Abstract
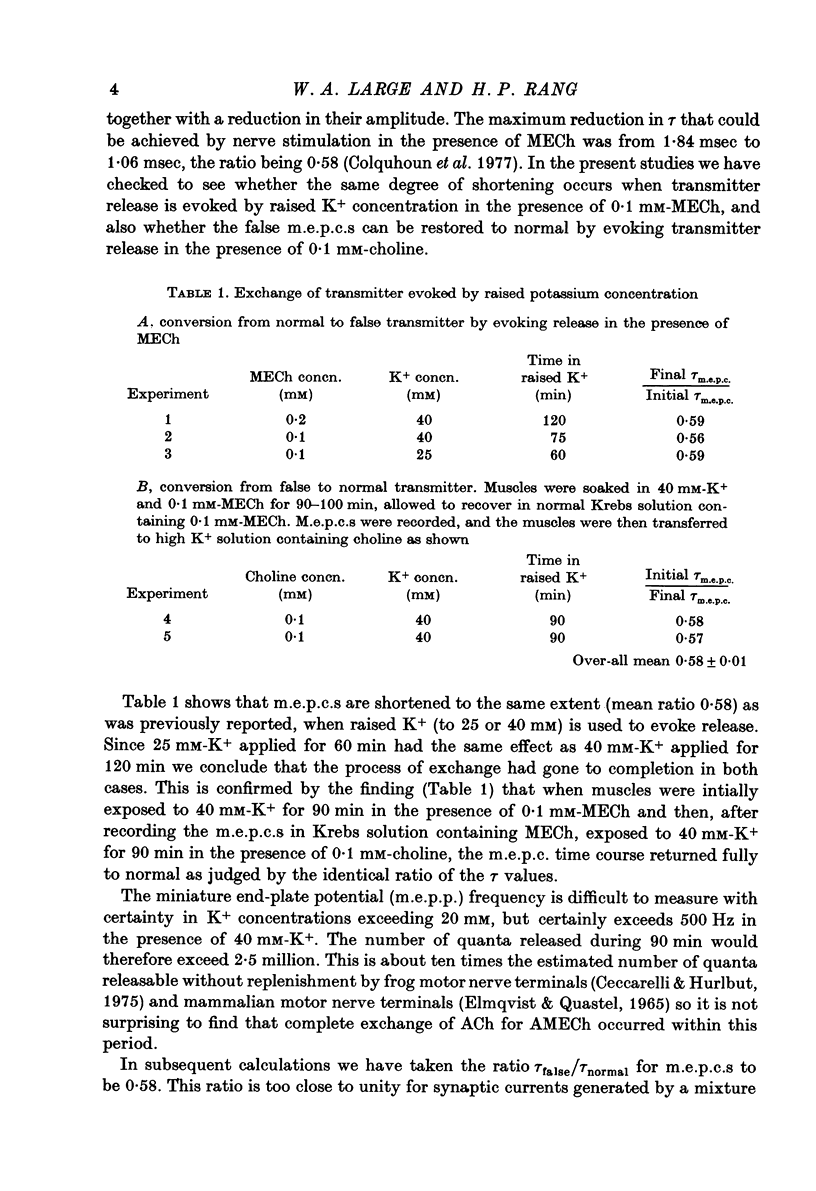
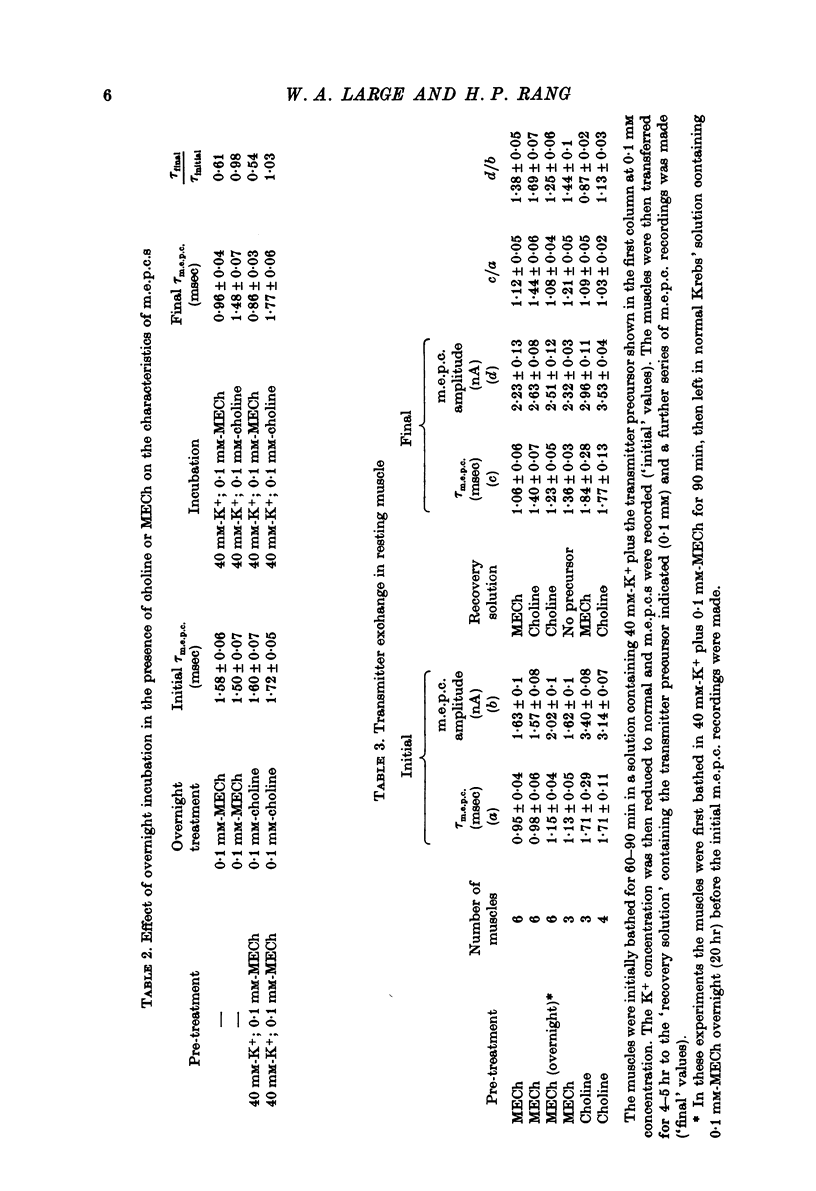
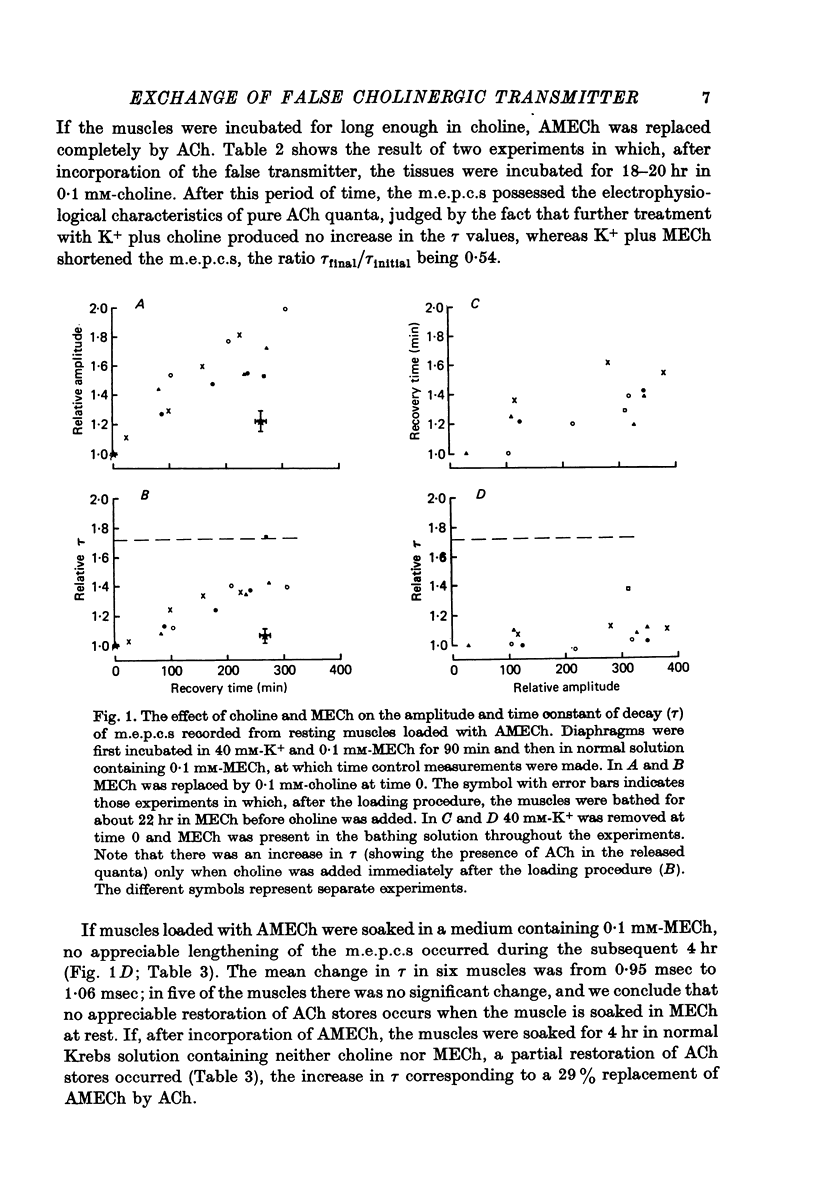
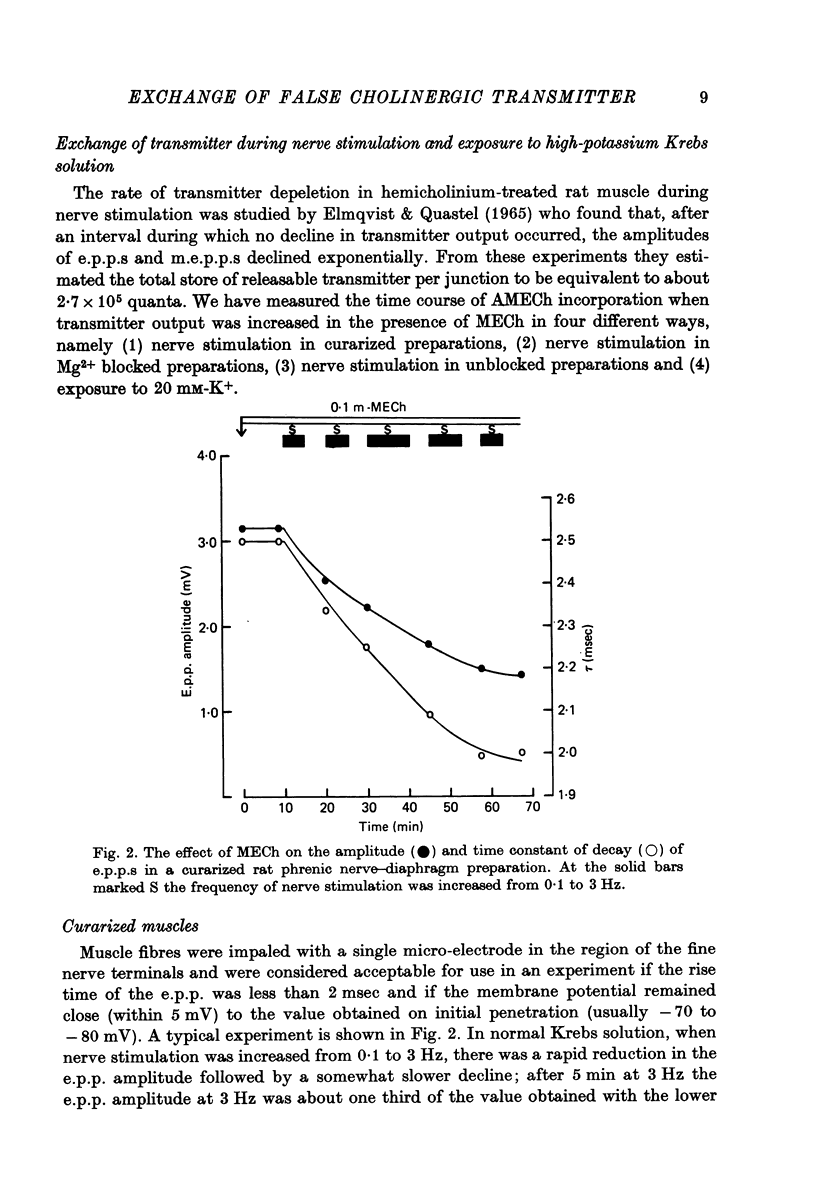
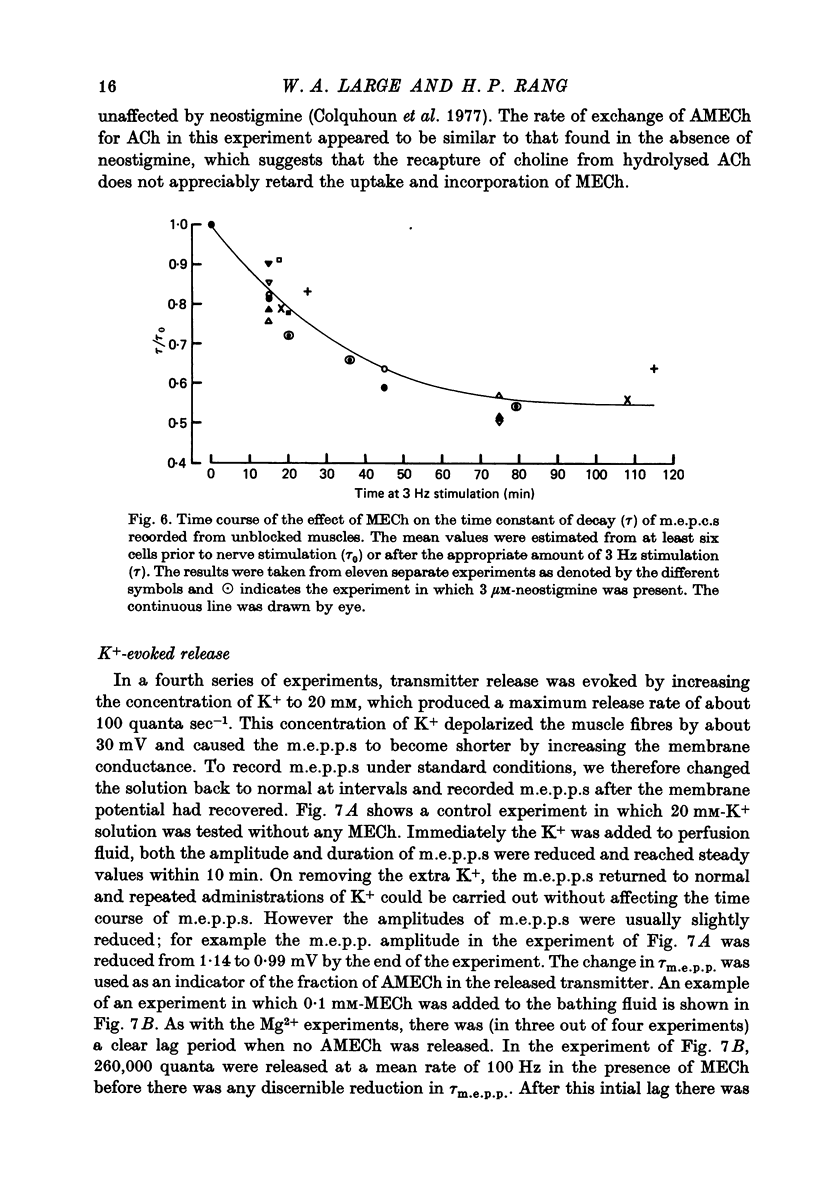
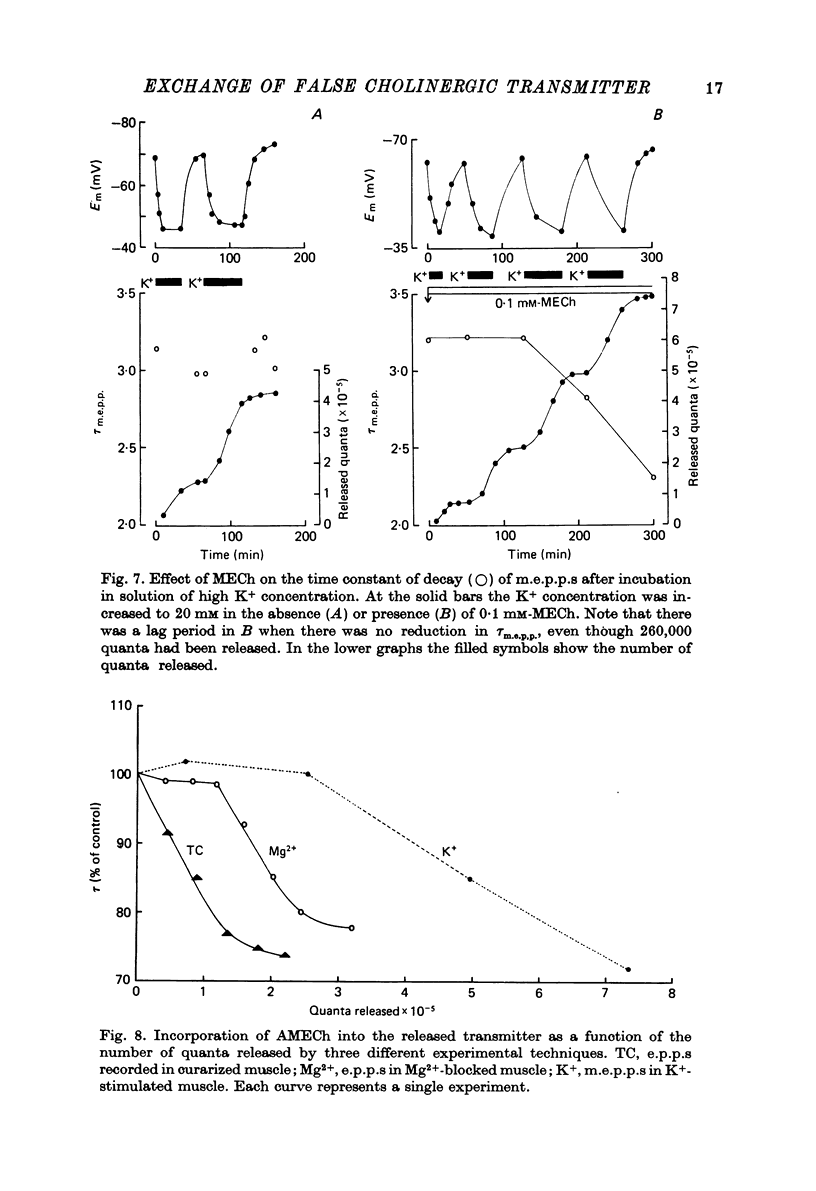
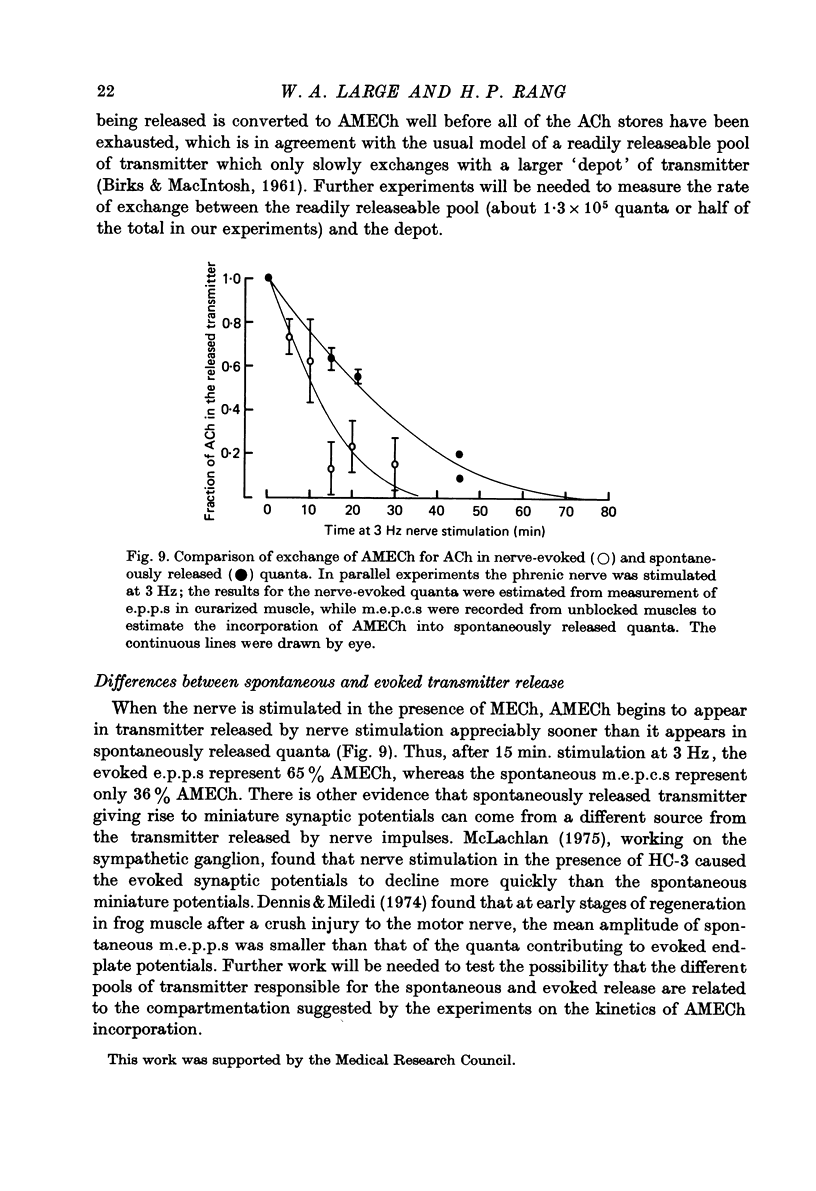
1. The incorporation of acetylmonoethylcholine (AMECh) into transmitter store at the mammalian neuromuscular junction has been studied using electrophysiological techniques. 2. Incubation of rat muscle in the presence of 0.1 mM-monoethylcholine (MECh) and 40 mM-K+ for 60-90 min produced a maximal reduction in the time constant of decay of synaptic currents and potentials, indicating that acetylcholine (ACh) had been replaced by AMECh in the released quanta. 3. Under resting conditions, muscles incubated for up to 24 hr in the presence of MECh showed no incorporation of AMECh into the released transmitter. In contrast, muscles pre-loaded with AMECh and then incubated in choline-containing medium showed a substantial reversion to ACh in the released transmitter within 4 hr. 4. It is suggested that this difference results from the rate of synthesis or packaging of transmitter being considerably slower with MECh than with choline, so that stimulation in the presence of MECh causes an over-all depletion of transmitter stores that does not occur with choline as the precursor. Measurements of m.e.p.c. amplitude following K+-evoked release in the presence of MECh or choline confirmed this interpretation. 5. In order to test whether newly formed AMECh is incorporated into a single homogeneous pool of transmitter from which the released quanta are derived, the rate of incorporation of AMECh into the released transmitter was measured as a function of the number of quanta released when transmitter output was increased by various methods. 6. The number of quanta released before 63% conversion of the released transmitter to AMECh was brought about varied from 1.3 X 10(5) (nerve stimulation at 3 Hz) to about 4 X 10(5) (release by high-K+ solution). With nerve stimulation in Mg2+-blocked muscles the value was 2.5 X 10(5). 7. Incorporation of AMECh into the quanta released by nerve stimulation appeared to take place more rapidly than its incorporation into spontaneously released quanta. 8. These results are discussed in terms of the compartmentation of transmitter stores in the nerve terminals.
Full text
PDF





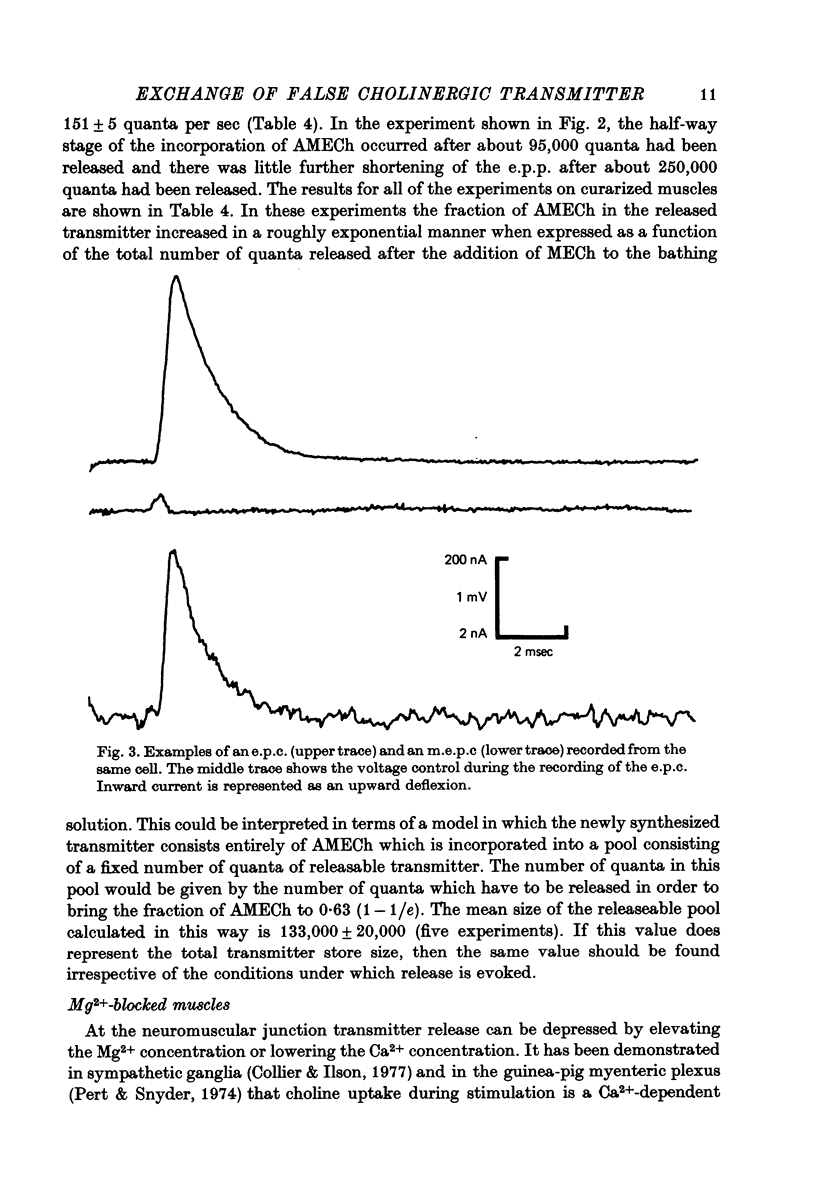
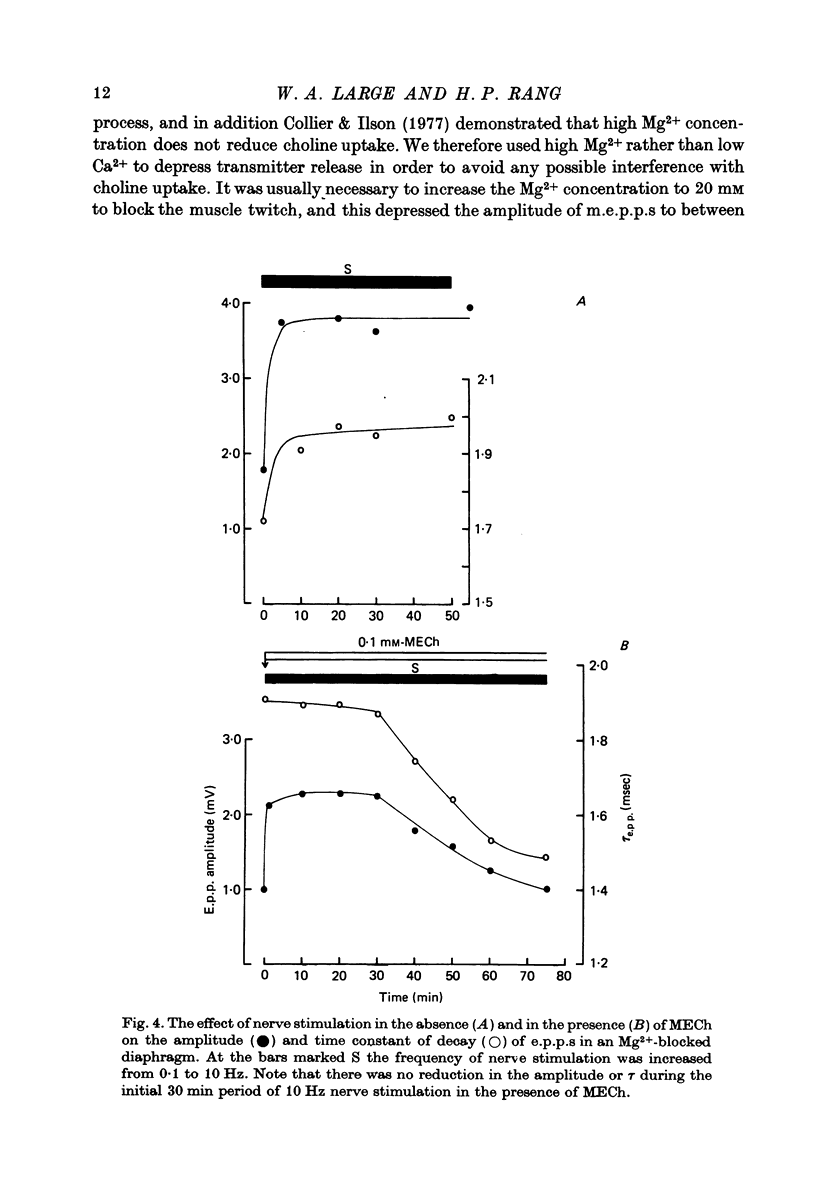
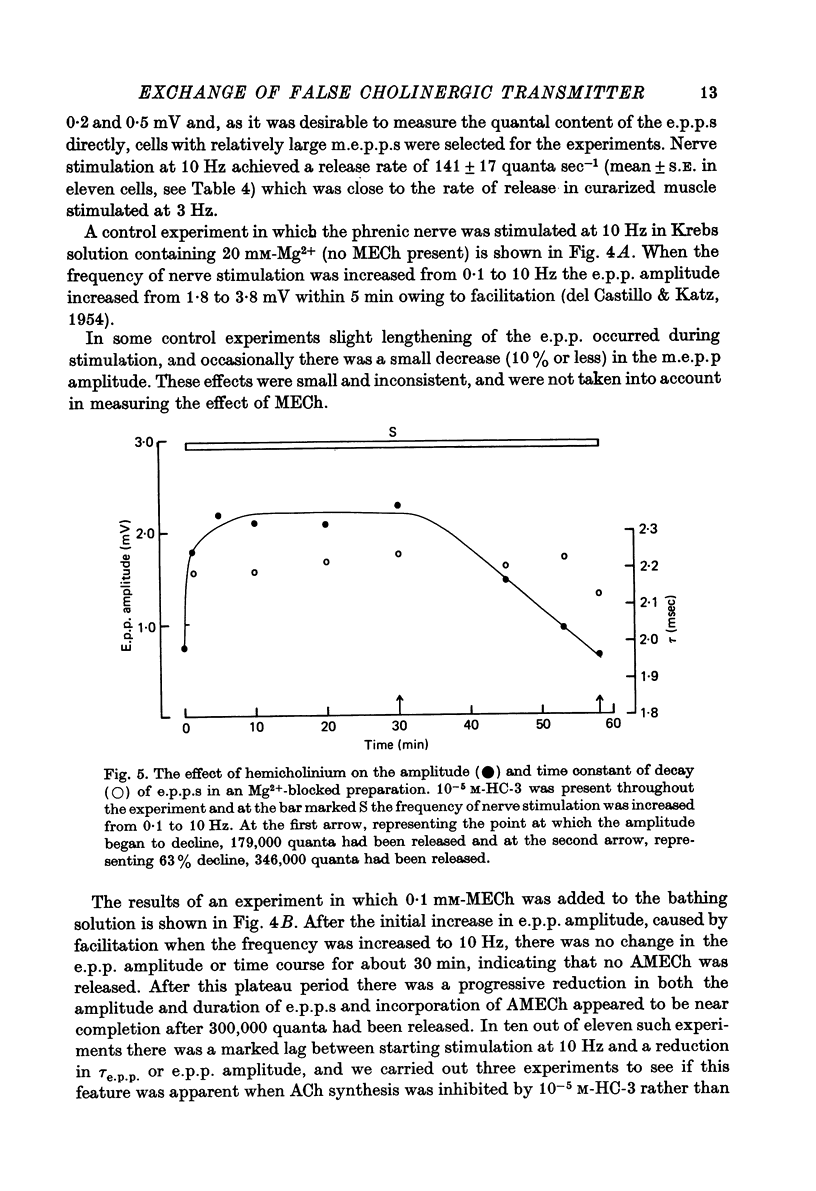

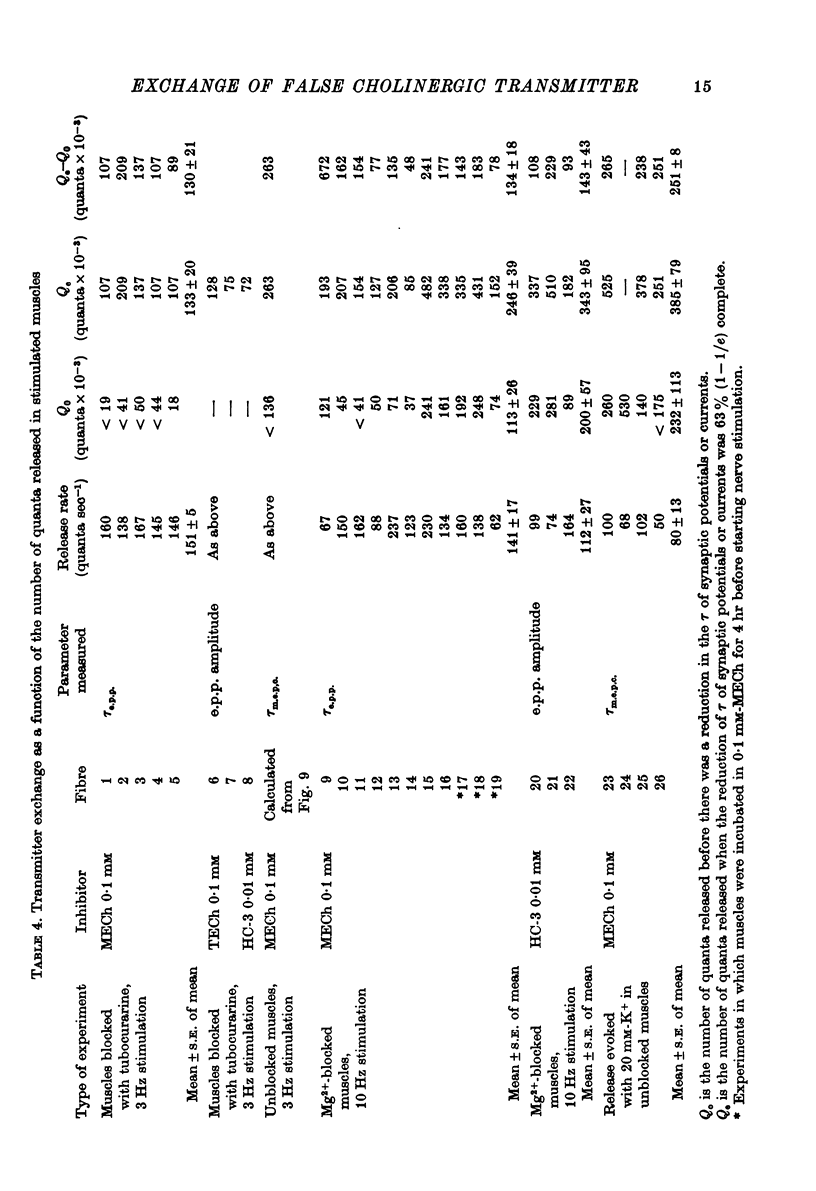






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach A., Betz W. Does curare affect transmitter release? J Physiol. 1971 Mar;213(3):691–705. doi: 10.1113/jphysiol.1971.sp009409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEANI L., BIANCHI C., LEDDA F. THE EFFECT OF TUBOCURARINE ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. J Physiol. 1964 Nov;174:172–183. doi: 10.1113/jphysiol.1964.sp007480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker L. A., Mittag T. W. Comparative studies of substrates and inhibitors of choline transport and choline acetyltransferase. J Pharmacol Exp Ther. 1975 Jan;192(1):86–94. [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M. An electrophysiological analysis of the synthesis of acetylcholine in preganglionic nerve terminals. J Physiol. 1972 Mar;221(3):669–682. doi: 10.1113/jphysiol.1972.sp009775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P. The effects of prolonged repetitive stimulation in hemicholinium on the frog neuromuscular junction. J Physiol. 1975 May;247(1):163–188. doi: 10.1113/jphysiol.1975.sp010926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier B., Ilson D. The effect of preganglionic nerve stimulation on the accumulation of certain analogues of choline by a sympathetic ganglion. J Physiol. 1977 Jan;264(2):489–509. doi: 10.1113/jphysiol.1977.sp011679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier B., Katz H. S. Acetylcholine synthesis from recaptured choline by a sympathetic ganglion. J Physiol. 1974 May;238(3):639–655. doi: 10.1113/jphysiol.1974.sp010548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier B. The preferential release of newly synthesized transmitter by a sympathetic ganglion. J Physiol. 1969 Nov;205(2):341–352. doi: 10.1113/jphysiol.1969.sp008969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Large W. A., Rang H. P. An analysis of the action of a false transmitter at the neuromuscular junction. J Physiol. 1977 Apr;266(2):361–395. doi: 10.1113/jphysiol.1977.sp011772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Miledi R. Characteristics of transmitter release at regenerating frog neuromuscular junctions. J Physiol. 1974 Jun;239(3):571–594. doi: 10.1113/jphysiol.1974.sp010583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunant Y., Gautron J., Israël M., Lesbats B., Manaranche R. Les compartiments d'acetylcholine de l'organe electrique de la torpille et leurs modifications par la stimulation. J Neurochem. 1972 Aug;19(8):1987–2002. doi: 10.1111/j.1471-4159.1972.tb01488.x. [DOI] [PubMed] [Google Scholar]
- ELMQVIST D., QUASTEL D. M. PRESYNAPTIC ACTION OF HEMICHOLINIUM AT THE NEUROMUSCULAR JUNCTION. J Physiol. 1965 Apr;177:463–482. doi: 10.1113/jphysiol.1965.sp007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet P., Lefresne P., Rossier J., Beaujouan J. C., Glowinski J. Inhibition by hemicholinium-3 of (14C)acetylcholine synthesis and (3H)choline high-affinity uptake in rat striatal synaptosomes. Mol Pharmacol. 1973 Sep;9(5):630–639. [PubMed] [Google Scholar]
- Hubbard J. I., Wilson D. F. Neuromuscular transmission in a mammalian preparation in the absence of blocking drugs and the effect of D-tubocurarine. J Physiol. 1973 Jan;228(2):307–325. doi: 10.1113/jphysiol.1973.sp010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. F., Kwanbunbumpen S. Some effects of nerve stimulation andhemicholinium on quantal transmitter release at the mammalian neuromuscular junction. J Physiol. 1970 Mar;207(1):51–61. doi: 10.1113/jphysiol.1970.sp009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Transmitter leakage from motor nerve endings. Proc R Soc Lond B Biol Sci. 1977 Feb 11;196(1122):59–72. doi: 10.1098/rspb.1977.0029. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The number of transmitter molecules in a quantum: an estimate from iontophoretic application of acetylcholine at the neuromuscular synapse. J Physiol. 1975 Oct;251(2):465–482. doi: 10.1113/jphysiol.1975.sp011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large W. A., Rang H. P. Variability of transmitter quanta released during incorporation of a false transmitter into cholinergic nerve terminals. J Physiol. 1978 Dec;285:25–34. doi: 10.1113/jphysiol.1978.sp012554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchbanks R. M. The uptake of [14C] choline into synaptosomes in vitro. Biochem J. 1968 Dec;110(3):533–541. doi: 10.1042/bj1100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M. Electrophysiological evidence for the second store of ACh in preganglionic nerve terminals. Brain Res. 1975 Nov 14;98(2):373–376. doi: 10.1016/0006-8993(75)90017-7. [DOI] [PubMed] [Google Scholar]
- Molenaar P. C., Polak R. L. Analysis of the preferential release of newly synthesized acetylcholine by cortical slices from rat brain with the aid of two different labelled precursors. J Neurochem. 1976 Jan;26(1):95–99. [PubMed] [Google Scholar]
- Pert C. B., Snyder S. H. High affinity transport of choline into the myenteric plexus of guinea-pig intestine. J Pharmacol Exp Ther. 1974 Oct;191(1):102–108. [PubMed] [Google Scholar]
- Potter L. T. Synthesis, storage and release of [14C]acetylcholine in isolated rat diaphragm muscles. J Physiol. 1970 Jan;206(1):145–166. doi: 10.1113/jphysiol.1970.sp009003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyskocil F., Illés P. Non-quantal release of transmitter at mouse neuromuscular junction and its dependence on the activity of Na+-K+ ATP-ase. Pflugers Arch. 1977 Sep 16;370(3):295–297. doi: 10.1007/BF00585542. [DOI] [PubMed] [Google Scholar]
- Wilson D. F. Estimates of quantal-release and binomial statistical-release parameters at rat neuromuscular junction. Am J Physiol. 1977 Nov;233(5):C157–C163. doi: 10.1152/ajpcell.1977.233.5.C157. [DOI] [PubMed] [Google Scholar]


