Abstract
Bartonella henselae is responsible for various disease syndromes that loosely correlate with the immune status of the host. In the immunocompromised individual, B. henselae-induced angiogenesis, or bacillary angiomatosis, is characterized by vascular proliferative lesions similar to those in Kaposi's sarcoma. We hypothesize that B. henselae-mediated interaction with immune cells, namely, macrophages, induces potential angiogenic growth factors and cytokines which contribute in a paracrine manner to the proliferation of endothelial cells. Vascular endothelial growth factor (VEGF), a direct inducer of angiogenesis, and interleukin-1β (IL-1β), a potentiator of VEGF, were detected within 12 and 6 h, respectively, in supernatants from phorbol 12-myristate 13-acetate-differentiated human THP-1 macrophages exposed to live B. henselae. Pretreatment of macrophages with cytochalasin D, a phagocytosis inhibitor, yielded comparable results, suggesting that bacterium-cell attachment is sufficient for VEGF and IL-1β induction. IL-8, an angiogenic cytokine with chemotactic properties, was induced in human microvascular endothelial cells (HMEC-1) within 6 h of infection, whereas no IL-8 induction was observed in infected THP-1 cells. In addition, conditioned medium from infected macrophages induced the proliferation of HMEC-1, thus demonstrating angiogenic potential. These data suggest that Bartonella modulation of host or target cell cytokines and growth factors, rather than a direct role of the bacterium as an endothelial cell mitogen, is the predominant mechanism responsible for angiogenesis. B. henselae induction of VEGF, IL-1β, and IL-8 outlines a broader potential paracrine angiogenic loop whereby macrophages play the predominant role as the effector cell and endothelial cells are the final target cell, resulting in their proliferation.
Bartonella henselae is a gram-negative, fastidious, facultative intracellular bacterium whose natural reservoir is the domestic cat. Infection with B. henselae may result in a range of syndromes, including cat-scratch disease (CSD), bacillary angiomatosis (BA), bacillary peliosis hepatis, and other multiorgan complications (2). Distinct clinical manifestations and pathological processes are determined primarily by the competency of the host's immune system. CSD, usually a benign syndrome, occurs predominantly in immunocompetent individuals following exposure to a cat. Conversely, the course of disease in the immunocompromised individual varies from a self-resolving infection to a systemic infection that may include fever with bacteremia and B. henselae-induced angiogenesis. BA is a manifestation of infection with B. henselae and Bartonella quintana in immunosuppressed individuals, including AIDS patients and chronic alcoholics (2).
Angiogenesis, or the proliferation of blood vessels, is a natural process required for growth and tissue reorganization, primarily during developmental stages (21). During adulthood, physiological angiogenesis is required but is mainly limited to processes associated with tissue healing and the menstrual cycle (21). Physiological angiogenesis is induced by positive regulators but declines due to the strict control of endogenous negative regulators (35). Besides physiological angiogenesis, pathological (inflammatory or tumor) angiogenesis, induced predominantly in neoplastic tissue and diabetic retinopathy, provides vascular nourishment required for tissue survival (35).
Endothelial, stromal, and immune chemoattracted cells generate some of the molecules involved in the regulatory coordination of angiogenesis. In particular, cells of the mononuclear phagocyte lineage, although highly heterogeneous in function, are capable of producing potent angiogenic factors upon activation (34). In fact, macrophage infiltration is frequently observed in tumor angiogenesis (34). The role of macrophages as effector cells depends on their stage of development and stimulation. Stimulation is required for macrophages to become functionally angiogenic; this may be triggered by metabolic signals, cytokines, bacterial lipopolysaccharide, or other factors and occurs by a different mechanism than activation for antigen presentation (36). Macrophages are thought to play a central role in the modulation of angiogenesis by means of their secreted molecules (22, 27, 34, 40). VEGF is one such potent endothelial cell mitogen induced and secreted by activated macrophages (13, 14, 26, 30, 32, 42, 47). Several other macrophage-derived mediators of angiogenesis have been described, including interleukin-8 (IL-8), IL-6, IL-1β, and tumor necrosis factor alpha (TNF-α) (34, 40).
The interaction between professional mononuclear phagocytic cells and bacteria may trigger different functional states that result in the production of inflammatory, microbicidal, or angiogenic cytokines and growth factors with autocrine or paracrine functions. Investigation of the role of macrophages in Bartonella-mediated disease has focused primarily on mechanisms of immune clearance (33). Histological examination of BA lesions shows the infiltration of polymorphonuclear leukocytes and the presence of macrophages (23, 24, 31). Thus, macrophages can be found in close proximity to proliferating endothelial cells. In this study we focused on the induction of secreted angiogenic factors by human THP-1 cells infected with B. henselae to determine if macrophages may play an effector cell role in mediating angiogenesis.
MATERIALS AND METHODS
Bacteria and plasmids.
B. henselae strains were grown on chocolate agar prepared with heart infusion agar base (Difco, Detroit, Mich.) supplemented with 1% bovine hemoglobin (Becton Dickinson, Cockeysville, Md.). B. henselae Houston-1 ATCC 49882 was recovered from a human immunodeficiency virus (HIV)-infected patient and has previously been described (37). B. henselae 882str, a streptomycin-resistant laboratory-adapted strain of ATCC 49882 (25), was grown on streptomycin (200 μg/ml) chocolate agar. A green fluorescent protein (GFP)-expressing derivative of 882str was previously developed in our laboratory by transformation with plasmid pVBGFPF (39) and was grown on kanamycin (25 μg/ml) chocolate agar. The B. henselae virB promoter used to drive the expression of the gfp gene in pVBGFPF is activated intracellularly (39). For certain experiments, bacteria were heat killed at 100°C for 30 min. Bacteria were plated on chocolate agar to assess viability. B. henselae cultures were maintained at 37°C with 5% CO2 and humidity to saturation.
Cell lines.
The human monocytic cell line THP-1, derived from a 1-year-old boy with acute monocytic leukemia (43), was seeded in 75-cm2 flasks containing RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (HyClone Laboratories, Logan, Utah), 5 × 10−6 M 2-mercaptoethanol (Sigma, St. Louis, Mo.), 10 μg of vancomycin (Sigma) per ml, 10 μg of gentamicin (Sigma) per ml, and 1 μg of amphotericin B (Sigma) per ml. Cells were subcultured every 2 days.
To generate supernatants for the analysis of secreted cytokines, THP-1 monocytes were placed into 24-well tissue culture plates (Costar, Cambridge, Mass.) at a concentration of 106 cells/well and differentiated by overnight incubation with 100 ng of phorbol 12-myristate 13-acetate (PMA) (Sigma) per ml. Nonadherent cells were removed by washing with supplemented RPMI. At this time, THP-1 macrophages were allowed to stabilize for 2 days prior to infection. For some experiments THP-1 monocytes were not differentiated with PMA. THP-1 cultures were incubated at 37°C with 5% CO2 and humidity to saturation.
HMEC-1 is a human microvascular endothelial cell line of dermal origin immortalized by transfection with plasmid pBR322 containing the gene for the simian virus 40 large T antigen (1) (Materials Transfer Agreement, Centers for Disease Control and Prevention, Atlanta, Ga.). Stock cultures of HMEC-1 were seeded in 75-cm2 flasks in MCDB131 as previously described (38). HMEC-1 cultures were incubated at 37°C with 5% CO2 and humidity to saturation.
Infection of macrophages, monocytes, and endothelial cells.
Induction of angiogenic factors by B. henselae was evaluated in THP-1 PMA-differentiated macrophages and in undifferentiated monocytes. PMA promotes not only cell differentiation but also cell activation (41). In fact, treatment with PMA induces vascular endothelial growth factor (VEGF) in other cell types (4, 15, 18, 19). Therefore, to control for the effect of PMA on induction of VEGF in the uninfected, control cells, we used a two-pronged strategy. First, differentiated cells were allowed to rest for 2 days prior to infection, and, second, we also evaluated VEGF induction in B. henselae-infected THP-1 undifferentiated monocytes.
Prior to infection, the culture medium containing antibiotics was removed from cell cultures, replaced with 20 ml of supplemented RPMI without antibiotics, and allowed to adapt overnight. This was done to avoid the potential effect of pinocytized antibiotics on intracellular bacteria. Bacteria were prepared by harvesting growth from chocolate agar plates inoculated with B. henselae and then incubated for 4 days and suspended in RPMI or MCDB131 medium. Suspensions were diluted to an optical density at 600 nm of 0.05, which we have shown corresponds to 108 CFU/ml by plate counts. THP-1 and HMEC-1 were infected with bacterial suspensions prepared in supplemented medium without antibiotics at a multiplicity of infection (MOI) of 100 or 1,000 bacteria per cell for 30 min, unless otherwise specified. Cells were infected with Escherichia coli JM109 as a control. Extracellular bacteria were removed by being washed three times with prewarmed supplemented medium. Cells were treated with supplemented RPMI containing 50 μg of gentamicin (Sigma) per ml for 1 h. Subsequently, cells were washed three times. In most experiments, cultures were replenished with medium containing 12.5 μg of gentamicin per ml. Supernatants were collected at specified times (hours) after infection.
For some experiments, undifferentiated THP-1 monocytes were resuspended in supplemented media without antibiotics. Because undifferentiated THP-1 monocytes grow in suspension, cells were infected with B. henselae in suspension as described above and subcultured at 106 cells/well in a 24-well plate. After incubation, supernatants were collected by centrifugation of the culture plates at 300 × g for 10 min. HMEC-1 were also adjusted to 106 cells/well prior to infection. All incubations involving infection of cells with B. henselae were done at 37°C with 5%CO2 and humidity to saturation.
Inhibition of phagocytosis with cytochalasin D.
Fluorescence microscopy was used to assess phagocytosis of GFP-expressing B. henselae by THP-1. Cells were seeded into sterile round coverslips placed inside 24-well plates at 105 cells/well and differentiated as described above. At this time, infection of THP-1 macrophages with B. henselae 882str/pVBGFPF was done at an MOI of 100. The virB promoter used to drive the expression of the gfp gene in pVBGFPF is activated intracellularly (39). After phagocytosis, cells were stained with 50 μg of ethidium bromide per ml to allow discrimination between intracellular (green, protected from dye) and extracellular (red-orange, exposed to dye) bacteria. This method was adapted from a previous application which used fluorescein isothiocyanate-labeled compounds (12). Coverslips were analyzed by fluorescence microscopy with a Nikon Eclipse E400 instrument (Southern Micro Instruments, Atlanta, Ga.) equipped with a Kodak DC290 Zoom camera. In selected cultures, THP-1 cells were pretreated with 1 μg of cytochalasin D (Sigma) per ml for 30 min prior to infection. Studies involving Legionella have shown that cytochalasin D can prevent phagocytosis by macrophages without affecting attachment (46). The ability of cytochalasin D to block phagocytosis and vacuole formation in this system was confirmed by fluorescence microscopy using GFP-expressing B. henselae and ethidium bromide as a quenching agent. In addition, supernatants from B. henselae-infected THP-1 macrophages, pretreated with cytochalasin D, were collected and assayed for VEGF, IL-1β, and IL-8 by enzyme-linked immunosorbent assay (ELISA).
ELISA.
To determine cytokine and growth factor levels in supernatants from B. henselae-infected cells, DuoSet ELISA development systems (R&D Systems, Minneapolis, Minn.) for human VEGF, IL-1β, and IL-8 were used according to the manufacturer's directions. The 3,3′,5,5′-tetramethylbenzidine Liquid Substrate System (Sigma) was added and left for 20 min. The horseradish peroxidase reaction was stopped with 2 N sulfuric acid. ELISA plates were analyzed using an Emax Microplate Reader (Molecular Devices, Sunnyvale, Calif.) at 450 nm.
HMEC-1 proliferation assay.
To determine if human macrophages produced sufficient levels of VEGF capable of causing proliferation of human microvascular endothelial cells in a paracrine manner, conditioned medium from B. henselae Houston-1-infected THP-1 macrophages was added to HMEC-1 cell cultures. HMEC-1 to be used for proliferation assays were grown to confluence in 75-cm2 flasks and harvested with trypsin. Cells were washed twice in Hanks' balanced salt solution (Sigma) and seeded into 24-well culture plates at a cell density of 105 cells/well. Conditioned medium from either uninfected or B. henselae Houston-1-infected THP-1 differentiated macrophages, collected 24 h after infection, was diluted 1:2 with MCDB131 with 10% fetal calf serum and added to the seeded 24-well culture plates. Control HMEC-1 were grown either in unconditioned medium or with medium containing 25 ng of human recombinant VEGF per ml. Cultures were incubated for 4 days without changing the medium, and cells were harvested and counted directly with a hemacytometer. Proliferation of HMEC-1 by live B. henselae was also examined by direct infection of HMEC-1 (in the absence of THP-1) at an MOI of 1,000 as described above. Proliferation is reported as an index calculated as number of cells harvested/number of cells seeded.
Statistical analysis.
Statistical significance was determined by Student's t test. P values of <0.05 were considered statistically significant. Analysis was performed using Excel 97 (Microsoft Corp., Redmond, Wash.). Experiments were performed two or more times in triplicate, and mean values ± standard deviations of the means are reported.
RESULTS
VEGF induction in THP-1 macrophages infected with B. henselae.
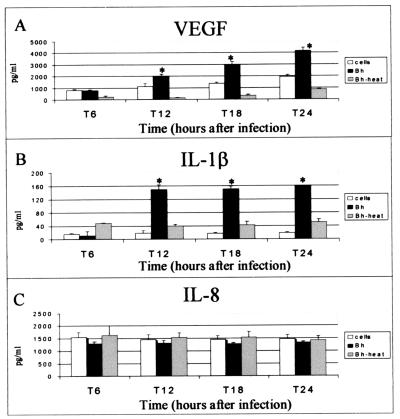
Supernatants analyzed by ELISA demonstrated that VEGF was induced in PMA-differentiated THP-1 macrophages at significant levels in B. henselae-infected cells compared to uninfected cells (Fig. 1A). The initial increase was observed within 6 to 12 h postinfection. Approximate levels ranged between 3,000 and 4,000 pg/ml per 106 cells at 24 h after infection (Fig. 1A). Also, heat-killed bacteria drastically decreased VEGF induction at all tested time points (Fig. 1A). No induction of VEGF by E. coli JM109-infected THP-1 cells above the level for uninfected controls was observed (not shown).
FIG. 1.
VEGF (A), IL-1β (B), and IL-8 (C) induction in THP-1 macrophages infected with viable or heat-killed B. henselae (Bh). Cells were seeded into 24-well plates at a concentration of 106 cells/well. THP-1 cells were differentiated by treatment with PMA. After a 2-day resting period, PMA-differentiated, adherent cells were infected at an MOI of 1,500. Heat-killed bacteria were boiled for 30 min prior to coculture with THP-1. Supernatants were analyzed by ELISA. The data depicted correspond to a representative experiment from three trials. Values are means and standard deviations; asterisks indicate statistical significance (P < 0.05).
IL-1β induction in THP-1 macrophages infected with B. henselae.
B. henselae infection of THP-1 induced IL-1β within 6 h of infection and resulted in approximately an eightfold induction by 12 h after infection (Fig. 1B). Infection with heat-killed bacteria did not induce IL-1β at levels comparable to those for viable bacteria (Fig. 1B).
IL-8 production by B. henselae-infected THP-1.
No evident induction of IL-8 by B. henselae in THP-1 cells was observed beyond constitutive levels in uninfected controls (Fig. 1C). Analogous results were obtained when cells were infected with heat-killed bacteria (Fig. 1C).
Cytochalasin D-treated THP-1 macrophages.
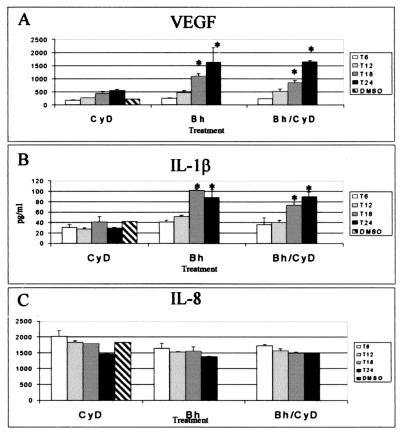
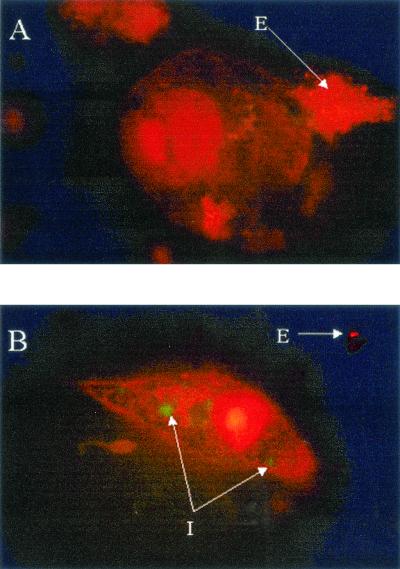
Pretreatment of THP-1 with cytochalasin D was performed to assess VEGF production in the absence of phagocytosis (46). Cytochalasin D interferes with cytoskeleton rearrangement. Cytochalasin D at 0.05 μg/ml (100 nM) prevents invasome formation in endothelial cells, whereas single B. henselae uptake remains unaffected (11). THP-1 were treated with 1 μg of cytochalasin D per ml, a concentration previously reported to inhibit phagocytosis but not adherence to the macrophage (46). No apparent changes were observed in the overall patterns of B. henselae-induced VEGF (Fig. 2A), IL-1β (Fig. 2B), and IL-8 (Fig. 2C) production between cytochalasin D-treated and untreated groups. However, at 18 h postinfection, a slight decrease (approximately 18%) was observed in VEGF and IL-1β production in the cytochalasin D-treated infected cells. These observations suggest that attachment is sufficient for the induction of these angiogenic factors. Absence of phagocytosis was confirmed by fluorescence microscopy (Fig. 3). Microscopic examination showed no intracellular (green) bacteria in cytochalasin D-treated macrophages (Fig. 3A), while vacuole formation was clearly evident in the untreated infected cells (Fig. 3B).
FIG. 2.
VEGF (A), IL-1β (B), and IL-8 (C) induction in B. henselae (Bh)-infected THP-1 macrophages pretreated with cytochalasin D (CyD). THP-1 cells were seeded into 24-well plates at a concentration of 106 cells/well and differentiated into macrophages by treatment with PMA. After a 2-day resting period, PMA-differentiated, adherent cells were infected at a MOI of 100. Selected groups were treated with 1 μg of CyD per ml prior to infection. Supernatants were collected at the indicated times and analyzed by ELISA for protein expression. The data depicted correspond to a representative experiment from three trials. Values are means and standard deviations; asterisks indicate statistical significance (P < 0.05). DMSO, dimethyl sulfoxide.
FIG. 3.
Fluorescence microscopy showing phagocytosis of GFP-expressing B. henselae by THP-1. THP-1 cells were infected with B. henselae 882str/pVBGFPF (39). After phagocytosis, cells were stained with 50 μg of ethidium bromide per ml to allow discrimination between intracellular (green, protected from dye) and extracellular (red-orange, exposed to dye) bacteria. (A) Cells pretreated with 1 μg of cytochalasin D per ml prior to infection. (B) Infected cells without cytochalasin D pretreatment. The data depicted correspond to a representative experiment from three trials. I, intracellular; E, extracellular. Total magnification, approximately ×2,800.
Endothelial cell proliferation.
HMEC-1 that have been cultured with conditioned medium from B. henselae-infected THP-1 cells result in significant proliferation compared to those cultured in both unconditioned medium (155%) and conditioned medium from uninfected THP-1 cells (137%) (Table 1). In addition, HMEC-1 cultured in conditioned medium from B. henselae-infected THP-1 cells resulted in a slightly increased proliferation (104%) over that observed in the human recombinant VEGF control (Table 1). The ability of B. henselae to cause direct proliferation of HMEC-1 (in the absence of conditioned medium or THP-1 cells) was not seen in any experiments, including those here (85% of control value). The percent proliferation values reported here are actual cell counts and reflect absolute increases in cell numbers.
TABLE 1.
Proliferation of HMEC-1 treated with conditioned medium from THP-1 cells infected with B. henselae, treated with uninfected control medium, or directly infected with B. henselae Houston-1
| HMEC-1 culture | No. of cells harvested/no. of cells seeded (mean ± SD)c |
|---|---|
| Unconditioned medium | 3.95 ± 0.64 |
| THP-1 conditioned medium | 4.47 ± 0.12 |
| THP-1 B. henselae conditioned mediuma | 6.13 ± 0.64* |
| B. henselae Houston-1b | 3.35 ± 0.20 |
| Human recombinant VEGF (25 ng/ml) | 5.85 ± 0.34* |
Collected 24 h after infection with B. henselae.
Direct infection of HMEC-1 with B. henselae Houston-1 at an MOI of 1,000.
*, significant at a P value of <0.01.
Production of angiogenic factors in undifferentiated THP-1 monocytes.
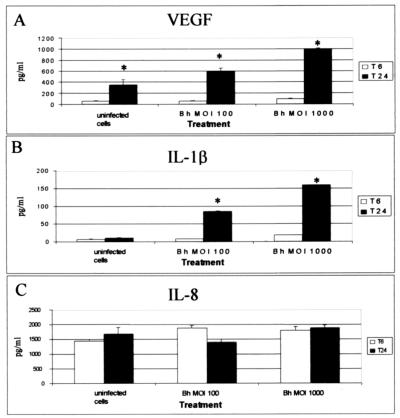
VEGF induction was detected in THP-1 monocytes within 24 h of infection (Fig. 4A). A similar pattern of induction was observed for IL-1β (Fig. 4B). This induction was proportional to the MOI used (Fig. 4A and B). Levels of VEGF and IL-1β in monocytes were lower than those detected in B. henselae-infected THP-1 macrophages. Monocytes produced approximately 600 pg of VEGF per ml (Fig. 4A), whereas macrophages produced close to 1,500 to 2,000 pg/ml (Fig. 2A) at an MOI of 100. Additionally, infected monocytes did not significantly induce the production of IL-8 (Fig. 4C).
FIG. 4.
Effect of bacterial load on VEGF (A), IL-1β (B), and IL-8 (C) induction in undifferentiated THP-1 monocytes. Cells in suspension were infected with viable B. henselae (Bh) at an MOI of 100 or 1,000. Cells were seeded into 24-well plates at a concentration of 106 cells/well. At the indicated times, plates were centrifuged and supernatants were collected and analyzed by ELISA for protein expression. The data depicted correspond to a representative experiment from two trials. Values are means and standard deviations; asterisks indicate statistical significance (P < 0.05).
Production of angiogenic substances in B. henselae-infected endothelial cells.
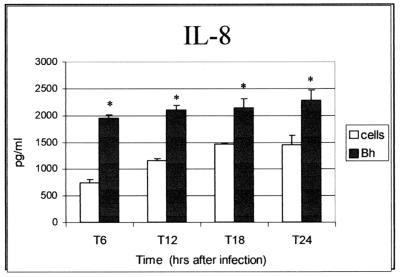
In B. henselae-infected endothelial cells (HMEC-1), an approximately fourfold increase in IL-8 was observed within 6 h of infection (Fig. 5). IL-8 induction continued throughout all examined time points. No evidence of VEGF or IL-1β induction by B. henselae was observed in HMEC-1 (not shown). This observation is in accord with the absence of VEGF induction reported for B. henselae-infected human umbilical vein endothelial cells (HUVECs) (18, 28).
FIG. 5.
IL-8 induction in B. henselae-infected human microvascular endothelial cells (HMEC-1). Cells were seeded into 24-well plates at a concentration of 106 cells/well and were infected with B. henselae Houston-1 (Bh) at an MOI of 500. Supernatants were collected at the indicated times and analyzed by ELISA for protein expression. The data depicted correspond to a representative experiment from three trials. Values are means and standard deviations; asterisks indicate statistical significance (P < 0.05).
DISCUSSION
B. henselae is one of three members of the genus Bartonella that are capable of causing disease characterized by vascular proliferative lesions (2). A close correlation between the host's immune status and disease severity during B. henselae infection is evidenced by the distinct clinical manifestations observed in CSD, which occurs predominantly in immunocompetent individuals, and in BA, which is a manifestation of infection seen primarily in the immunosuppressed. Therefore, in this study we considered the involvement of immune cells of the mononuclear phagocytic lineage in the development of B. henselae-induced angiogenesis. As part of the innate arm of the immune system, professional phagocytic cells are involved in the elimination of particulate targets such as bacteria. The interaction between these scavenger cells and bacteria results in the production of cytokines and growth factors with autocrine and paracrine functions, some of which are known regulators of angiogenesis (34, 40). The interaction of monocytes or macrophages with vascular endothelium during pathological angiogenesis and arteriogenesis may induce the production of angiogenic mediators (17, 27). In addition, macrophage infiltration is a common occurrence in BA lesions (23, 24, 31).
Our results suggest that more than one angiogenic factor may be induced during infection of THP-1 macrophages and monocytes with B. henselae. Our data implicate VEGF, a direct endothelial cell mitogen, as a significant mediator induced during B. henselae infection of macrophages and monocytes. VEGF induction by B. henselae was previously reported in EA.hy926, a cell line resulting from the fusion of HUVECs with A549, a permanent human lung carcinoma cell line (18). Also, IL-1β, a proinflammatory cytokine that indirectly upregulates angiogenesis in vivo and induces direct positive mediators from stromal or recruited inflammatory cells (35), was induced by B. henselae in THP-1 monocytes and macrophages. In the tumor microenvironment, macrophage chemoattractant protein-1 (MCP-1), an indirect positive inducer of angiogenesis, recruits macrophages (16). Production of MCP-1 by tumor cells induces macrophage-derived proinflammatory cytokines, such as IL-1β, that in turn support output of other direct mediators of angiogenesis (27). Presumably, a comparable phenomenon may also take place during B. henselae infection by interaction between macrophages and other cells in the stroma adjacent to vascular endothelium.
Heat-killed bacteria did not induce VEGF or IL-1β at levels equivalent to those for viable bacteria. To determine if VEGF induction was due to the activity of bacterial endotoxin, TNF-α was examined. Interestingly, heat-killed B. henselae induced TNF-α (data not shown), a hallmark of inflammation typically elicited in response to lipopolysaccharide in gram-negative bacteria. Inhibition of phagocytosis by pretreatment with cytochalasin D did not significantly affect VEGF or IL-1β induction patterns in THP-1 cells. Our results are consistent with those reported for EA.hy926 (18). Taken together, the data suggest that VEGF induction is caused by the interaction of bacterial proteins with macrophages and does not involve endotoxin. Pretreatment of THP-1 with purified B. henselae OMP43 (6) induced VEGF but at an approximately fourfold lower level than for viable bacteria. Furthermore, consistent with the report involving EA.hy926 (18), induction of VEGF in THP-1 macrophages is specific to B. henselae, since infection with E. coli JM109 did not induce its production (data not shown).
Angiogenic activity in conditioned medium from B. henselae-infected THP-1 cultures was evidenced by induction of endothelial cell proliferation. The levels of proliferation were comparable to those seen with recombinant VEGF and similar to those observed for other endothelial cell mitogens with HMEC-1 (44). Our results are in agreement with a recent study by Kempf et al. showing the importance of VEGF in B. henselae-mediated endothelial cell proliferation but also showing that endothelial cells are not the major cell type producing VEGF (18). Maeno et al. previously showed that endothelial cells do not produce VEGF in response to B. henselae but are able to proliferate in response to a B. henselae factor (28). It is also important to indicate that direct comparison with other reports showing proliferation of endothelial cells by B. henselae is difficult, since these reports used HUVECs (7, 28). A more recent study showed that B. henselae and B. quintana are able to suppress caspase activity in endothelial cells, resulting in inhibition of apoptosis (20). Those authors propose that inhibition of apoptosis accounts in part for the ability of Bartonella to induce vascular proliferation in vivo (20).
Macrophage-derived IL-8 is also a direct positive mediator of angiogenesis (22). However, in this system IL-8 production was upregulated in HMEC-1 (Fig. 5) within 6 h of infection with B. henselae but not in B. henselae-infected THP-1 macrophages and monocytes. Levels of secreted IL-8 in THP-1-infected cells were similar to the levels observed in uninfected cells. HMEC-1-derived IL-8 may have an autocrine role in disease progression associated with B. henselae infection. In addition to VEGF (3), IL-8 has been associated with the pathogenesis of Kaposi's sarcoma, a neoplasm caused by Kaposi's sarcoma-associated herpesvirus 8 that is seen most often in HIV-infected individuals in the United States (29). However, the autocrine role of IL-8 in causing endothelial cell proliferation seems minimal in the absence of other cell types, since we were unable to demonstrate direct proliferation of HMEC-1 by B. henselae (Table 1).
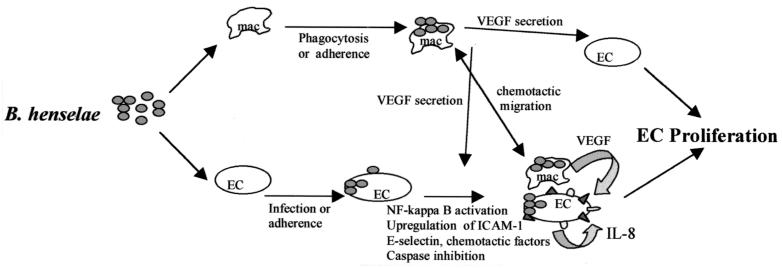
Taken together, the data suggest that macrophage-derived VEGF and IL-1β, as well as HMEC-1-derived IL-8, are linked to a paracrine angiogenic loop that could promote the development of BA during B. henselae infection. THP-1 conditioned medium from infected cultures confirmed the potential angiogenic activity present in supernatants by promoting the proliferation of HMEC-1. The steps central to such a paracrine angiogenic loop are outlined in Fig. 6. Clearly, the role of additional cytokines, chemokines such as MCP-1, growth factors, other regulators of angiogenesis, and antiapoptotic factors of Bartonella need to be further defined and clarified. However, the central concept of this model is the role of the macrophage as the secondary effector cell in promoting angiogenesis.
FIG. 6.
Hypothetical paracrine angiogenic loop model of B. henselae-mediated angiogenesis. B. henselae is able to adhere to and invade human macrophages (mac) and induce production of VEGF. This secreted VEGF functions in a paracrine manner and acts as an endothelial cell mitogen. Infection of endothelial cells (EC) with B. henselae may serve to further enhance proliferation by induction of IL-8, activation of chemotactic migration of macrophages, or inhibition of apoptosis.
We have proposed that macrophages are an efficient source of VEGF upon B. henselae stimulation to fulfill this effector role. However, others have shown proliferation of endothelial cells upon exposure to B. henselae or fractions of the bacterium (7, 28), implying the presence of an angiogenic factor with direct mitogenic activity for endothelial cells. In each of these studies early-passage primary HUVECs were used. We have not been able to reproduce consistent proliferation of HUVECs by B. henselae. In addition, we observe no direct proliferation of HMEC-1 by B. henselae, despite the fact that HMEC-1 responds well to known endothelial cell mitogens such as VEGF and basic fibroblast growth factor (44). We and others have shown that endothelial cells are not efficient producers of VEGF upon B. henselae stimulation (18, 28). Two possibilities may serve to explain this apparent contradiction in the mechanisms by which endothelial cell proliferation is activated. First, there may be some direct mitogenic effect of B. henselae on endothelial cells that we did not observe. This mitogenic effect may be through an antiapoptotic mechanism like that recently described (20) or through an IL-8-dependent (VEGF-independent) mechanism. The induction of IL-8 in HMEC-1 by B. henselae supports this possibility. Either mechanism may serve to complement the VEGF production by the macrophage effector cell. Alternatively and less likely, the proliferation of HUVECs may be due to the presence of small numbers of other cell types capable of producing VEGF in these low-passage primary endothelial cells which function in the role of effector cells.
We further speculate that these angiogenic molecules may be differentially regulated in immunosuppressed and immunocompetent individuals. Monocytes and macrophages are a primary target for HIV infection and may become viral reservoirs in HIV-infected individuals. Impairment of macrophage phagocytic function and increased production of proinflammatory cytokines are observed during HIV infection (8, 9). For example, activated macrophages are able to control intracellular infections with Candida albicans and Toxoplasma gondii, whereas intracellular killing of these opportunistic pathogens is impaired in HIV-infected macrophages (5, 10). Phagocytosis is a complex process, and clearly further experimentation is required to elucidate whether a similar occurrence takes place during B. henselae infection of macrophages.
In conclusion, we report that B. henselae triggers the type of activation required for the induction of secreted macrophage-derived mediators of angiogenesis. The primary factor involved appears to be VEGF, since the level of VEGF induction is mitogenic for endothelial cells. The role of other cell and bacterial factors in inducing angiogenesis must be further examined, since a number of both positive and negative regulators of angiogenesis are likely to be involved. In addition, the establishment of an animal model will prove invaluable to studies aimed at understanding B. henselae-mediated angiogenesis. A recent study by Wong et al. showed that VEGF-secreting tumor cells caused a paraneoplastic disease similar to bacillary peliosis hepatis in SCID mice (45). Similar models may prove useful in the study of B. henselae-induced angiogenesis.
Acknowledgments
This work was supported by Public Health Service grant R01-AI 38178 from the National Institute of Allergy and Infectious Diseases.
We thank Thomas Lawley of Emory University and The Biological Products Branch, Centers for Disease Control and Prevention, for providing the HMEC-1 cell line used in these studies.
Editor: J. T. Barbieri
REFERENCES
- 1.Ades, E. W., F. J. Candal, R. A. Swerlick, V. G. George, S. Summers, D. C. Bosse, and T. J. Lawley. 1992. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J. Investig. Dermatol. 99:683-690. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, B. E., and M. A. Neuman. 1997. Bartonella spp. as emerging human pathogens. Clin. Microbiol. Rev. 10:203-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arasteh, K., and A. Hannah. 2000. The role of vascular endothelial growth factor (VEGF) in AIDS-related Kaposi's sarcoma. Oncologist 5(Suppl. 1):28-31. [DOI] [PubMed] [Google Scholar]
- 4.Bamba, H., S. Ota, A. Kato, C. Kawamoto, and F. Matsuzaki. 2000. Effect of prostaglandin E1 on vascular endothelial growth factor production by human macrophages and colon cancer cells. J. Exp. Clin. Cancer Res. 19:219-223. [PubMed] [Google Scholar]
- 5.Biggs, B. A., M. Hewish, S. Kent, K. Hayes, and S. M. Crowe. 1995. HIV-1 infection of human macrophages impairs phagocytosis and killing of Toxoplasma gondii. J. Immunol. 154:6132-6139. [PubMed] [Google Scholar]
- 6.Burgess, A. W., J. Y. Paquet, J. J. Letesson, and B. E. Anderson. 2000. Isolation, sequencing and expression of Bartonella henselae omp43 and predicted membrane topology of the deduced protein. Microb. Pathog. 29:73-80. [DOI] [PubMed] [Google Scholar]
- 7.Conley, T., L. Slater, and K. Hamilton. 1994. Rochalimaea species stimulate human endothelial cell proliferation and migration in vitro. J. Lab. Clin. Med. 124:521-528. [PubMed] [Google Scholar]
- 8.Covelli, V., S. Pece, G. Giuliani, C. De Simone, and E. Jirillo. 1997. Pathogenetic role of phagocytic abnormalities in human virus immunodeficiency infection: possible therapeutical approaches. Immunopharmacol. Immunotoxicol. 19:147-164. [DOI] [PubMed] [Google Scholar]
- 9.Crowe, S. M. 1995. Role of macrophages in the pathogenesis of human immunodeficiency virus (HIV) infection. Aust. N. Z. J. Med. 25:777-783. [DOI] [PubMed] [Google Scholar]
- 10.Crowe, S. M., N. J. Vardaxis, S. J. Kent, A. L. Maerz, M. J. Hewish, M. S. McGrath, and J. Mills. 1994. HIV infection of monocyte-derived macrophages in vitro reduces phagocytosis of Candida albicans. J. Leukoc. Biol. 56:318-327. [DOI] [PubMed] [Google Scholar]
- 11.Dehio, C., M. Meyer, J. Berger, H. Schwarz, and C. Lanz. 1997. Interaction of Bartonella henselae with endothelial cells results in bacterial aggregation on the cell surface and the subsequent engulfment and internalization of the bacterial aggregate by a unique structure, the invasome. J. Cell Sci. 110:2141-2154. [DOI] [PubMed] [Google Scholar]
- 12.Drevets, D. A., and P. A. Campbell. 1991. Macrophage phagocytosis: use of fluorescence microscopy to distinguish between extracellular and intracellular bacteria. J. Immunol. Methods 142:31-38. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara, N. 2000. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog. Horm. Res. 55:15-35. [PubMed] [Google Scholar]
- 14.Fujimoto, J., H. Sakaguchi, R. Hirose, H. Wen, and T. Tamaya. 1999. Angiogenesis in endometriosis and angiogenic factors. Gynecol. Obstet. Investig. 48(Suppl. 1):14-20. [DOI] [PubMed] [Google Scholar]
- 15.Gaudry, M., O. Bregerie, V. Andrieu, J. El Benna, M. A. Pocidalo, and J. Hakim. 1997. Intracellular pool of vascular endothelial growth factor in human neutrophils. Blood 90:4153-4161. [PubMed] [Google Scholar]
- 16.Goede, V., L. Brogelli, M. Ziche, and H. G. Augustin. 1999. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int. J. Cancer 82:765-770. [DOI] [PubMed] [Google Scholar]
- 17.Heil, M., M. Clauss, K. Suzuki, I. R. Buschmann, A. Willuweit, S. Fischer, and W. Schaper. 2000. Vascular endothelial growth factor (VEGF) stimulates monocyte migration through endothelial monolayers via increased integrin expression. Eur. J. Cell Biol. 79:850-857. [DOI] [PubMed] [Google Scholar]
- 18.Kempf, V. A., B. Volkmann, M. Schaller, C. A. Sander, K. Alitalo, T. Riess, and I. B. Autenrieth. 2001. Evidence of a leading role for VEGF in Bartonella henselae-induced endothelial cell proliferations. Cell. Microbiol. 3:623-632. [DOI] [PubMed] [Google Scholar]
- 19.Kim, Y., R. Y. Imdad, A. H. Stephenson, R. S. Sprague, and A. J. Lonigro. 1998. Vascular endothelial growth factor mRNA in pericytes is upregulated by phorbol myristate acetate. Hypertension 31:511-515. [DOI] [PubMed] [Google Scholar]
- 20.Kirby, J. E., and D. M. Nekorchuk. 2002. Bartonella-associated endothelial proliferation depends on inhibition of apoptosis. Proc. Natl. Acad. Sci. USA 99:4656-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klagsbrun, M., and P. A. D'Amore. 1991. Regulators of angiogenesis. Annu. Rev. Physiol. 53:217-239. [DOI] [PubMed] [Google Scholar]
- 22.Koch, A. E., P. J. Polverini, S. L. Kunkel, L. A. Harlow, L. A. DiPietro, V. M. Elner, S. G. Elner, and R. M. Strieter. 1992. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 258:1798-1801. [DOI] [PubMed] [Google Scholar]
- 23.LeBoit, P. E., T. G. Berger, B. M. Egbert, J. H. Beckstead, T. S. Yen, and M. H. Stoler. 1989. Bacillary angiomatosis. The histopathology and differential diagnosis of a pseudoneoplastic infection in patients with human immunodeficiency virus disease. Am. J. Surg. Pathol. 13:909-920. [PubMed] [Google Scholar]
- 24.LeBoit, P. E., T. G. Berger, B. M. Egbert, T. S. Yen, M. H. Stoler, T. A. Bonfiglio, J. A. Strauchen, C. K. English, and D. J. Wear. 1988. Epithelioid haemangioma-like vascular proliferation in AIDS: manifestation of cat scratch disease bacillus infection? Lancet i:960-963. [DOI] [PubMed] [Google Scholar]
- 25.Lee, A. K., and S. Falkow. 1998. Constitutive and inducible green fluorescent protein expression in Bartonella henselae. Infect. Immun. 66:3964-3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung, D. W., G. Cachianes, W. J. Kuang, D. V. Goeddel, and N. Ferrara. 1989. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246:1306-1309. [DOI] [PubMed] [Google Scholar]
- 27.Liss, C., M. J. Fekete, R. Hasina, C. D. Lam, and M. W. Lingen. 2001. Paracrine angiogenic loop between head-and-neck squamous-cell carcinomas and macrophages. Int. J. Cancer 93:781-785. [DOI] [PubMed] [Google Scholar]
- 28.Maeno, N., H. Oda, K. Yoshiie, M. R. Wahid, T. Fujimura, and S. Matayoshi. 1999. Live Bartonella henselae enhances endothelial cell proliferation without direct contact. Microb. Pathog. 27:419-427. [DOI] [PubMed] [Google Scholar]
- 29.Masood, R., J. Cai, A. Tulpule, T. Zheng, A. Hamilton, S. Sharma, B. M. Espina, D. L. Smith, and P. S. Gill. 2001. Interleukin 8 is an autocrine growth factor and a surrogate marker for Kaposi's sarcoma. Clin. Cancer Res. 7:2693-2702. [PubMed] [Google Scholar]
- 30.McLaren, J., A. Prentice, D. S. Charnock-Jones, S. A. Millican, K. H. Muller, A. M. Sharkey, and S. K. Smith. 1996. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J. Clin. Investig. 98:482-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monteil, R. A., J. F. Michiels, P. Hofman, M. C. Saint-Paul, C. Hitzig, C. Perrin, and J. Santini. 1994. Histological and ultrastructural study of one case of oral bacillary angiomatosis in HIV disease and review of the literature. Eur. J. Cancer 30B:65-71. [DOI] [PubMed] [Google Scholar]
- 32.Mukutmoni, M., N. E. Hubbard, and K. L. Erickson. 2001. Prostaglandin E(2) modulation of vascular endothelial growth factor production in murine macrophages. Prostaglandins Leukot. Essent. Fatty Acids 65:123-131. [DOI] [PubMed] [Google Scholar]
- 33.Musso, T., R. Badolato, D. Ravarino, S. Stornello, P. Panzanelli, C. Merlino, D. Savoia, R. Cavallo, A. N. Ponzi, and M. Zucca. 2001. Interaction of Bartonella henselae with the murine macrophage cell line J774: infection and proinflammatory response. Infect. Immun. 69:5974-5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ono, M., H. Torisu, J. Fukushi, A. Nishie, and M. Kuwano. 1999. Biological implications of macrophage infiltration in human tumor angiogenesis. Cancer Chemother. Pharmacol. 43(Suppl.):S69-S71. [DOI] [PubMed] [Google Scholar]
- 35.Pepper, M. S., S. J. Mandriota, J. D. Vassalli, L. Orci, and R. Montesano. 1996. Angiogenesis-regulating cytokines: activities and interactions. Curr. Top. Microbiol. Immunol. 213:31-67. [DOI] [PubMed] [Google Scholar]
- 36.Polverini, P. J., P. S. Cotran, M. A. Gimbrone, Jr., and E. R. Unanue. 1977. Activated macrophages induce vascular proliferation. Nature 269:804-806. [DOI] [PubMed] [Google Scholar]
- 37.Regnery, R. L., B. E. Anderson, J. E. Clarridge 3rd, M. C. Rodriguez-Barradas, D. C. Jones, and J. H. Carr. 1992. Characterization of a novel Rochalimaea species, R. henselae sp. nov., isolated from blood of a febrile, human immunodeficiency virus-positive patient. J. Clin. Microbiol. 30:265-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Resto-Ruiz, S. I., D. Sweger, R. H. Widen, N. Valkov, and B. E. Anderson. 2000. Transcriptional activation of the htrA (high-temperature requirement A) gene from Bartonella henselae. Infect. Immun. 68:5970-5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmiederer, M., R. Arcenas, R. Widen, N. Valkov, and B. Anderson. 2001. Intracellular induction of the Bartonella henselae virB operon by human endothelial cells. Infect. Immun. 69:6495-6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sunderkotter, C., K. Steinbrink, M. Goebeler, R. Bhardwaj, and C. Sorg. 1994. Macrophages and angiogenesis. J. Leukoc. Biol. 55:410-422. [DOI] [PubMed] [Google Scholar]
- 41.Todd, R. F., 3rd, and D. Y. Liu. 1986. Mononuclear phagocyte activation: activation-associated antigens. Fed. Proc. 45:2829-2836. [PubMed] [Google Scholar]
- 42.Torisu, H., M. Ono, H. Kiryu, M. Furue, Y. Ohmoto, J. Nakayama, Y. Nishioka, S. Sone, and M. Kuwano. 2000. Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: possible involvement of TNFalpha and IL-1alpha. Int. J. Cancer 85:182-188. [PubMed] [Google Scholar]
- 43.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26:171-176. [DOI] [PubMed] [Google Scholar]
- 44.Vasse, M., J. Pourtau, V. Trochon, M. Muraine, J. P. Vannier, H. Lu, J. Soria, and C. Soria. 1999. Oncostatin M induces angiogenesis in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 19:1835-1842. [DOI] [PubMed] [Google Scholar]
- 45.Wong, A. K., M. Alfert, D. H. Castrillon, Q. Shen, J. Holash, G. D. Yancopoulos, and L. Chin. 2001. Excessive tumor-elaborated VEGF and its neutralization define a lethal paraneoplastic syndrome. Proc. Natl. Acad. Sci. USA 98:7481-7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto, Y., S. Okubo, T. W. Klein, K. Onozaki, T. Saito, and H. Friedman. 1994. Binding of Legionella pneumophila to macrophages increases cellular cytokine mRNA. Infect. Immun. 62:3947-3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zachary, I. 1998. Vascular endothelial growth factor. Int. J. Biochem. Cell. Biol. 30:1169-1174. [DOI] [PubMed] [Google Scholar]