Abstract
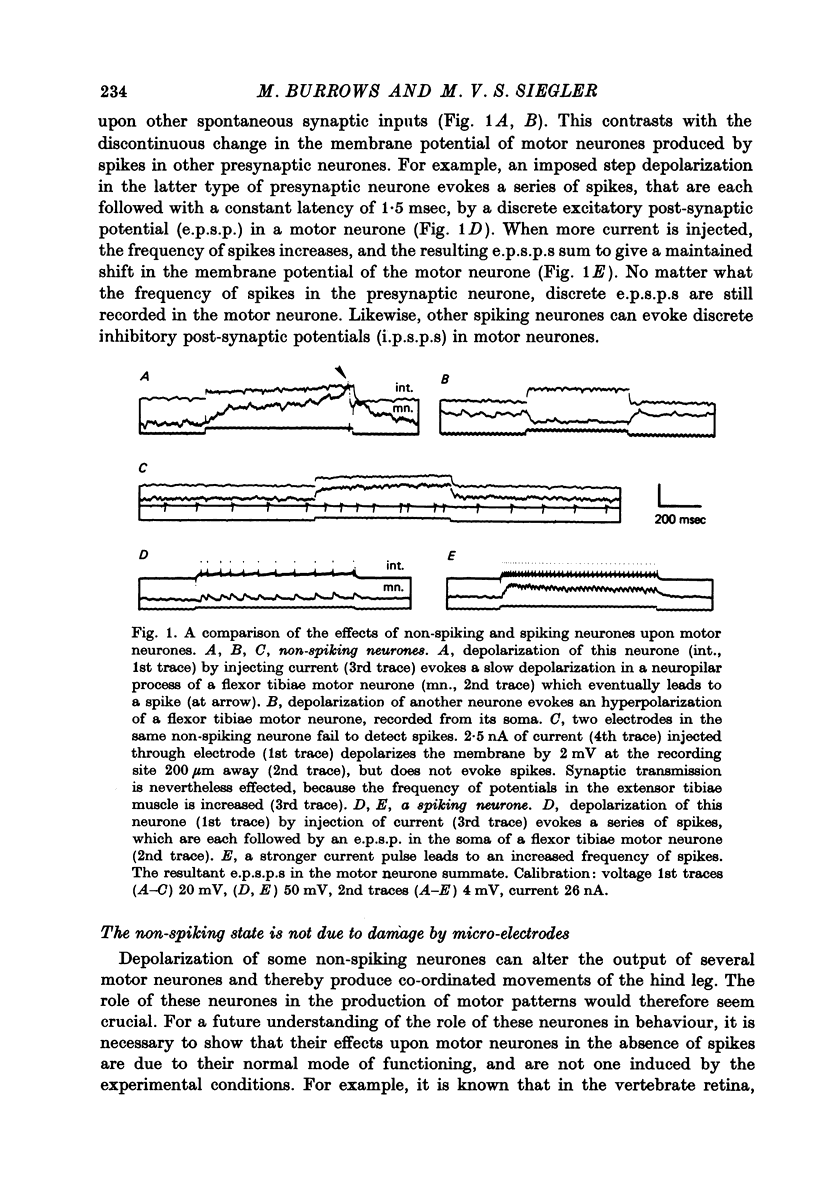
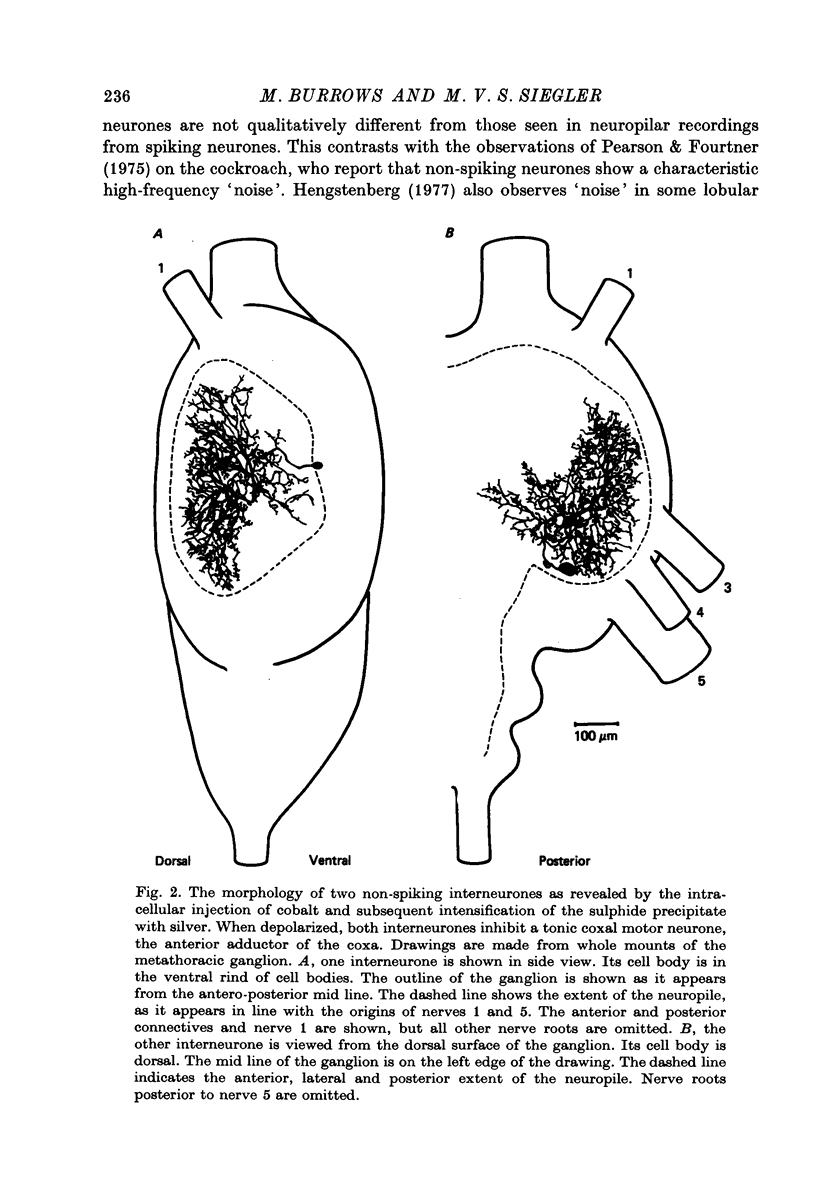
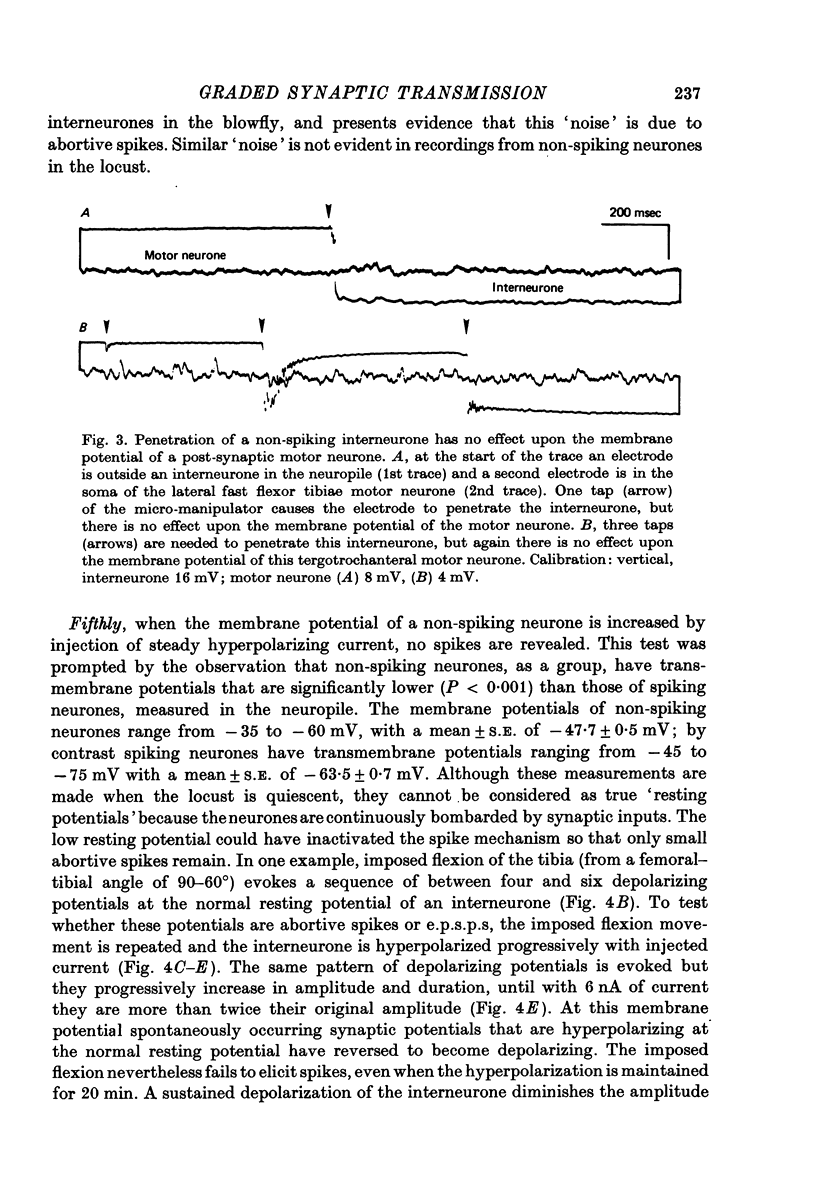
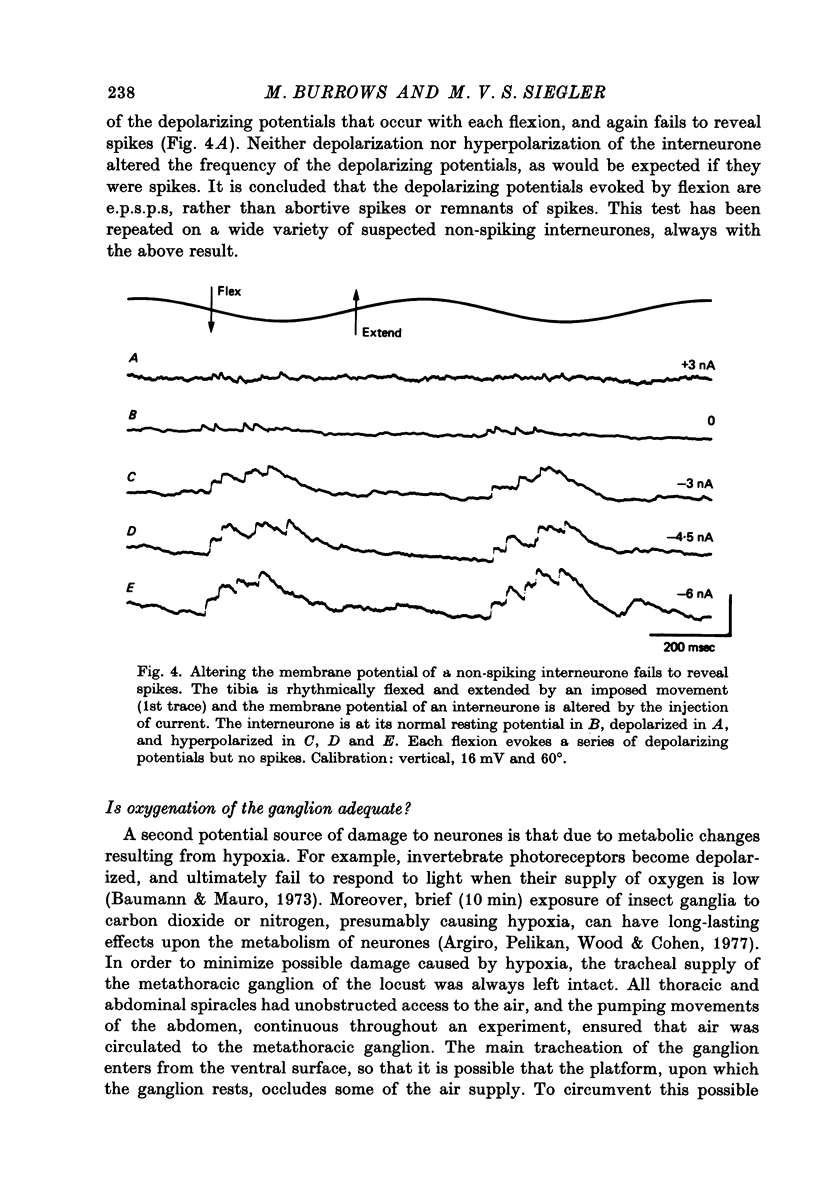
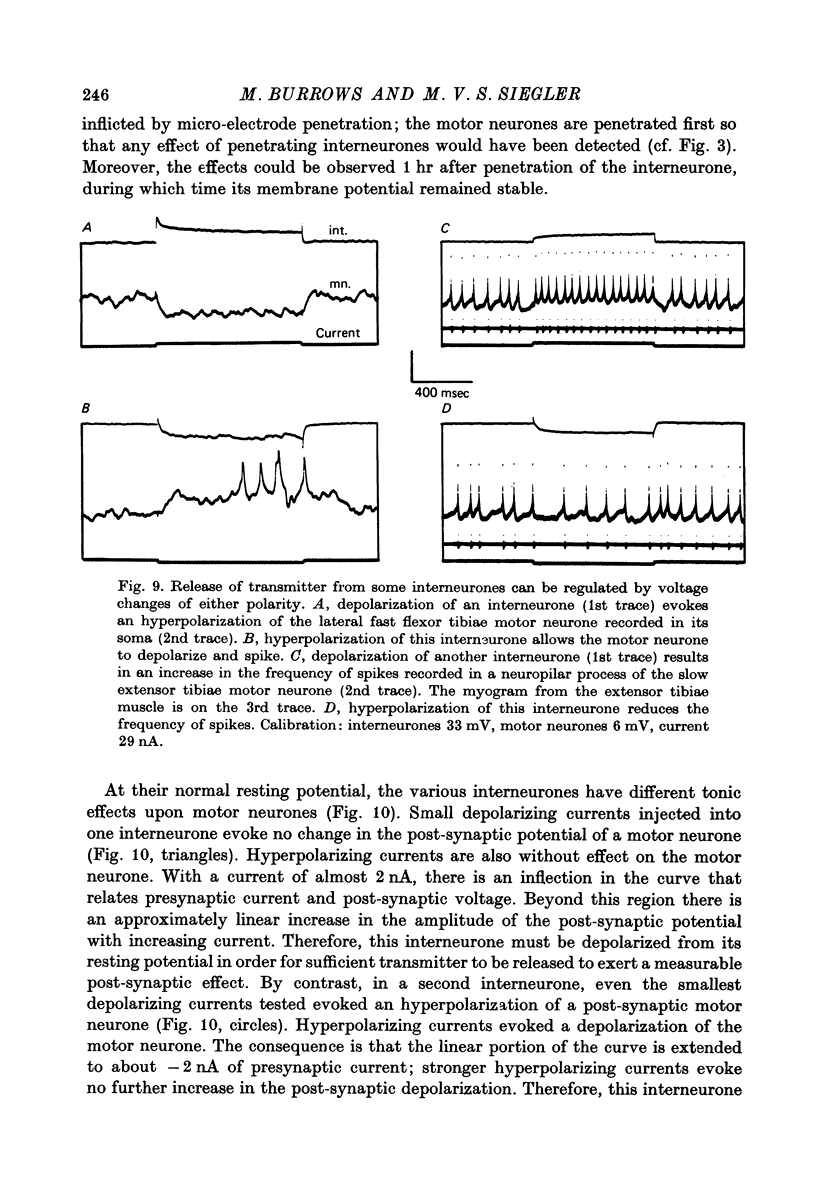
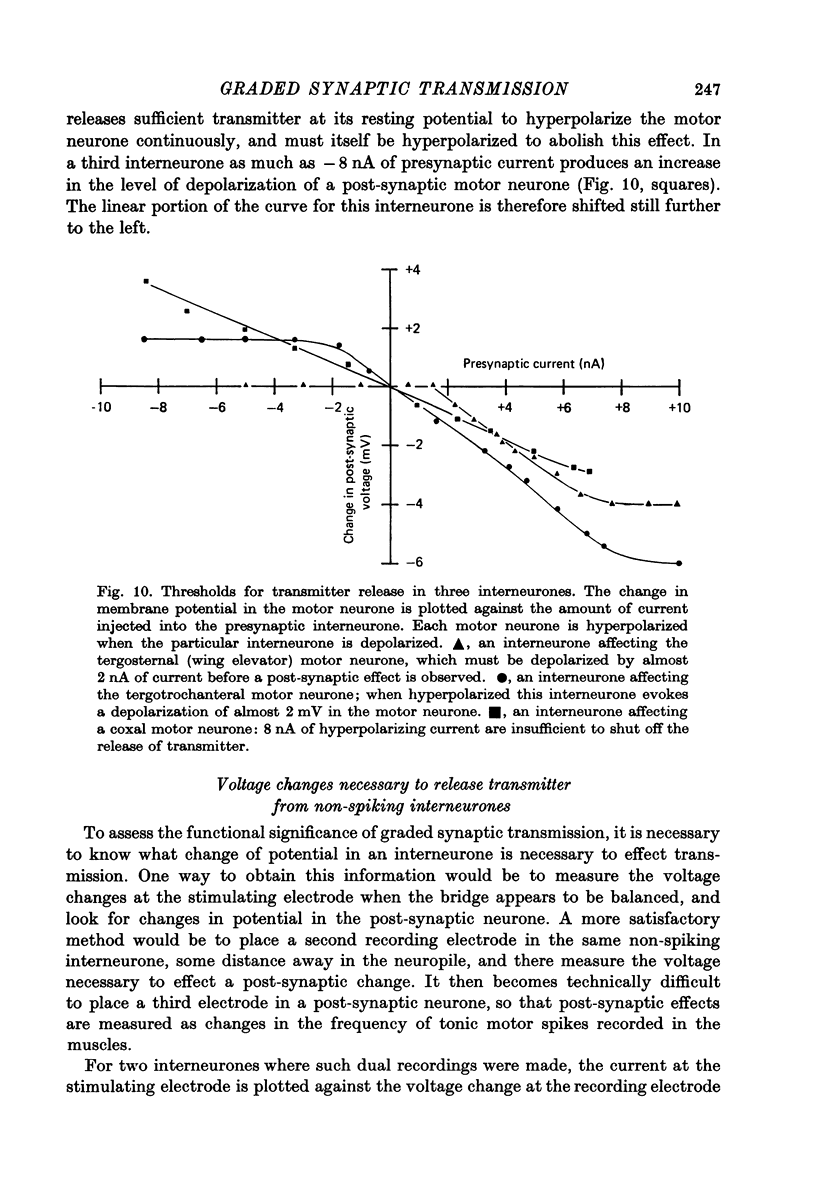
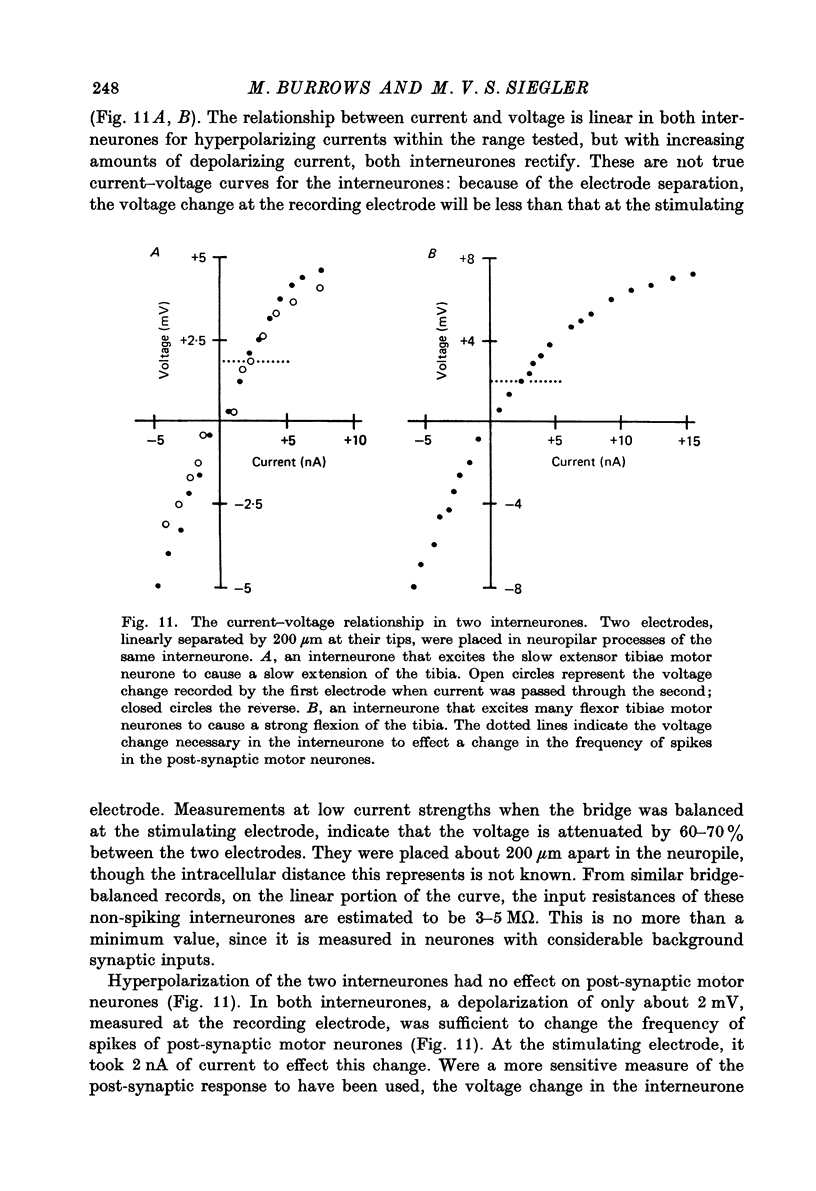
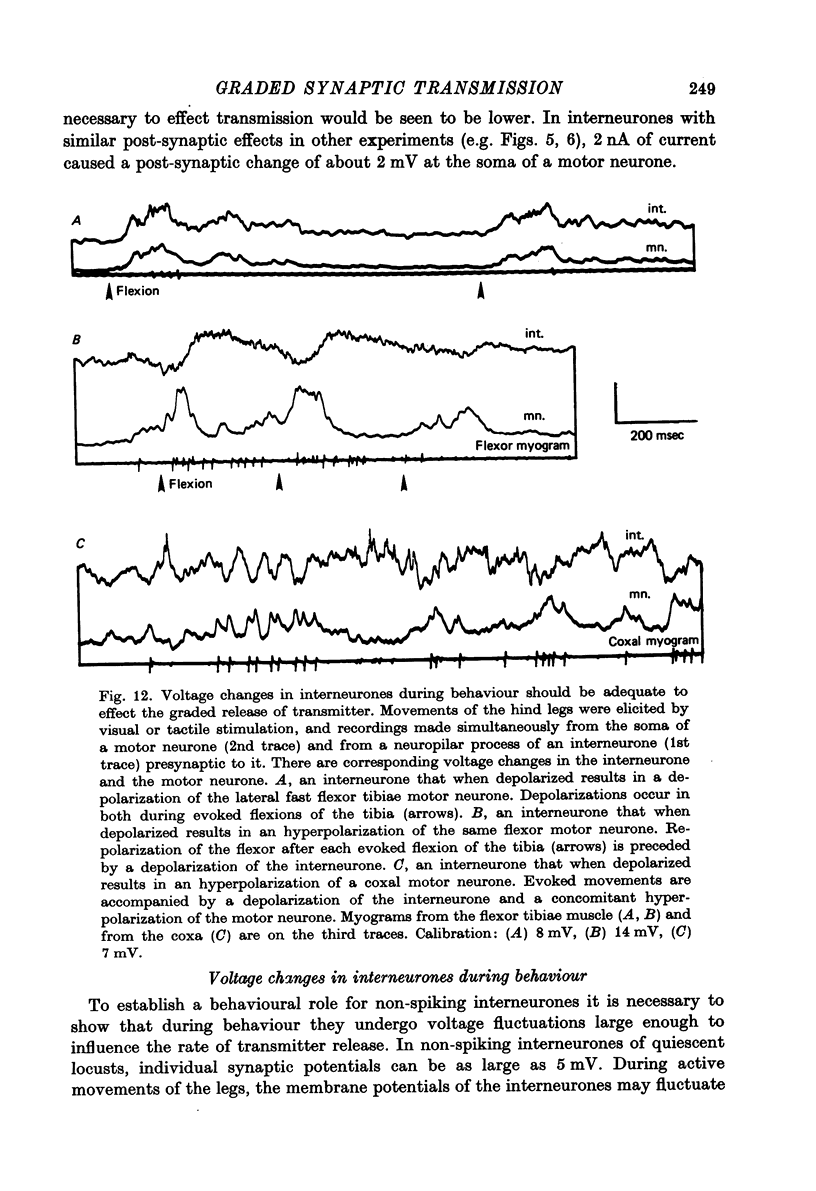
1. In the metathoracic ganglion of the locust some neurones can effect changes in the membrane potential of identified post-synaptic motor neurones without themselves spiking. 2. These 'non-spiking' neurones have processes only within the metathoracic ganglion, and therefore are local intraganglionic interneurones. 3. The absence of spikes in the interneurones reflects their normal physiological state and is not due to the experimental conditions. 4. When the interneurones are depolarized by the injection of current pulses lasting several hundred milliseconds, post-synaptic motor neurones are either depolarized, or hyperpolarized, for the duration of the pulse. 5. The magnitude of the change in post-synaptic voltage is graded according to the amount of presynaptic current. 6. A number of physiological tests indicate that the graded effects upon motor neurones are mediated by chemical synaptic transmission. For example, an evoked hyperpolarization of a motor neurone can be reversed in polarity by simultaneously hyperpolarizing the motor neurone with injected current. 7. At their resting potential some interneurones tonically release sufficient transmitter to have a measurable post-synaptic effect. The injection of depolarizing and hyperpolarizing currents into these interneurones effects opposite changes in post-synaptic potential. 8. Other interneurones must be depolarized from resting potential before a post-synaptic effect is observed, and hyperpolarizing currents have no post-synaptic effect. In these interneurones it is estimated that a depolarization of only 2 mV is sufficient to effect the release of transmitter. 9. The membrane potentials of non-spiking interneurones can fluctuate by as much as 15 mV during active movements of the hind legs and individual p.s.p.s as large as 5 mV can be recorded. Therefore, summed p.s.p.s or even single ones are expected to be the electrophysiological signals effecting transmitter release from these interneurones.
Full text
PDF




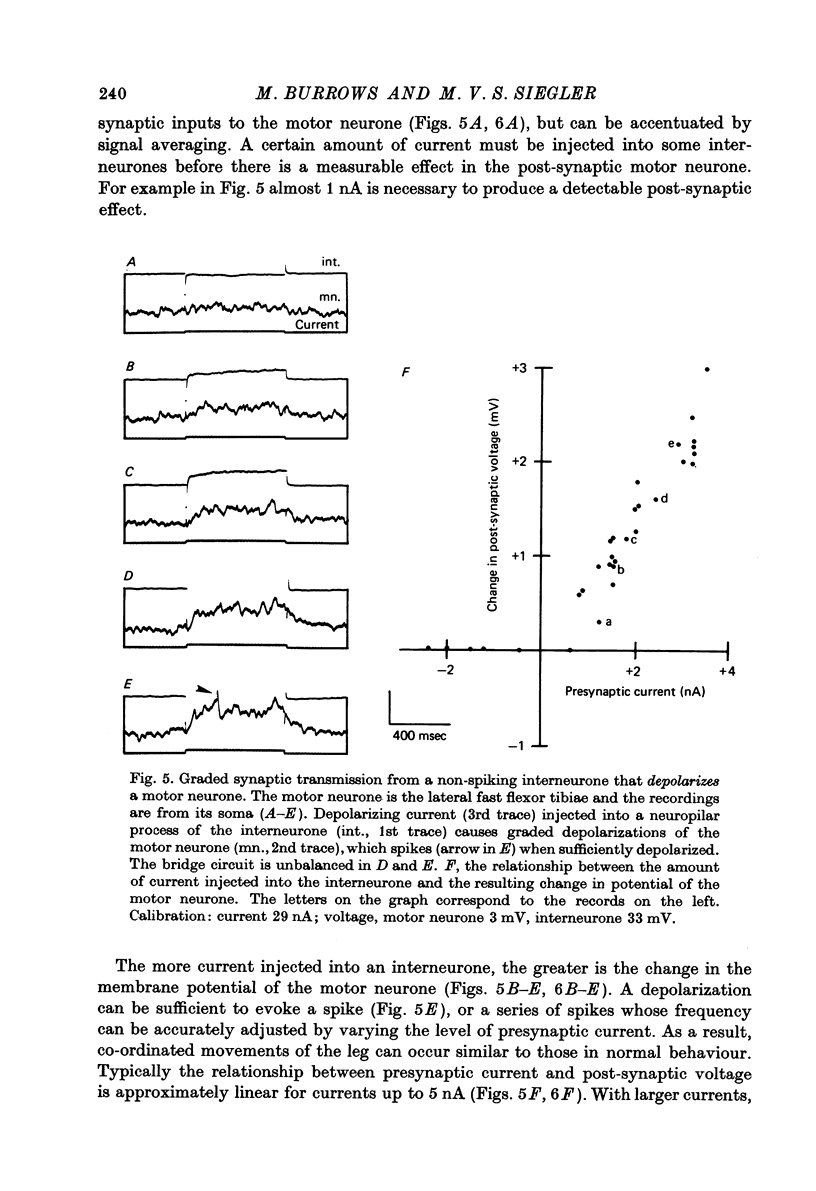








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
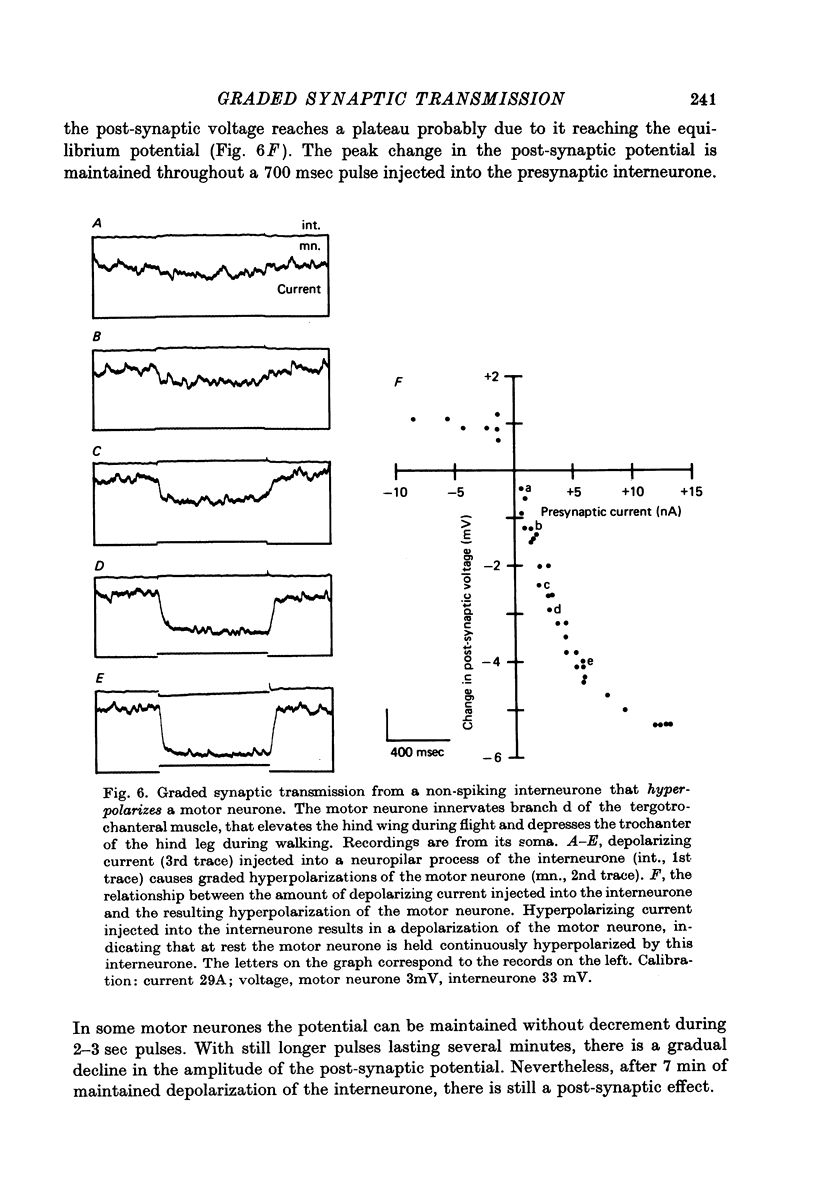
- Bacon J. P., Altman J. S. A silver intensification method for cobalt-filled neurones in wholemount preparations. Brain Res. 1977 Dec 16;138(2):359–363. doi: 10.1016/0006-8993(77)90753-3. [DOI] [PubMed] [Google Scholar]
- Baumann F., Mauro A. Effect of hypoxia on the change in membrane conductance evoked by illumination in arthropod photoreceptors. Nat New Biol. 1973 Aug 1;244(135):146–148. doi: 10.1038/newbio244146b0. [DOI] [PubMed] [Google Scholar]
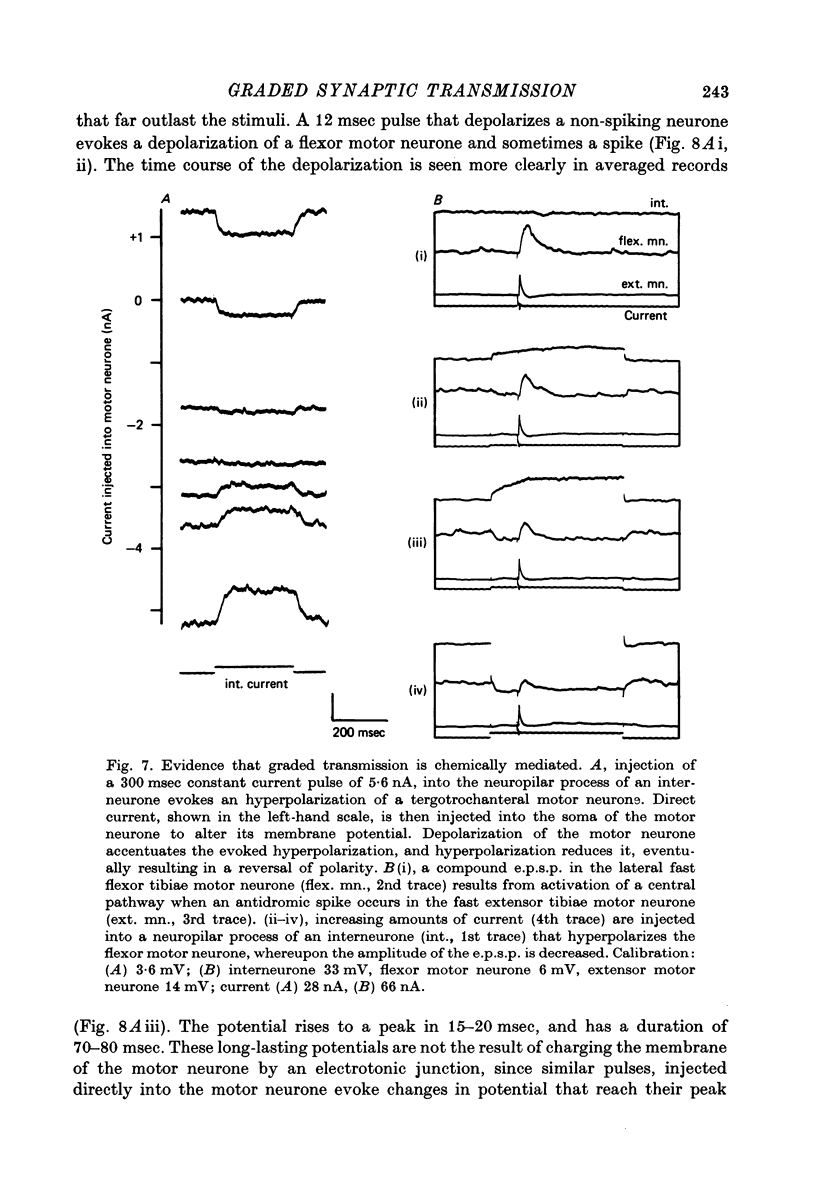
- Baylor D. A., Fettiplace R. Transmission of signals from photoreceptors to ganglion cells in the eye of the turtle. Cold Spring Harb Symp Quant Biol. 1976;40:529–536. doi: 10.1101/sqb.1976.040.01.049. [DOI] [PubMed] [Google Scholar]
- Berry M. S., Pentreath V. W. Criteria for distinguishing between monosynaptic and polysynaptic transmission. Brain Res. 1976 Mar 19;105(1):1–20. doi: 10.1016/0006-8993(76)90919-7. [DOI] [PubMed] [Google Scholar]
- Burrows M., Horridge G. A. The organization of inputs to motoneurons of the locust metathoracic leg. Philos Trans R Soc Lond B Biol Sci. 1974 Sep 12;269(896):49–94. doi: 10.1098/rstb.1974.0041. [DOI] [PubMed] [Google Scholar]
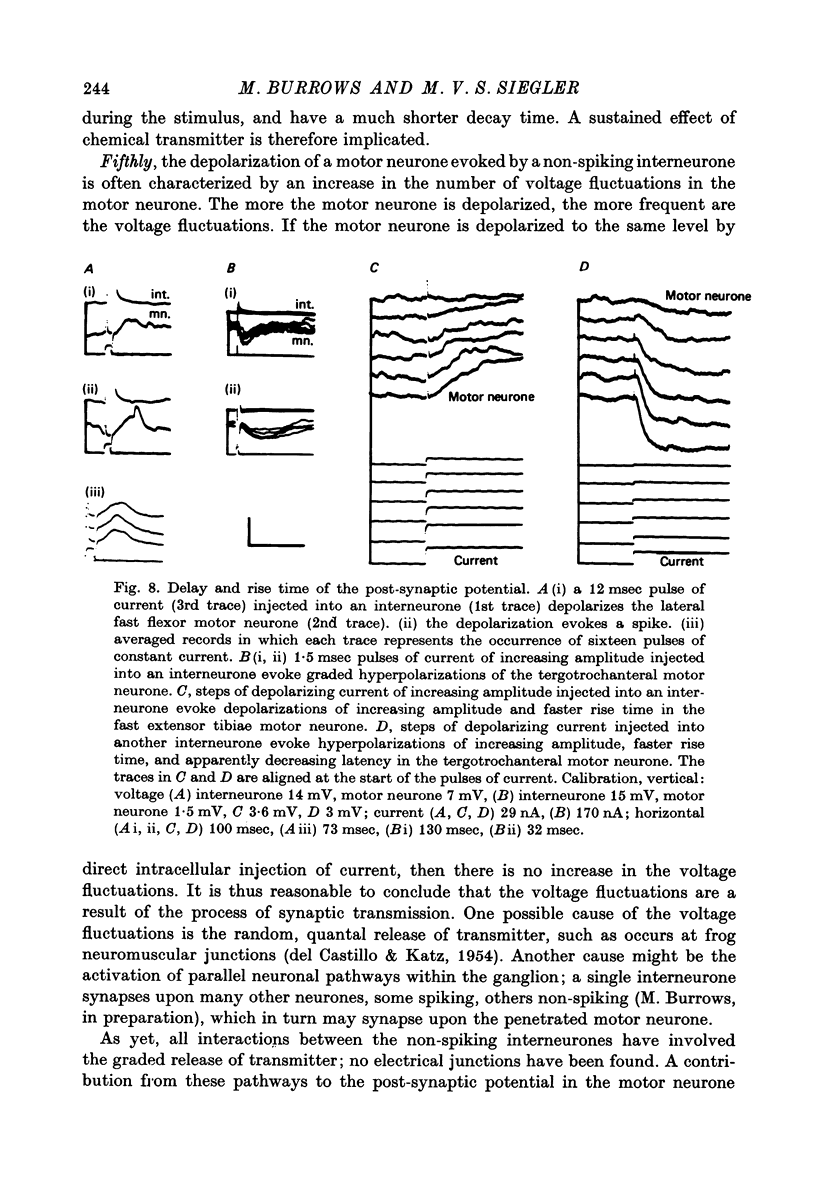
- Burrows M. Monosynaptic connexions between wing stretch receptors and flight motoneurones of the locust. J Exp Biol. 1975 Feb;62(1):189–219. doi: 10.1242/jeb.62.1.189. [DOI] [PubMed] [Google Scholar]
- Burrows M., Siegler M. V. Transmission without spikes between locust interneurones and motoneurones. Nature. 1976 Jul 15;262(5565):222–224. doi: 10.1038/262222a0. [DOI] [PubMed] [Google Scholar]
- Chappell R. L., Dowling J. E. Neural organization of the median ocellus of the dragonfly. I. Intracellular electrical activity. J Gen Physiol. 1972 Aug;60(2):121–147. doi: 10.1085/jgp.60.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Changes in end-plate activity produced by presynaptic polarization. J Physiol. 1954 Jun 28;124(3):586–604. doi: 10.1113/jphysiol.1954.sp005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitler W. J., Burrows M. The locust jump. I. The motor programme. J Exp Biol. 1977 Feb;66(1):203–219. doi: 10.1242/jeb.66.1.203. [DOI] [PubMed] [Google Scholar]
- Hengstenberg R. Spike responses of 'non-spiking' visual interneurone. Nature. 1977 Nov 24;270(5635):338–340. doi: 10.1038/270338a0. [DOI] [PubMed] [Google Scholar]
- Hoyle G., Burrows M. Neural mechanisms underlying behavior in the locust Schistocerca gregaria. I. Physiology of identified motorneurons in the metathoracic ganglion. J Neurobiol. 1973;4(1):3–41. doi: 10.1002/neu.480040104. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. R., Ringham G. L. Synaptic transfer at a vertebrate central nervous system synapse. J Physiol. 1975 Oct;251(2):409–426. doi: 10.1113/jphysiol.1975.sp011101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson M. Oscillator neurons in crustacean ganglia. Science. 1971 Mar 19;171(3976):1170–1173. doi: 10.1126/science.171.3976.1170. [DOI] [PubMed] [Google Scholar]
- Murakami M., Shimoda Y. Identification of amacrine and ganglion cells in the carp retina. J Physiol. 1977 Jan;264(3):801–818. doi: 10.1113/jphysiol.1977.sp011695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson K. G., Fourtner C. R. Nonspiking interneurons in walking system of the cockroach. J Neurophysiol. 1975 Jan;38(1):33–52. doi: 10.1152/jn.1975.38.1.33. [DOI] [PubMed] [Google Scholar]
- Pitman R. M., Tweedle C. D., Cohen M. J. Branching of central neurons: intracellular cobalt injection for light and electron microscopy. Science. 1972 Apr 28;176(4033):412–414. doi: 10.1126/science.176.4033.412. [DOI] [PubMed] [Google Scholar]
- Rall W., Shepherd G. M. Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. J Neurophysiol. 1968 Nov;31(6):884–915. doi: 10.1152/jn.1968.31.6.884. [DOI] [PubMed] [Google Scholar]
- Ralston H. J., 3rd Evidence for presynaptic dendrites and a proposal for their mechanism of action. Nature. 1971 Apr 30;230(5296):585–587. doi: 10.1038/230585a0. [DOI] [PubMed] [Google Scholar]
- Ripley S. H., Bush B. M., Roberts A. Crab muscle receptor which responds without impulses. Nature. 1968 Jun 22;218(5147):1170–1171. doi: 10.1038/2181170a0. [DOI] [PubMed] [Google Scholar]
- USHERWOOD P. N., GRUNDFEST H. PERIPHERAL INHIBITION IN SKELETAL MUSCLE OF INSECTS. J Neurophysiol. 1965 May;28:497–518. doi: 10.1152/jn.1965.28.3.497. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Regenerative amacrine cell depolarization and formation of on-off ganglion cell response. J Physiol. 1977 Jan;264(3):767–785. doi: 10.1113/jphysiol.1977.sp011693. [DOI] [PMC free article] [PubMed] [Google Scholar]


