infected phagocytes can initiate central nervous system infection in mice. Infect. Immun. 69:1344-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drevets, D. A., R. T. Sawyer, T. A. Potter, and P. A. Campbell. 1995. Listeria monocytogenes infects human endothelial cells by two distinct mechanisms. Infect. Immun. 63:4268-4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eck, H. 1957. Encephalomyelitis listeria apostematosa. Schweiz. Med. Wochenschr. 87:210-214. [PubMed]
- 10.Greiffenberg, L., W. Goebel, K. S. Kim, I. Weiglein, A. Bubert, F. Engelbrecht, M. Stins, and M. Kuhn. 1998. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: InlB-dependent invasion, long-term intracellular growth, and spread from macrophages to endothelial cells. Infect. Immun. 66:5260-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guleria, I., and J. W. Pollard. 2001. Aberrant macrophage and neutrophil population dynamics and impaired Th1 response to Listeria monocytogenes in colony-stimulating factor 1-deficient mice. Infect. Immun. 69:1795-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin, Y., L. Dons, K. Kristensson, and M. E. Rottenberg. 2001. Neural route of cerebral Listeria monocytogenes murine infection: role of immune response mechanisms in controlling bacterial neuroinvasion. Infect. Immun. 69:1093-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otter, A., and W. F. Blakemore. 1989. Observation on the presence of Listeria monocytogenes in axons. Acta Microbiol. Hung. 36:125-131. [PubMed] [Google Scholar]
- 14.Peters, M., and M. Hewicker-Trautwein. 1996. Studies on the cell tropism of Listeria monocytogenes in ovine fetal brain cell cultures. Vet. Microbiol. 49:169-179. [DOI] [PubMed] [Google Scholar]
- 15.Pollock, S. S., T. M. Pollock, and M. J. Harrison. 1984. Infection of the central nervous system by Listeria monocytogenes: a review of 54 adult and juvenile cases. Q. J. Med 53:331-340. [PubMed] [Google Scholar]
- 16.Randolph, G. J., K. Inaba, D. F. Robbiani, R. M. Steinman, and W. A. Muller. 1999. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 11:753-761. [DOI] [PubMed] [Google Scholar]
- 17.Rosen, H., S. Gordon, and R. J. North. 1989. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelomonocytic cells. Absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J. Exp. Med. 170:27-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samsom, J. N., A. Annema, P. H. Groeneveld, N. van Rooijen, J. A. Langermans, and R. van Furth. 1997. Elimination of resident macrophages from the livers and spleens of immune mice impairs acquired resistance against a secondary Listeria monocytogenes infection. Infect. Immun. 65:986-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schluter, D., E. Domann, C. Buck, T. Hain, H. Hof, T. Chakraborty, and M. Deckert-Schluter. 1998. Phosphatidylcholine-specific phospholipase C from Listeria monocytogenes is an important virulence factor in murine cerebral listeriosis. Infect. Immun. 66:5930-5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanley, E. R., L. J. Guilbert, R. J. Tushinski, and S. H. Bartelmez. 1983. CSF-1—a mononuclear phagocyte lineage-specific hemopoietic growth factor. J. Cell. Biochem. 21:151-159. [DOI] [PubMed] [Google Scholar]
- 21.Teitelbaum, R., W. Schubert, L. Gunther, Y. Kress, F. Macaluso, J. W. Pollard, D. N. McMurray, and B. R. Bloom. 1999. The M cell as a portal of entry to the lung for the bacterial pathogen Mycobacterium tuberculosis. Immunity 10:641-650. [DOI] [PubMed] [Google Scholar]
- 22.Unanue, E. R. 1997. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T-cell response. Immunol. Rev. 158:11-25. [DOI] [PubMed] [Google Scholar]
- 23.Vazquez-Boland, J. A., C. Kocks, S. Dramsi, H. Ohayon, C. Geoffroy, J. Mengaud, and P. Cossart. 1992. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect. Immun. 60:219-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiktor-Jedrzejczak, W., A. Bartocci, A. W. Ferrante, Jr., A. Ahmed-Ansari, K. W. Sell, J. W. Pollard, and E. R. Stanley. 1990. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc. Natl. Acad. Sci. USA 87:4828-4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiktor-Jedrzejczak, W., B. Dzwigala, M. Szperl, M. Maruszynski, E. Urbanowska, and P. Szwech. 1996. Colony-stimulating factor 1-dependent resident macrophages play a regulatory role in fighting Escherichia coli fecal peritonitis. Infect. Immun. 64:1577-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiktor-Jedrzejczak, W., M. Z. Ratajczak, A. Ptasznik, K. W. Sell, A. Ahmed-Ansari, and W. Ostertag. 1992. CSF-1 deficiency in the op/op mouse has differential effects on macrophage populations and differentiation stages. Exp. Hematol. 20:1004-1010. [PubMed] [Google Scholar]
- 27.Witmer-Pack, M. D., D. A. Hughes, G. Schuler, L. Lawson, A. McWilliam, K. Inaba, R. M. Steinman, and S. Gordon. 1993. Identification of macrophages and dendritic cells in the osteopetrotic (op/op) mouse. J. Cell Sci. 104:1021-1029. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida, H., S. Hayashi, T. Kunisada, M. Ogawa, S. Nishikawa, H. Okamura, T. Sudo, and L. D. Shultz. 1990. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345:442-444. [DOI] [PubMed] [Google Scholar]