Abstract
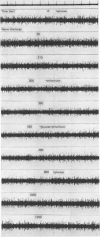
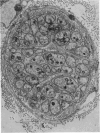
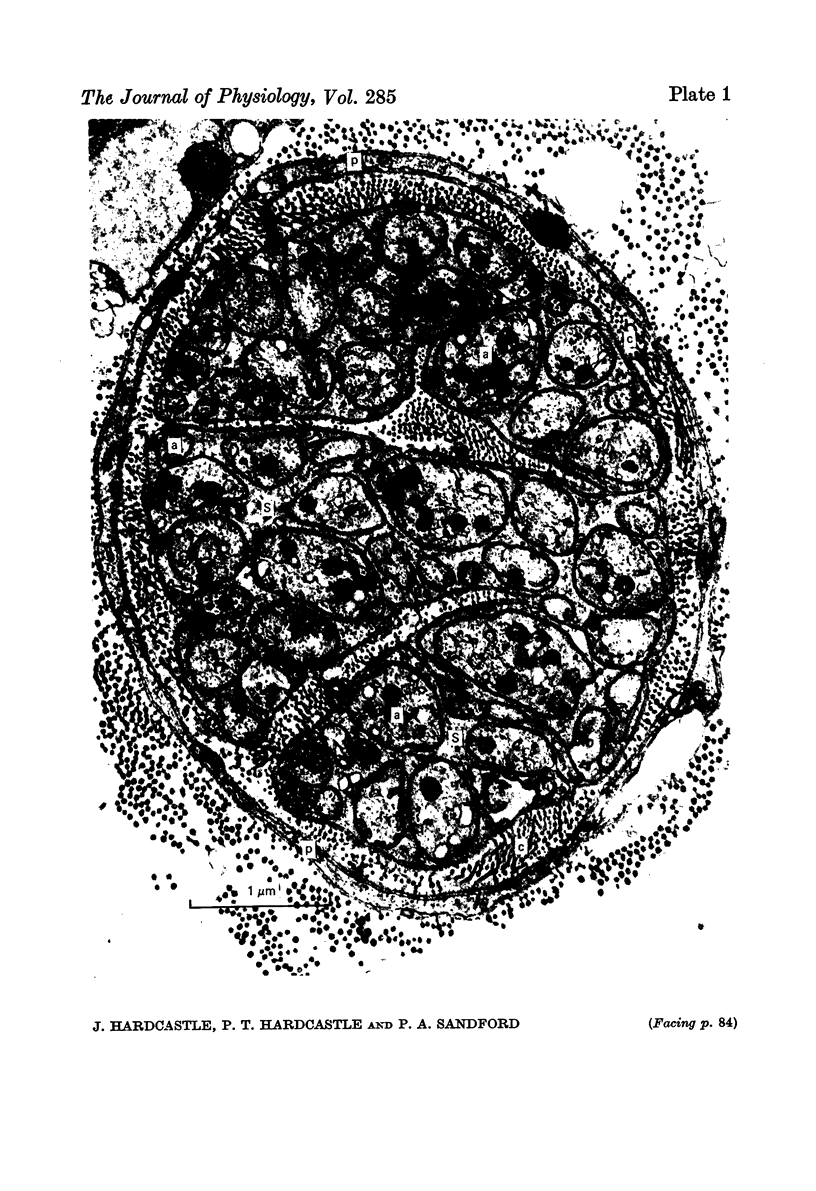
1. The afferent discharge of mesenteric nerves has been recorded while perfusing salines of different composition through the lumen of rat ileum. The p.d. across the ileum and hexose absorption have also been measured. 2. The ileal mesenteric nerves were sensitive to actively transported hexoses. The discharge recorded increased dramatically on perfusion with 10 or 50 mM-glucose, quietening within 3 min of returning to glucose-free saline. A similar response was obtained with galactose. 3. No change in afferent discharge could be detected on perfusion of mannitol or the slowly transferred hexose, mannose. It is concluded that the response to glucose and galactose is not dependent on the stimulation of non-specific luminal osmoreceptors. 4. Phlorhizin prevented glucose from increasing the afferent nerve discharge. Subsequent perfusion with saline removed the phlorhizin and an afferent nerve response to glucose was restored. It is concluded that this response requires glucose transfer beyond the phlorhizin-sensitive glucose entry mechanism at the luminal side of the mucosal epithelial cell. 5. Very small amounts of glucose or galactose were absorbed under the conditions employed. The time course for the change in p.d. correlated well with that described for the alteration in afferent nerve discharge. 6. Electron micrographs show that ileal nerves consist of bundles of small non-myelinated fibres of approximately 1 micron diameter. 7. The significance of the findings is discussed remembering that carbohydrate absorption is considered to be completed normally in the jejunum.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALVARADO F., CRANE R. K. Phlorizin as a competitive inhibitor of the active transport of sugars by hamster small intestine, in vitro. Biochim Biophys Acta. 1962 Jan 1;56:170–172. doi: 10.1016/0006-3002(62)90543-7. [DOI] [PubMed] [Google Scholar]
- Andrews C. J., Andrews W. H. Receptors, activated by acid, in the duodenal wall of rabbits. Q J Exp Physiol Cogn Med Sci. 1971 Oct;56(4):221–230. doi: 10.1113/expphysiol.1971.sp002123. [DOI] [PubMed] [Google Scholar]
- Browning J. G., Hardcastle J., Hardcastle P. T., Sanford P. A. The role of acetylcholine in the regulation of ion transport by rat colon mucosa. J Physiol. 1977 Nov;272(3):737–754. doi: 10.1113/jphysiol.1977.sp012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C. C., Hsieh C. P., Yu Y. M., Kvietys P., Yu L. C., Pittman R., Dabney J. M. Localization of mesenteric hyperemia during digestion in dogs. Am J Physiol. 1976 Mar;230(3):583–589. doi: 10.1152/ajplegacy.1976.230.3.583. [DOI] [PubMed] [Google Scholar]
- Clothier M. G., Green J. H., Hardcastle J., Hardcastle P. T., Sanford P. A. Proceedings: Afferent nerve discharge from rat isolated small intestine in vitro. J Physiol. 1975 May;247(1):11P–12P. [PubMed] [Google Scholar]
- DAHLQVIST A., THOMSON D. L. THE DIGESTION AND ABSORPTION OF MALTOSE AND TREHALOSE BY THE INTACT RAT. Acta Physiol Scand. 1963 Sep-Oct;59:111–125. doi: 10.1111/j.1748-1716.1963.tb02728.x. [DOI] [PubMed] [Google Scholar]
- Davison J. S. Response of single vagal afferent fibres to mechanical and chemical stimulation of the gastric and duodenal mucosa in cats. Q J Exp Physiol Cogn Med Sci. 1972 Oct;57(4):405–416. doi: 10.1113/expphysiol.1972.sp002176. [DOI] [PubMed] [Google Scholar]
- Field M., McColl I. Ion transport in rabbit ileal mucosa. 3. Effects of catecholamines. Am J Physiol. 1973 Oct;225(4):852–857. doi: 10.1152/ajplegacy.1973.225.4.852. [DOI] [PubMed] [Google Scholar]
- GROISSER V. W., FARRAR J. T. Absorption of radioactive sodium from the intestinal tract of man. I. Effect of intestinal motility. II. Effect of an organomercurial. J Clin Invest. 1960 Nov;39:1607–1618. doi: 10.1172/JCI104183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabella G. Proceedings: A special muscle layer in the intestinal muscular coat. J Physiol. 1974 Jul;240(2):1P–3P. [PMC free article] [PubMed] [Google Scholar]
- Hardcastle P. T., Eggenton J. The effect of acetylcholine on the electrical activity of intestinal epithelial cells. Biochim Biophys Acta. 1973 Feb 27;298(1):95–100. doi: 10.1016/0005-2736(73)90013-8. [DOI] [PubMed] [Google Scholar]
- Isaacs P. E., Corbett C. L., Riley A. K., Hawker P. C., Turnberg L. A. In vitro behavior of human intestinal mucosa. The influence of acetyl choline on ion transport. J Clin Invest. 1976 Sep;58(3):535–542. doi: 10.1172/JCI108498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itina L. V. Impul'satsiia tsentral'nykh i perifericheskikh otrezkov chrevnogo i bluzhdaiushchego nervov pri khimicheskom razdrazhenii slizistoi obolochki tonkoi kishki. Fiziol Zh SSSR Im I M Sechenova. 1971 Jan;57(1):95–102. [PubMed] [Google Scholar]
- Jenkins D. H., Macleod I. B. Proximal jejunal and distal ileal extrinsic innervation in the rabbit and dog. Br J Surg. 1972 May;59(5):394–400. doi: 10.1002/bjs.1800590518. [DOI] [PubMed] [Google Scholar]
- Luciano L., Reale E., Ruska H. Bürstenzellen im Alveolarepithel der Rattenlunge. Z Zellforsch Mikrosk Anat. 1969;95(2):198–201. [PubMed] [Google Scholar]
- NEWEY H., SANFORD P. A., SMYTH D. H., WILLIAMS A. H. THE EFFECT OF SOME ANALOGUES OF PHLORRHIZIN ON INTESTINAL HEXOSE AND FLUID TRANSFER. J Physiol. 1963 Nov;169:229–236. doi: 10.1113/jphysiol.1963.sp007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeyama A., Leblond C. P. "Caveolated cells" characterized by deep surface invaginations and abundant filaments in mouse gastro-intestinal epithelia. Am J Anat. 1974 Jun;140(2):147–165. doi: 10.1002/aja.1001400203. [DOI] [PubMed] [Google Scholar]
- PARSONS B. J., SMYTH D. H., TAYLOR C. B. The action of phlorrhizin on the intestinal transfer of glucose and water in vitro. J Physiol. 1958 Dec 30;144(3):387–402. doi: 10.1113/jphysiol.1958.sp006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezek M., Novin D. Duodenal nutrient infusion: effects on feeding in intact and vagotomized rabbits. J Nutr. 1976 Jun;106(6):812–820. doi: 10.1093/jn/106.6.812. [DOI] [PubMed] [Google Scholar]
- SHARMA K. N., NASSET E. S. Electrical activity in mesenteric nerves after perfusion of gut lumen. Am J Physiol. 1962 Apr;202:725–730. doi: 10.1152/ajplegacy.1962.202.4.725. [DOI] [PubMed] [Google Scholar]
- Sanford P. A. Proceedings: Effect of actively transported hexoses on afferent nerve discharge from rat small intestine in vivo. J Physiol. 1976 Jan;254(1):75P–76P. [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Stach W. Uber die Nervengeflechte der Duodenalzotten. Licht- und elektronenmikroskopische Untersuchungen. Acta Anat (Basel) 1973;85(2):216–231. [PubMed] [Google Scholar]