Abstract
Lesions caused by Leishmania amazonensis normally heal, but relapses occur due to parasite persistence in host tissues. It has been proposed that infection of fibroblasts plays an important role in this process by providing the parasites with a safe haven in which to replicate. However, most previous studies have focused on the entry of Leishmania into macrophages, a process mediated by serum opsonins. To gain insight into a possible role of nonopsonic entry in the intracellular persistence of amastigotes, we examined the invasion of Chinese hamster ovary (CHO) cells. Amastigotes entered CHO cells by a cytochalasin D, genistein, wortmannin, and 2,3-butanedione monoxime-sensitive pathway and replicated within phagolysosomes. However, unlike most phagocytic processes described to date, amastigote internalization in CHO cells involved activation of the GTPases Rho and Cdc42 but not Rac-1. When uptake was mediated by fibronectin or when amastigotes were opsonized with immunoglobulin G and internalized by Fc receptor-expressing CHO cells, Rac-1 activation was restored and found to be required for parasite internalization. Given the essential role of Rac in assembly of the respiratory burst oxidase, invasion through this nonopsonic, Rac-1-independent pathway may play a central role in the intracellular survival of Leishmania in immune hosts.
Leishmaniasis affects several million people throughout the world (26). It is a serious parasitic disease transmitted by sandflies and can remain latent within the host for years. With increasing numbers of people infected with both human immunodeficiency virus and Leishmania, the need to understand how latent forms of the parasite survive in the mammalian host is becoming more critical (30, 40). Macrophages are believed to be the primary cell type supporting intracellular replication of Leishmania in the mammalian host. Accordingly, numerous studies have focused on invasion, survival, and replication of several Leishmania species within macrophages, on the spread of amastigotes from macrophage to macrophage within the mammalian host, and on the role of macrophages in the clearance of infections (2, 47). The gradual healing of Leishmania-induced lesions has been linked to a slow development of antigen-specific gamma interferon (IFN-γ) responses, which activate macrophages for intracellular killing (21, 35).
It has remained unclear how, in several clinical forms of leishmaniasis, small numbers of parasites persist in lymphoid tissue and/or the skin long after lesions are healed, giving rise to recurrent infections (43, 46). Previous studies suggested that cell types distinct from macrophages and lacking potent microbicidal responses harbor the parasites during the chronic phase of the disease (3, 10, 37, 44). Lymph node fibroblasts from mice chronically infected with Leishmania major were shown to contain replicating amastigotes and have been proposed to play an important role in persistent infection due to their inability to sustain effective inducible nitric oxide synthase-mediated killing (8).
The uptake of Leishmania amastigotes by macrophages in vivo is thought to be mediated primarily through opsonization with immunoglobulins (Igs) and complement (23, 39). Experiments performed with antibody- and FcR-deficient mice actually showed directly that IgG opsonization plays an important role in the development of Leishmania lesions in vivo (29a, 39). Recent studies revealed that ligation of the Fcγ receptor (FcR) or complement receptor 3 results in downregulation of interleukin-12 (IL-12) secretion by macrophages (34, 50). Importantly, IL-12 downregulation has been specifically linked to the slow development of cell-mediated immunity during Leishmania infections (35). Several lines of evidence therefore suggest that this slow-developing immune response would favor an initial rapid uptake of IgG- or complement-opsonized parasites by macrophages, with intracellular replication proceeding until the activation of intracellular killing mechanisms. At this point, additional cell types infected through a distinct, nonopsonic pathway might become important players by sustaining parasite replication even after the development of an effective immune response.
As a model for a nonopsonic cell entry pathway, we examined the interaction of Leishmania amazonensis amastigotes with Chinese hamster ovary (CHO) cells, previously reported to support the intracellular growth of Leishmania (53). Direct comparisons with opsonin-mediated uptake pathways were performed by following in parallel the internalization of fibronectin-coated beads or amastigotes in CHO cells and of IgG-coated amastigotes in FcR-expressing CHO cells. Surprisingly, we found that the small guanosine triphosphatase (GTPase) Rac-1 is only activated when cell entry is mediated by opsonization. Uncoated amastigotes invaded CHO cells through a unique actin-dependent, Rac-1-independent pathway that may play an important role in intracellular survival.
MATERIALS AND METHODS
Materials.
CHO-K1 cells were obtained from the American Type Culture Collection (Manassas, Va.) and grown in alpha minimal essential medium (α-MEM)-10% fetal calf serum (FCS) at 37°C and 5% CO2. The 3A.1 anti-L. amazonensis mouse monoclonal antibody (MAb) was a gift from David Sacks (Laboratory of Parasitic Diseases, National Institutes of Health), rabbit anti-fibronectin was purchased from Life Technologies (Grand Island, N.Y.), and the anti-hamster lysosome-associated membrane glycoprotein 1 (LAMP-1) UH1 MAb was developed by B. L. Granger and S. Uthayakumar and obtained from the Developmental Studies Hybridoma Bank, University of Iowa, Iowa City. Mouse anti-human vinculin antibodies, 2,3-butanedione monoxime (BDM), cytochalasin D (CD), 3-μm-diameter polystyrene latex beads, Clostridium difficile toxin B, 4,6-diamidino-2-phenylindole (DAPI), wortmannin (WM), genistein (GT), and LY294002 (LY) were purchased from Sigma Chemical Co. (St. Louis, Mo.); C3 exotoxin from Clostridium botulinum was purchased from Upstate Pharmaceuticals (Lake Placid, N.Y.); secondary antibodies, rhodamine-phalloidin, and Texas-red-conjugated dextran (molecular weight, 10,000) were purchased from Molecular Probes (Eugene, Oreg.); RGE and RGD peptides, rat fibronectin, rabbit anti-fibronectin antibodies, and α-MEM and M199 cell culture medium were purchased from Life Technologies (Rockville, Md.); antibodies to Rac-1 and Cdc42 were purchased from Santa Cruz Biotech (Santa Cruz, Calif.); and FuGENE 6 was purchased from Boehringer Mannheim (Indianapolis, Ind.).
Parasites.
Leishmania amazonensis amastigotes (strain IFLA/BR/67/PH8) harvested from infected footpads of BALB/c mice were obtained from David Sacks (Laboratory of Parasitic Diseases, National Institutes of Health) and propagated as promastigotes at 27°C in M199 medium with 5% penicillin-streptomycin, 0.1% hemin (25 mg/ml in 0.1 N NaOH), 10 mM adenine (pH 7.5), and 10% FCS. To generate amastigotes, log-phase promastigotes were diluted 1:10 in the same medium supplemented with 0.25% glucose, 0.5% Trypticase, 40 mM Na succinate (pH 5.4), and 20% FCS and incubated at 31°C. Once all parasites differentiated into amastigotes and reached late log phase, cultures were maintained by 1:10 passage dilutions at 31°C. Amastigotes were washed three times in phosphate-buffered saline (PBS) and resuspended in serum-free α-MEM before use in invasion assays. Fibronectin-coated latex beads (FNLB) were prepared by incubating 3-μm-diameter latex beads (Sigma Chemical Co.) overnight under rotation at room temperature with 200 μg of fibronectin/ml in PBS. Coating of amastigotes was done in PBS with 200 μg of fibronectin/ml or 1 μl of 3A.1 MAb ascites/ml for 60 min at 37°C. Heat killing of amastigotes was done by incubation at 56°C for 5 min. Amastigotes and FNLB were washed three times in PBS and resuspended in serum-free α-MEM for internalization assays.
Internalization assays.
CHO cells were plated at 104 cells/cm2 on 12-mm-diameter glass coverslips in 24-well dishes 24 h prior to internalization assays. FNLB were resuspended in serum-free α-MEM at 106 beads/ml, and L. amazonensis amastigotes were resuspended in serum-free α-MEM at 107/ml. For RGE/RGD assays, CHO cells in 24-well plates were incubated with 100 μM RGE or RGD peptide at 37°C for 2 h followed by three washes with PBS prior to exposure to FNLB or amastigotes. Internalization assays were performed by exposing CHO cell monolayers to 4 × 105 FNLB or 4 × 106 L. amazonensis amastigotes in 500 μl of serum-free α-MEM for the indicated times at 34°C. Coverslips were then washed three times with PBS, fixed in 2% paraformaldehyde for 60 min, and stained with either rabbit anti-fibronectin or mouse anti-L. amazonensis MAb 3A.1 for 30 min, followed by treatment with the appropriate secondary antibodies. The total number of beads or parasites associated with the cells was determined microscopically by phase contrast, and the number of internalized particles was calculated by subtracting the number of fluorescently stained, extracellularly associated beads or parasites. For each experimental point, a minimum of 200 CHO cells was analyzed in triplicate. For LAMP-1, vinculin, and actin cup staining, fixed cells were permeabilized with 0.2% Triton X-100 for 10 min and incubated with rhodamine-phalloidin and mouse anti-vinculin or mouse anti-LAMP-1 antibodies, followed by treatment with the appropriate secondary antibodies. DAPI staining was performed to discern nuclear DNA as well as amastigote kinetoplast DNA. Images were acquired on a Zeiss Axiovert 135 microscope equipped with an Orca II digital camera (Hamamatsu) controlled by Metamorph software (Universal Imaging).
Electron microscopy.
Amastigotes, FNLB, or low-density glycoprotein (LDL)-labeled gold particles were added at the time points indicated to CHO cells plated on 12-cm2 petri dishes 24 h earlier. Human LDL (density, 1.019 to 1.063 g/ml) was isolated from fresh plasma by zonal density gradient ultracentrifugation (42); gold particles of 15 (±1)-nm diameter were obtained by reduction of 0.3 mM tetrachloroauric solution with 1.2 mM sodium citrate (15), and LDL-gold complexes were prepared as previously described (25). After 24-h incubation in medium containing 10% lipoprotein-deficient serum, CHO cells were pulse labeled with 0.1 mg of LDL-gold particles/ml for 3 h at 37°C, washed, and infected with L. amazonensis amastigotes for 60 min. Infected monolayers were washed three times with PBS, fixed in 2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) for 60 min, processed for transmission electron microscopy (TEM), and photographed with a Philips 410 electron microscope operating at 80 kV.
Rho GTPase expression constructs and transfection.
Dominant negative and constitutively active mutant constructs of Cdc42 (Cdc42N17 and Cdc42L61) and Rac-1 (RacN17 and RacV12) were kindly provided by Jorge Galán, Yale University (11). Plasmids were digested and inserted into the EcoRI site of pIRES2-EGFP vector (Clontech). Endonuclease-free DNA was concentrated to 1 μg/μl and used to transfect CHO cells plated 24 h earlier. A FuGENE 6-to-DNA ratio of 3 μl to 1 μg was used for all transfection assays. Internalization assays were performed 24 h posttransfection, and transfected cells were identified based on cytosolic green fluorescent protein fluorescence.
Microinjection.
CHO cells plated on gridded coverslips (CELLocate; Eppendorf, Hamburg, Germany) were kept at 37°C on a heated microscope stage. Microinjection was performed with a 5242 microinjector and a 5170 micromanipulator (Eppendorf) using finely pulled glass capillaries (Femtotips; Eppendorf). PGEX-CRIB (Cdc42/Rac binding domain of p21-activated kinase [PAK], kindly provided by Jorge Galán, Yale University) was expressed in Escherichia coli, and CRIB-glutathione transferase (CRIB-GST) was purified as previously described (22). CRIB-GST (1 mg/ml), GST alone, or 750 μg of C3 exotoxin (Upstate Pharmaceuticals)/ml in microinjection buffer (26 mM KH2PO4, 27 mM K2HPO4, and 8 mM Na2HPO4) containing 1 mg of Texas red-dextran (molecular weight, 10,000) (Molecular Probes)/ml was microinjected into approximately 200 cells over a 30-min period. The cells were placed at 37°C and 5% CO2 for 60 min and then exposed to amastigotes or FNLB for phagocytosis assays. Microinjected cells were identified based on cytosolic Texas red-dextran fluorescence.
PAK-CRIB pull-down assays.
Purified CRIB-GST (20 μg) bound to glutathione-Sepharose beads was incubated for 60 min with 0.5 ml of clarified CHO cell lysates (lysis buffer: 20 mM Tris-HCl [pH 8], 1% Triton X-100, 500 mM NaCl, 15% glycerol, 10 mM MgCl2, and 0.5 mM dithiothreitol) after exposure to various stimuli for the indicated time periods. Beads were then washed (wash buffer: Tris-buffered saline [pH 8], 1 mM Na orthovanadate), resuspended in 2× sodium dodecyl sulfate sample buffer, and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting. Enhanced chemiluminescence (NEN Life Science, Boston, Mass.) was used for protein detection.
RESULTS
Internalization of amastigotes in CHO cells resembles classical phagocytosis.
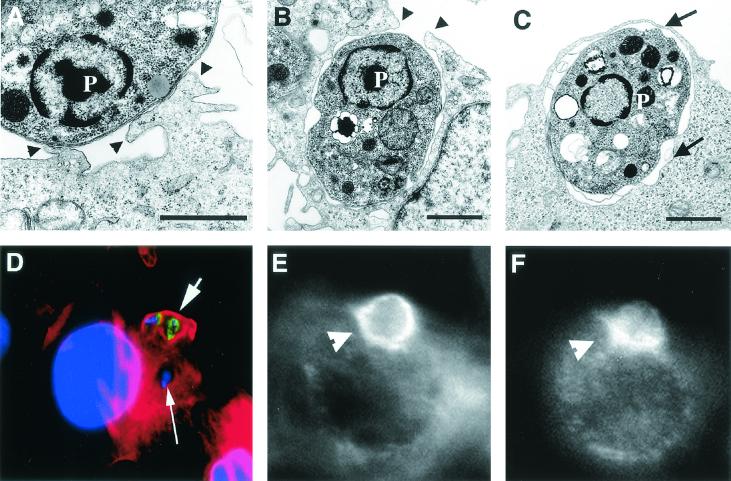
We initially examined the mechanism by which L. amazonensis amastigotes were internalized in CHO cells. To minimize the association of host molecules with the parasite's surface, we utilized axenically grown amastigotes and performed all infections in serum-free media. These axenically grown amastigotes retained the capability of causing footpad lesions in mice after several passages in culture (data not shown). After 20 min of interaction, TEM observations showed pseudopod extension at the sites of amastigote attachment and fully internalized parasites in tight vacuoles close to the cell periphery (Fig. 1A, B, and C). Phalloidin staining revealed the presence of “cups” of polymerized actin around amastigotes which were in the process of being internalized, structures no longer evident once the parasites were completely intracellular (Fig. 1D). As observed in other phagocytic processes, vinculin was also detected in association with the F-actin cups surrounding amastigotes during cell entry (Fig. 1E and F).
FIG. 1.
L. amazonensis amastigotes enter CHO cells by phagocytosis. CHO cells were exposed to 5 × 107 amastigotes/ml for 20 min, fixed, and processed for TEM. (A) Pseudopod formation at the amastigote attachment site. (B) Partially formed phagosome. (C) Complete phagosome. Arrowheads point to pseudopod extensions, and arrows point to the membrane of a recently formed phagosome. P, parasite. Sizing bars, 1 μm. (D) Digital fluorescence microscopy image of a CHO cell containing one internalized amastigote (long arrow) and with two amastigotes in the process of being phagocytosed (short arrow). The cells were fixed and stained with rhodamine-phalloidin (to reveal the cups of polymerized actin surrounding the parasites), DAPI (to reveal the CHO and amastigote nuclei), and fluorescein isothiocyanate-labeled anti-L. amazonensis MAb (to reveal extracellular portions of the parasites). (E) Rhodamine-phalloidin staining of an infected CHO cell, showing the F-actin cup surrounding an amastigote (arrowhead). (F) Same cell (shown in panel E) stained with anti-vinculin antibodies, showing vinculin associated with the phagocytic cup (arrowhead).
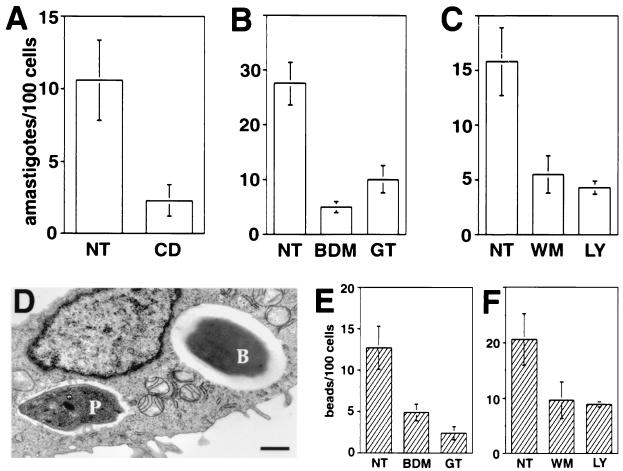
Because the morphology of the internalization of amastigotes in CHO cells resembled that of a classical phagocytic event, we asked whether known inhibitors of phagocytosis would affect parasite uptake. We found that amastigote entry was strongly inhibited when CHO cells were pretreated with the microfilament-disrupting agent CD, the myosin inhibitor BDM, the tyrosine kinase inhibitor GT, and the phosphatidylinositol 3-kinase inhibitors WM and LY (Fig. 2A, B, and C). In parallel experiments, we examined the effect of the same agents on the uptake of FNLB, a well-characterized phagocytic process mediated by β1 integrins (36, 52). Latex beads of similar size and shape to Leishmania amastigotes were chosen for these experiments (Fig. 2D), and their internalization in CHO cells was also blocked by CD (data not shown), BDM, GT (Fig. 2E), and WM or LY (Fig. 2F). We thus conclude that several hallmarks of the entry of L. amazonensis amastigotes into CHO cells resemble those of classical phagocytosis.
FIG. 2.
Agents that block phagocytosis inhibit internalization of FNLB and L. amazonensis amastigotes in CHO cells. NT, not treated. (A) Number of intracellular parasites in cells not treated or treated with 10 μM CD. (B) Number of intracellular parasites in cells not treated or treated with 25 mM BDM or 20 μM GT. (C) Number of intracellular parasites in cells not treated or treated with 100 nM WM or 100 μM LY. CHO cells were pretreated with the indicated drugs for 20 min, followed by exposure to amastigotes for 60 min. (D) TEM image of a CHO cell containing an amastigote and an FNLB. CHO cells were simultaneously exposed to FNLB and amastigotes for 60 min. P, parasite; B, FNLB. Sizing bar, 1 μm. (E) Number of intracellular FNLB in CHO cells not treated or treated with BDM or GT as described for panel B. (F) Number of intracellular FNLB in CHO cells not treated or treated with WM or LY as described for panel C. CHO cells were pretreated with the indicated drugs for 20 min, followed by exposure to FNLB for 60 min. The data represent the means and standard deviations (SD) of triplicate experiments.
Amastigotes target to and replicate within lysosomal compartments of CHO cells.
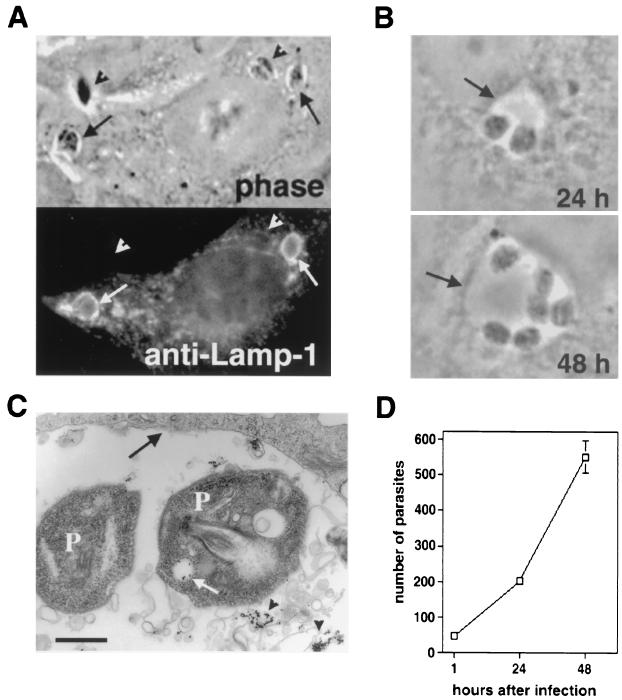
When Leishmania amastigotes are phagocytosed by macrophages, phagosomes fuse with lysosomes, generating a parasitophorous vacuole with lysosomal properties whereby the parasites replicate throughout their intracellular cycle (4, 14, 45). We therefore verified whether this same process occurred in CHO cells after amastigote internalization. Shortly after entry (60 min), amastigotes were found in vesicular compartments containing the lysosomal glycoprotein LAMP-1 (Fig. 3A). After increasing periods of incubation at 34°C, parasites were found to reside inside large phagolysosomes (Fig. 3B) that accumulated endocytosed LDL-gold particles (Fig. 3C), a marker for lysosomal trafficking. Amastigotes replicated within these compartments (Fig. 3B and D), and LDL-gold particles were observed inside vesicular compartments of the amastigotes (Fig. 3C, white arrow), an indication that the parasites were fully viable and capable of active endocytosis.
FIG. 3.
L. amazonensis replicates within phagolysosomes in CHO cells. (A) Phase-contrast image (top) and immunofluorescence staining with anti-LAMP-1 MAbs (bottom) of CHO cells infected with L. amazonensis amastigotes. Arrows point to intracellular parasites, and arrowheads point to extracellularly attached parasites. (B) Phagolysosome containing replicating amastigotes at 24 (top) and 48 (bottom) h after infection. (C) TEM showing amastigotes replicating in a phagolysosome containing LDL-gold particles 24 h after infection. Arrowheads point to LDL-gold particles in the lumen of the phagolysosome, the white arrow points to LDL-gold particles internalized by one of the parasites, and the black arrow points to the phagolysosome membrane. P, parasite. Sizing bar, 1 μm. (D) Intracellular growth curve of L. amazonensis in CHO cells. Cells were exposed to amastigotes for 60 min, washed, and further incubated at 34°C for 24 and 48 h. After fixation, the total number of intracellular parasites per five microscopic fields (×100) was determined in triplicate (the data represent the averages ± SD of triplicate experiments).
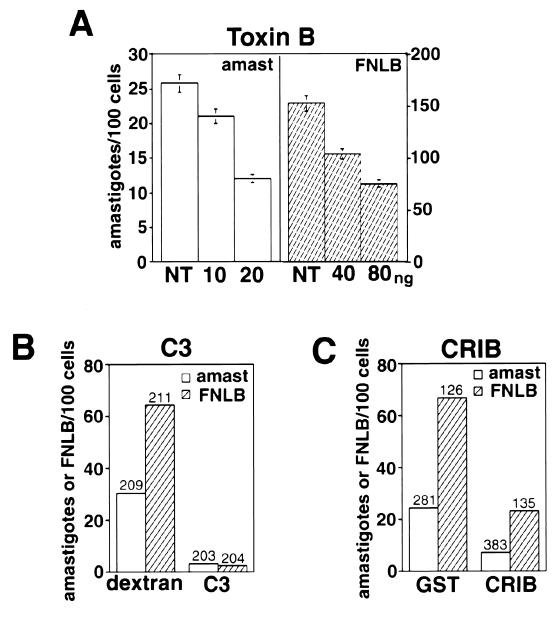
Rho GTPases are necessary for amastigote internalization in CHO cells. Members of the Rho family of small GTPases are known to be involved in the reorganization of F-actin in response to a variety of stimuli, including phagocytosis (12, 24, 38). We found that pretreatment of CHO cells with C. difficile toxin B, an inhibitor of all members of the Rho family, inhibited the uptake of both L. amazonensis amastigotes and FNLB (Fig. 4A). And as previously reported for complement (9) and IgG-mediated phagocytosis (12), the Rho inhibitor C3 transferase strongly blocked uptake of amastigotes and FNLB after microinjection into CHO cells (Fig. 4B). Microinjection of the CRIB domain of PAK protein (which specifically binds Cdc42 and Rac in their active GTP-bound states) (6, 51) fused to GST also significantly reduced amastigote and FNLB entry, indicating a requirement for activated Cdc42 and/or Rac-1 (Fig. 4C). All invasion assays were performed utilizing parasite concentrations and interaction periods, resulting in internalization values within the linear range previously determined from time course-multiplicity of infection experiments (unpublished data).
FIG. 4.
Nonopsonic cell entry by L. amazonensis requires activation of Rho GTPases. (A) Number of intracellular amastigotes (amast) or FNLB in cells not treated (NT) or treated with the indicated amounts of toxin B/ml for 60 min. After microinjection, CHO cells were exposed to amastigotes or FNLB for 60 min at 34°C and fixed and the number of internalized particles was determined in triplicate (the data correspond to the means and SD of triplicate experiments). (B) Number of intracellular amastigotes or FNLB in cells microinjected with dextran alone or with C3 exotoxin. (C) Number of intracellular amastigotes or FNLB in cells microinjected with GST alone or with CRIB-GST. After microinjection, CHO cells were exposed to amastigotes or FNLB for 60 min at 34°C and fixed and the number of internalized particles was determined in the microinjected cells (the numbers above the columns indicate the total number of microinjected cells analyzed).
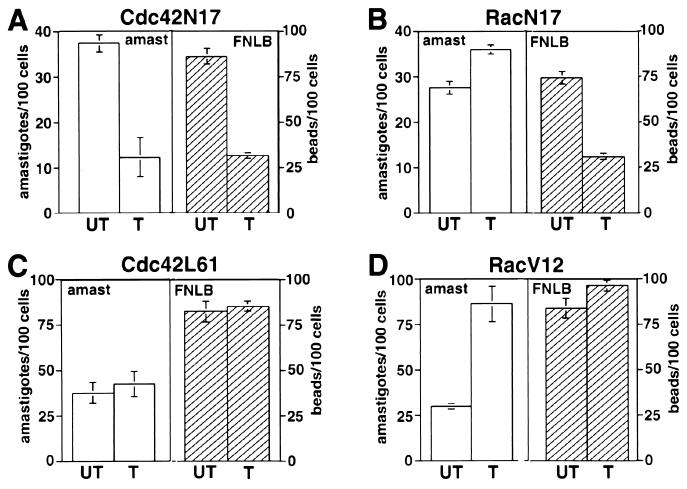
Cdc42 activation, but not Rac-1 activation, is necessary for amastigote internalization.
Consistent with the findings described above, expression in CHO cells of the dominant-negative Cdc42 construct Cdc42N17 (9) inhibited the internalization of both FNLB and amastigotes (Fig. 5A) (similar numbers of intracellular amastigotes were consistently observed in CHO cells not transfected or transfected with a plasmid encoding green fluorescent protein alone [data not shown]). Surprisingly, however, the dominant-negative Rac-1 construct RacN17 inhibited the uptake of FNLB but not of L. amazonensis amastigotes (Fig. 5B). When the effects of the presence of constitutively active Cdc42 (Cdc42L61) and Rac-1 (RacV12) constructs were analyzed, a marked difference was again observed: the presence of Cdc42L61 did not affect uptake of either FNLB or amastigotes (Fig. 5C) but that of the equivalent, constitutively active RacV12 construct specifically enhanced amastigote entry (Fig. 5D). This enhanced uptake probably reflects the stimulation in membrane ruffling and lamellipodium formation that is caused by the presence of activated Rac in many cell types (38). These findings thus suggest that the internalization of L. amazonensis amastigotes in CHO cells normally does not involve Rac-1 activation.
FIG. 5.
Nonopsonic cell entry by L. amazonensis requires activation of Cdc42 but not of Rac-1. (A) Number of intracellular amastigotes or FNLB in cells not transfected (UT) or transfected with Cdc42N17. (B) Number of intracellular amastigotes or FNLB in cells not transfected (UT) or transfected with RacN17. (C) Number of intracellular amastigotes or FNLB in cells not transfected (UT) or transfected with Cdc42L61. (D) Number of intracellular amastigotes or FNLB in cells not transfected (UT) or transfected with RacV12. Transfected CHO cells were exposed to amastigotes or FNLB for 60 min at 34°C and fixed, and the number of internalized particles was determined in triplicate (the data correspond to the means and SD of triplicate experiments).
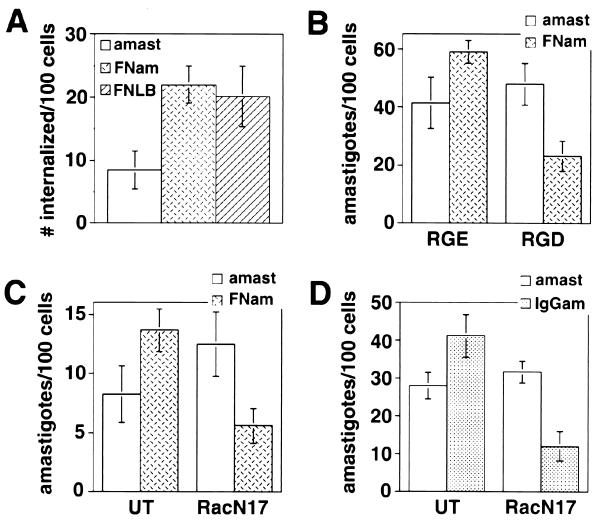
Opsonization with fibronectin or IgG targets amastigotes to a Rac-1-dependent internalization pathway.
We found that heat-killed L. amazonensis amastigotes are not internalized by CHO cells (unpublished data), suggesting that unique properties of live L. amazonensis amastigotes might be responsible for directing their entry towards a Rac-1-independent invasion pathway. To directly investigate this possibility, we verified whether altering the ligands involved in amastigote internalization could modulate the requirement for Rac-1 activation. When L. amazonensis amastigotes were coated with fibronectin, their internalization was enhanced to the same levels normally observed with FNLB (Fig. 6A). The RGD peptide, a specific inhibitor of β1 integrin-mediated interactions, did not alter the internalization of uncoated amastigotes but markedly reduced the uptake of fibronectin-coated amastigotes (Fig. 6B). A shift of fibronectin-coated amastigotes towards a β1 integrin-mediated uptake pathway was confirmed when the effect of the presence of dominant-negative RacN17 construct was examined: internalization of fibronectin-coated amastigotes was now inhibited in cells transfected with RacN17 (Fig. 6C). This result demonstrates that the presence of an alternative ligand-receptor pair is sufficient to alter the requirement for Rac-1 activation in L. amazonensis uptake, and suggests that soluble factors secreted by the parasites do not play a dominant role. This conclusion is reinforced by the finding that ATP depletion in amastigotes (60-min treatment with 5 mM sodium azide and 50 mM 2-deoxyglucose), a condition known to block secretion, had no effect on the uptake of amastigotes by CHO cells (unpublished data).
FIG. 6.
Rac-1 activation is required for opsonin-mediated uptake of L. amazonensis. (A) Number of intracellular amastigotes (amast), fibronectin-coated amastigotes (FNam), or FNLB in CHO cells incubated at 4°C with amastigotes or FNLB for 30 min, followed by incubation at 34°C for 30 min. (B) Number of amastigotes or fibronectin-coated amastigotes internalized in the presence of 100 μM RGE or RGD peptides (Life Technologies). CHO cells were exposed to amastigotes for 60 min at 34°C. (C) Number of amastigotes or fibronectin-coated amastigotes internalized in cells not transfected (UT) or transfected with RacN17. Transfected CHO cells were exposed to amastigotes for 60 min at 34°C. (D) Number of amastigotes or IgG-coated amastigotes (IgGam) internalized in FcRII-B2 cells not transfected (UT) or transfected with RacN17. FcRII-B2-transfected CHO cells were exposed to amastigotes for 60 min at 34°C. After fixation, the number of internalized particles was determined in triplicate (the data correspond to the means and SD of triplicate experiments).
To verify whether a distinct ligand-receptor pair was also capable of redirecting the parasites towards a Rac-1-dependent entry pathway, amastigotes were coated with a MAb specific for L. amazonensis glycoconjugates (16) and incubated with CHO cells stably transfected with the mFcRII isoform of the Fc receptor (FcRII-B2) (28). Antibody coating, as expected, enhanced amastigote entry into FcRII-CHO cells and shifted their uptake into a pathway sensitive to inhibition by the dominant-negative RacN17 construct (Fig. 6D). However, coating with IgG did not inhibit amastigote entry into wild-type CHO cells (unpublished data), indicating that the endogenous uptake pathway was not blocked but was overridden when IgG receptors were available. Consistent with these observations, at least 60% of Leishmania mexicana amastigotes recovered from lesions were reported to have surface-bound IgG molecules but could still infect cultured COS cells and replicate intracellularly (39).
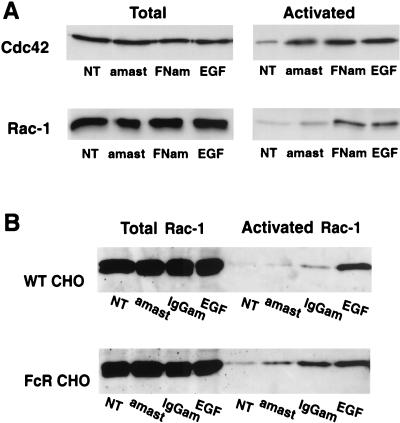
Rac-1 is not activated during the internalization of nonopsonized amastigotes in CHO cells.
The results described above strongly suggested that the cell entry pathway of nonopsonized L. amazonensis amastigotes involved the activation of Cdc42 but not of Rac-1. To directly investigate this issue, we determined the levels of GTP-bound Cdc42 and Rac-1 in CHO cells infected with amastigotes by assaying the ability of the endogenous GTPases to bind to GST-CRIB. As previously mentioned, PAK-CRIB will only bind Cdc42 or Rac-1 in their activated, GTP-bound forms (7, 51). Cell lysates were incubated with GST-CRIB coupled to Sepharose beads, and the total cell lysate fractions and bound fractions were probed for Cdc42 or Rac-1 by Western blotting. As shown in Fig. 7, cells not treated, cells infected with either uncoated or fibronectin-coated amastigotes, and cells treated with epidermal growth factor (EGF) (a known activator of the Rac-1 and Cdc42 pathways) contained similar amounts of total Cdc42 and Rac-1 (Fig. 7A, left panels). However, a marked difference was detected between the levels of activated GTP-bound Cdc42 and Rac-1 (Fig. 7A, right panels). Whereas both GTPases were activated when CHO cells were exposed to EGF and to fibronectin-coated amastigotes, only Cdc42 was markedly activated during the uptake of uncoated amastigotes. GTP-bound Rac-1 was detected only in small amounts above background in CHO cells infected with uncoated amastigotes, and this pattern was shifted to a full activation when the amastigotes were coated with fibronectin (Fig. 7A, lower right panel). The small increase (∼20%) in the levels of activated Cdc42 in cells infected with fibronectin-coated versus uncoated amastigotes probably reflects the more efficient parasite internalization observed under these conditions (Fig. 6). In contrast, the increase in the levels of activated Rac-1 in cells infected with fibronectin-coated amastigotes was much higher (on the order of 200%), reinforcing the interpretation that Rac-1 is only minimally activated during the entry of amastigotes via a nonopsonization-dependent pathway. Importantly, very similar results were obtained when amastigotes were opsonized with IgG and internalized by CHO cells expressing or not expressing the FcRII-B2 receptor. Whereas a partial elevation (∼50%) in the levels of activated Rac-1 was observed in wild-type CHO cells infected with IgG-coated amastigotes (Fig. 7B, upper panel), a much more vigorous, 500% increase in Rac-1 activation was observed in FcR-transfected cells infected with antibody-opsonized parasites (Fig. 7B, lower panel).
FIG. 7.
Nonopsonic cell entry by L. amazonensis does not trigger Rac-1 activation. The total endogenous levels of Cdc42 and Rac-1 were determined by Western blotting in lysates of CHO cells. The levels of activated (GTP-bound) forms of the same GTPases were determined according to their ability to bind GST-CRIB coupled to glutathione-Sepharose beads. (A) Total and activated Cdc42 and Rac-1 in wild-type CHO cells. (B) Total and activated Rac-1 in wild-type (WT CHO) and FcR-transfected (FcR CHO) cells. NT, no treatment; amast, uncoated amastigotes; FNam, fibronectin-coated amastigotes; IgGam, IgG-coated amastigotes; EGF, EGF treated.
DISCUSSION
In this work we have identified an important difference between opsonic and nonopsonic pathways leading to the internalization of L. amazonensis amastigotes in host cells. Consistent with the results of previous studies in several phagocytic systems (12), we found that the uptake of fibronectin or IgG-opsonized amastigotes by CHO cells requires activation of the small GTPases Rho, Cdc42, and Rac-1. Remarkably, however, we found that nonopsonized amastigotes enter these cells by a phagocyte-like, actin-mediated process that does not involve Rac-1 activation.
Although heparin-binding moieties have been proposed to be necessary for the attachment of Leishmania amastigotes to CHO cells (33), the receptor promoting internalization of nonopsonized parasites into these cells is still unknown. β1 integrin receptors do not appear to be involved in this unique, Rac-1-independent amastigote invasion pathway, since beads or amastigotes coated with fibronectin are clearly shifted to an invasion pathway that requires activation of Rac-1 in addition to that of Rho and Cdc42. These results are consistent with those of previous studies showing that RhoA, Rac-1, and Cdc42 are activated during cytoskeletal reorganization events triggered by integrin-mediated adhesion to the extracellular matrix (20).
The identification of a Rac-1-independent cell entry pathway has important implications for our understanding of the intracellular survival strategy of L. amazonensis and its persistence in the tissues of immune hosts. Several recent studies established that Rac, together with the cytosolic components p47phox and p67phox and the membrane-associated flavocytochrome b558 (gp91phox), is essential for assembly of the multicomponent respiratory burst oxidase on phagosome membranes (1, 54). Although the exact mechanism by which Rac regulates activation of the NADPH oxidase is still unclear, recent data suggest that Rac may act directly on flavocytochrome b558, facilitating its binding to p67phox and thereby promoting completion of electron transfer and superoxide formation (17). Importantly, it is known that superoxide and other toxic oxygen metabolites play a central role in host defense during the early stages of infection with Leishmania, as demonstrated by the markedly increased parasite load observed in gp91phox-deficient mice (X-linked chronic granulomatous disease) (37a).
Rac-2 appears to be the major isoform involved in NADPH oxidase activation in phagocytes (18), but several lines of evidence increasingly point to the involvement of Rac-1 in superoxide production in “nonprofessional” phagocytic cells (27, 29, 49). A homologue of gp91phox, Mox-1, was detected in several tissues and shown to mediate the production of reactive oxygen species (48). Although little is known about additional ubiquitously expressed oxidase components, additional gp91phox homologues are expressed in several superoxide-producing cells such as fibroblasts and endothelial, kidney, and thyroid cells (17). Of direct relevance to our findings, oxidative activity has been detected in primary fibroblasts during internalization of collagen and IgG-coated beads in cutaneous wounds (5) and in CHO cells during activation of the p38-mitogen-activated protein kinase cascade (41).
The fact that both professional and nonprofessional phagocytic cells seem capable of generating superoxide and other oxidase-dependent reactive oxygen species directly suggests that parasite intracellular survival depends on a mechanism for bypassing Rac-1 activation. Our findings suggest that the lack of opsonin receptors in host cells may be the key for this survival strategy. Leishmania amastigotes are known to be opsonized with IgG in vivo (29a, 39), and our results show that IgG-coated amastigotes enter cells lacking Fc receptors without triggering Rac-1 activation. In contrast, in a situation that mimics the interaction with macrophages in vivo, entry of these parasites in FcR-expressing CHO cells requires Rac-1 activation. One can therefore envision a scenario in which, even after development of an effective microbicidal response in macrophages, IgG-opsonized parasites would survive when internalized in fibroblasts or other cell types lacking receptors for IgG or for other serum opsonins such as complement.
Our studies did not detect a significant difference in intracellular replication between IgG-coated and uncoated amastigotes after internalization in FcR-expressing CHO cells (unpublished data). This is not entirely surprising, since the capacity of nonphagocytic cells to generate superoxide and other toxic oxygen metabolites is likely to be subject to regulation by cytokines generated in vivo during Leishmania infections. A recent study showed that IL-10, in particular, plays an essential role in promoting persistence of L. major in vivo, possibly by reducing the sensitivity of host cells to IFN-γ activation for intracellular killing (7a). It is still not clear whether IL-10 can specifically modulate the intracellular killing capacity of fibroblasts, but compared to macrophages, fibroblasts activated by IFN-γ and other cytokines have a reduced ability to express the type 2 nitric oxide synthase and to kill intracellular L. major (14, 54). It is thus conceivable that the Rac-1-independent invasion pathway we identified here, which is active in cells lacking opsonin receptors, may promote parasite survival by preventing oxidase activation in a background of already markedly reduced NO production. Under conditions favoring NO production, however, intracellular killing may still occur, explaining the sterile cure of L. major infections achieved in the absence of IL-10 or in the presence of antibodies against its receptor (7a). The likelihood of this scenario is reinforced by the demonstration that L. major organisms residing in fibroblasts are susceptible to killing mediated by NO produced by neighboring macrophages (14).
In this study, we also showed that after uptake, L. amazonensis amastigotes reside in large phagolysosomal compartments, where they remain viable and undergo several rounds of replication. These findings are consistent with previous observations of L. amazonensis infections in CHO cells and of L. mexicana infections in COS cells. Although the entry mechanism and the intracellular compartment where the parasites resided were not fully characterized in these previous studies, intracellular survival and replication of amastigotes were reported (39). Our findings thus reinforce the view that, although macrophages and FcR-mediated invasion clearly play a central role in the establishment of Leishmania infections in vivo (17), these parasites are also capable of establishing replicative compartments in cells devoid of classical phagocytic receptors.
In contrast to what has been previously described for opsonin-mediated phagocytosis (12) and for the uptake of several intracellular bacteria (13, 19, 31, 32), our results show that nonopsonized L. amazonensis amastigotes enter CHO cells without triggering significant levels of Rac-1 activation. Our studies also demonstrate that antibody-coated parasites can efficiently enter FcR-deficient cells in a Rac-1-independent fashion, an important finding considering that amastigotes present in lesions in vivo are likely to be opsonized with IgG (29a, 39). The importance of Rac-1 in the activation of oxidative killing mechanisms points directly to this unique invasion pathway of Leishmania as a possible strategy for intracellular survival in immune hosts. Our observations (unpublished data) and those of others (28) indicate that the FcR-expressing CHO cells do not produce a detectable oxidative burst during the uptake of antibody-coated parasites; therefore, further studies involving primary cells and the establishment of Leishmania infections in vivo will be required to test this hypothesis directly.
Acknowledgments
We are very grateful to J. Galán and B. Kazmierczak for reagents and advice with GTPase experiments, D. Sacks for Leishmania strains and reagents, J. Carlyon for help with superoxide detection experiments, K. Joiner and D. McMahon-Pratt for thoughtful input, B. Burleigh and A. Rodríguez for critical reading of the manuscript, members of the Andrews lab for helpful suggestions, and H. Tan for excellent technical assistance.
This work was supported by an NIH K08-AI01584-02 grant to J.M. and by an NIH grant and a Burroughs Wellcome Scholar award to N.W.A.
Editor: J. M. Mansfield
REFERENCES
- 1.Abo, A., M. R. Webb, A. Grogan, and A. W. Segal. 1994. Activation of NADPH oxidase involves the dissociation of p21rac from its inhibitory GDP/GTP exchange protein (rhoGDI) followed by its translocation to the plasma membrane. Biochem. J. 298:585-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander, J., A. R. Satoskar, and D. G. Russell. 1999. Leishmania species: models of intracellular parasitism. J. Cell Sci. 112:2993-3002. [DOI] [PubMed] [Google Scholar]
- 3.Andrade, Z. A., S. G. Reed, S. B. Roters, and M. Sadigursky. 1984. Immunopathology of experimental cutaneous leishmaniasis. Am. J. Pathol. 114:137-148. [PMC free article] [PubMed] [Google Scholar]
- 4.Antoine, J. C., E. Prina, T. Lang, and N. Courret. 1998. The biogenesis and properties of the parasitophorous vacuoles that harbour Leishmania in murine macrophages. Trends Microbiol. 6:392-401. [DOI] [PubMed] [Google Scholar]
- 5.Arlein, W. J., J. D. Shearer, and M. D. Caldwell. 1998. Continuity between wound macrophage and fibroblast phenotype: analysis of wound fibroblast phagocytosis. Am. J. Physiol. 275:R1041-R1048. [DOI] [PubMed] [Google Scholar]
- 6.Bagrodia, S., S. J. Taylor, C. L. Creasy, J. Chernoff, and R. A. Cerione. 1995. Identification of a mouse p21Cdc42/Rac activated kinase. J. Biol. Chem. 270:22731-22737. [DOI] [PubMed] [Google Scholar]
- 7.Bagrodia, S., S. J. Taylor, K. A. Jordon, L. Van Aelst, and R. A. Cerione. 1998. A novel regulator of p21-activated kinases. J. Biol. Chem. 273:23633-23636. [DOI] [PubMed] [Google Scholar]
- 7a.Belkaid, Y., K. F. Hoffmann, S. Mendez, S. Kamhawi, M. C. Udey, T. A. Wynn, and D. L. Sacks. 2001. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 194:1497-1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogdan, C., N. Donhauser, R. Doring, M. Rollinghoff, A. Diefenbach, and M. G. Rittig. 2000. Fibroblasts as host cells in latent leishmaniosis. J. Exp. Med. 191:2121-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caron, E., and A. Hall. 1998. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282:1717-1721. [DOI] [PubMed] [Google Scholar]
- 10.Chang, K. P. 1981. Leishmanicidal mechanisms of human polymorphonuclear phagocytes. Am. J. Trop. Med. Hyg. 30:322-333. [DOI] [PubMed] [Google Scholar]
- 11.Chen, L. M., S. Hobbie, and J. E. Galan. 1996. Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science 274:2115-2118. [DOI] [PubMed] [Google Scholar]
- 12.Chimini, G., and P. Chavrier. 2000. Function of Rho family proteins in actin dynamics during phagocytosis and engulfment. Nat. Cell Biol. 2:E191-E196. [DOI] [PubMed] [Google Scholar]
- 13.Churin, Y., E. Kardalinou, T. F. Meyer, and M. Naumann. 2001. Pathogenicity island-dependent activation of Rho GTPases Rac1 and Cdc42 in Helicobacter pylori infection. Mol. Microbiol. 40:815-823. [DOI] [PubMed] [Google Scholar]
- 14.Collins, H. L., U. E. Schaible, J. D. Ernst, and D. G. Russell. 1997. Transfer of phagocytosed particles to the parasitophorous vacuole of Leishmania mexicana is a transient phenomenon preceding the acquisition of annexin I by the phagosome. J. Cell Sci. 110:191-200. [DOI] [PubMed] [Google Scholar]
- 15.Coppens, I., F. R. Opperdoes, P. J. Courtoy, and P. Baudhuin. 1987. Receptor-mediated endocytosis in the bloodstream form of Trypanosoma brucei. J. Protozool. 34:465-473. [DOI] [PubMed] [Google Scholar]
- 16.Courret, N., E. Prina, E. Mougneau, E. M. Saraiva, D. L. Sacks, N. Glaichenhaus, and J. C. Antoine. 1999. Presentation of the Leishmania antigen LACK by infected macrophages is dependent upon the virulence of the phagocytosed parasites. Eur. J. Immunol. 29:762-773. [DOI] [PubMed] [Google Scholar]
- 17.Diebold, B. A., and G. M. Bokoch. 2001. Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat. Immunol. 2:211-215. [DOI] [PubMed] [Google Scholar]
- 18.Dorseuil, O., L. Reibel, G. M. Bokoch, J. Camonis, and G. Gacon. 1996. The Rac target NADPH oxidase p67phox interacts preferentially with Rac2 rather than Rac1. J. Biol. Chem. 271:83-88. [DOI] [PubMed] [Google Scholar]
- 19.Galan, J. E., and D. Zhou. 2000. Striking a balance: modulation of the actin cytoskeleton by Salmonella. Proc. Natl. Acad. Sci. USA 97:8754-8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giancotti, F. G., and E. Ruoslahti. 1999. Integrin signaling. Science 285:1028-1032. [DOI] [PubMed] [Google Scholar]
- 21.Green, S. J., R. M. Crawford, J. T. Hockmeyer, M. S. Meltzer, and C. A. Nacy. 1990. Leishmania major amastigotes initiate the l-arginine-dependent killing mechanism in IFN-γ-stimulated macrophages by induction of tumor necrosis factor-alpha. J. Immunol. 145:4290-4297. [PubMed] [Google Scholar]
- 22.Guan, K. L., and J. E. Dixon. 1991. Evidence for protein-tyrosine-phosphatase catalysis proceeding via a cysteine-phosphate intermediate. J. Biol. Chem. 266:17026-17030. [PubMed] [Google Scholar]
- 23.Guy, R. A., and M. Belosevic. 1993. Comparison of receptors required for entry of Leishmania major amastigotes into macrophages. Infect. Immun. 61:1553-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hackam, D. J., O. D. Rotstein, A. Schreiber, W. Zhang, and S. Grinstein. 1997. Rho is required for the initiation of calcium signaling and phagocytosis by Fcγ receptors in macrophages. J. Exp. Med. 186:955-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Handley, D. A., C. M. Arbeeny, L. D. Witte, and S. Chien. 1981. Colloidal gold—low density lipoprotein conjugates as membrane receptor probes. Proc. Natl. Acad. Sci. USA 78:368-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herwaldt, B. L. 1999. Leishmaniasis. Lancet 354:1191-1199. [DOI] [PubMed] [Google Scholar]
- 27.Irani, K., Y. Xia, J. L. Zweier, S. J. Sollott, C. J. Der, E. R. Fearon, M. Sundaresan, T. Finkel, and P. J. Goldschmidt-Clermont. 1997. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science 275:1649-1652. [DOI] [PubMed] [Google Scholar]
- 28.Joiner, K. A., S. A. Fuhrman, H. M. Miettinen, L. H. Kasper, and I. Mellman. 1990. Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc receptor-transfected fibroblasts. Science 249:641-646. [DOI] [PubMed] [Google Scholar]
- 29.Joneson, T., M. McDonough, D. Bar-Sagi, and L. Van Aelst. 1996. RAC regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase. Science 274:1374-1376. [DOI] [PubMed] [Google Scholar]
- 29a.Kima, P. E., S. L. Constant, L. Hannum, M. Colmenares, K. S. Lee, A. M. Haberman, J. Shlomchik, and D. McMahon-Pratt. 2000. Internalization of Leishmania mexicana complex via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J. Exp. Med. 191:1063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kubar, J., P. Marty, A. Lelievre, J. F. Quaranta, P. Staccini, C. Caroli-Bosc, and Y. Le Fichoux. 1998. Visceral leishmaniosis in HIV-positive patients: primary infection, reactivation and latent infection. Impact of the CD4+ T-lymphocyte counts. AIDS 12:2147-2153. [DOI] [PubMed] [Google Scholar]
- 31.Lee, D. J., D. Cox, J. Li, and S. Greenberg. 2000. Rac1 and Cdc42 are required for phagocytosis, but not NF-κB-dependent gene expression, in macrophages challenged with Pseudomonas aeruginosa. J. Biol. Chem. 275:141-146. [DOI] [PubMed] [Google Scholar]
- 32.Linder, S., C. Heimerl, V. Fingerle, M. Aepfelbacher, and B. Wilske. 2001. Coiling phagocytosis of Borrelia burgdorferi by primary human macrophages is controlled by CDC42Hs and Rac1 and involves recruitment of Wiskott-Aldrich syndrome protein and Arp2/3 complex. Infect. Immun. 69:1739-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Love, D. C., J. D. Esko, and D. M. Mosser. 1993. A heparin-binding activity on Leishmania amastigotes which mediates adhesion to cellular proteoglycans. J. Cell Biol. 123:759-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marth, T., and B. L. Kelsall. 1997. Regulation of interleukin-12 by complement receptor 3 signaling. J. Exp. Med. 185:1987-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDowell, M. A., and D. L. Sacks. 1999. Inhibition of host cell signal transduction by Leishmania: observations relevant to the selective impairment of IL-12 responses. Curr. Opin. Microbiol. 2:438-443. [DOI] [PubMed] [Google Scholar]
- 36.McKeown, M., G. Knowles, and C. A. McCulloch. 1990. Role of the cellular attachment domain of fibronectin in the phagocytosis of beads by human gingival fibroblasts in vitro. Cell Tissue Res. 262:523-530. [DOI] [PubMed] [Google Scholar]
- 37.Moll, H., S. Flohe, and C. Blank. 1995. Dendritic cells seclude Leishmania parasites that persist in cured mice—a role in the maintenance of T-cell memory? Adv. Exp. Med. Biol. 378:507-509. [DOI] [PubMed] [Google Scholar]
- 37a.Murray, H. W., and C. F. Nathan. 1999. Macrophage microbicidal mechanisms in vivo: reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J. Exp. Med. 189:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nobes, C. D., and A. Hall. 1995. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81:53-62. [DOI] [PubMed] [Google Scholar]
- 39.Peters, C., T. Aebischer, Y. D. Stierhof, M. Fuchs, and P. Overath. 1995. The role of macrophage receptors in adhesion and uptake of Leishmania mexicana amastigotes. J. Cell Sci. 108:3715-3724. [DOI] [PubMed] [Google Scholar]
- 40.Pineda, J. A., J. A. Gallardo, J. Macias, J. Delgado, C. Regordan, F. Morillas, F. Relimpio, J. Martin-Sanchez, A. Sanchez-Quijano, M. Leal, and E. Lissen. 1998. Prevalence of and factors associated with visceral leishmaniasis in human immunodeficiency virus type 1-infected patients in southern Spain. J. Clin. Microbiol. 36:2419-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pomerance, M., H. B. Abdullah, S. Kamerji, C. Correze, and J. P. Blondeau. 2000. Thyroid-stimulating hormone and cyclic AMP activate p38 mitogen-activated protein kinase cascade. Involvement of protein kinase A, rac1, and reactive oxygen species. J. Biol. Chem. 275:40539-40546. [DOI] [PubMed] [Google Scholar]
- 42.Poumay, Y., and M. F. Ronveaux-Dupal. 1985. Rapid preparative isolation of concentrated low density lipoproteins and of lipoprotein-deficient serum using vertical rotor gradient ultracentrifugation. J. Lipid Res. 26:1476-1480. [PubMed] [Google Scholar]
- 43.Ramirez, J. L., and P. Guevara. 1997. Persistent infections by Leishmania (Viannia) braziliensis. Mem. Inst. Oswaldo Cruz 92:333-338. [DOI] [PubMed] [Google Scholar]
- 44.Ridley, D. S. 1980. A histological classification of cutaneous leishmaniasis and its geographical expression. Trans. R. Soc. Trop. Med. Hyg. 74:515-521. [DOI] [PubMed] [Google Scholar]
- 45.Russell, D. G., S. Xu, and P. Chakraborty. 1992. Intracellular trafficking and the parasitophorous vacuole of Leishmania mexicana-infected macrophages. J. Cell Sci. 103:1193-1210. [DOI] [PubMed] [Google Scholar]
- 46.Schubach, A., F. Haddad, M. P. Oliveira-Neto, W. Degrave, C. Pirmez, G. Grimaldi, Jr., and O. Fernandes. 1998. Detection of Leishmania DNA by polymerase chain reaction in scars of treated human patients. J. Infect. Dis. 178:911-914. [DOI] [PubMed] [Google Scholar]
- 47.Solbach, W., and T. Laskay. 2000. The host response to Leishmania infection. Adv. Immunol. 74:275-317. [DOI] [PubMed] [Google Scholar]
- 48.Suh, Y. A., R. S. Arnold, B. Lassegue, J. Shi, X. Xu, D. Sorescu, A. B. Chung, K. K. Griendling, and J. D. Lambeth. 1999. Cell transformation by the superoxide-generating oxidase Mox1. Nature 401:79-82. [DOI] [PubMed] [Google Scholar]
- 49.Sundaresan, M., Z. X. Yu, V. J. Ferrans, D. J. Sulciner, J. S. Gutkind, K. Irani, P. J. Goldschmidt-Clermont, and T. Finkel. 1996. Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem. J. 318:379-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutterwala, F. S., G. J. Noel, R. Clynes, and D. M. Mosser. 1997. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J. Exp. Med. 185:1977-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor, S. J., and D. Shalloway. 1996. Cell cycle-dependent activation of Ras. Curr. Biol. 6:1621-1627. [DOI] [PubMed] [Google Scholar]
- 52.Tran Van Nhieu, G., and R. R. Isberg. 1993. Bacterial internalization mediated by beta 1 chain integrins is determined by ligand affinity and receptor density. EMBO J. 12:1887-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veras, P. S., C. Moulia, C. Dauguet, C. T. Tunis, M. Thibon, and M. Rabinovitch. 1995. Entry and survival of Leishmania amazonensis amastigotes within phagolysosome-like vacuoles that shelter Coxiella burnetii in Chinese hamster ovary cells. Infect. Immun. 63:3502-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voncken, J. W., H. van Schaick, V. Kaartinen, K. Deemer, T. Coates, B. Landing, P. Pattengale, O. Dorseuil, G. M. Bokoch, J. Groffen, et al. 1995. Increased neutrophil respiratory burst in bcr-null mutants. Cell 80:719-728. [DOI] [PubMed] [Google Scholar]