Abstract
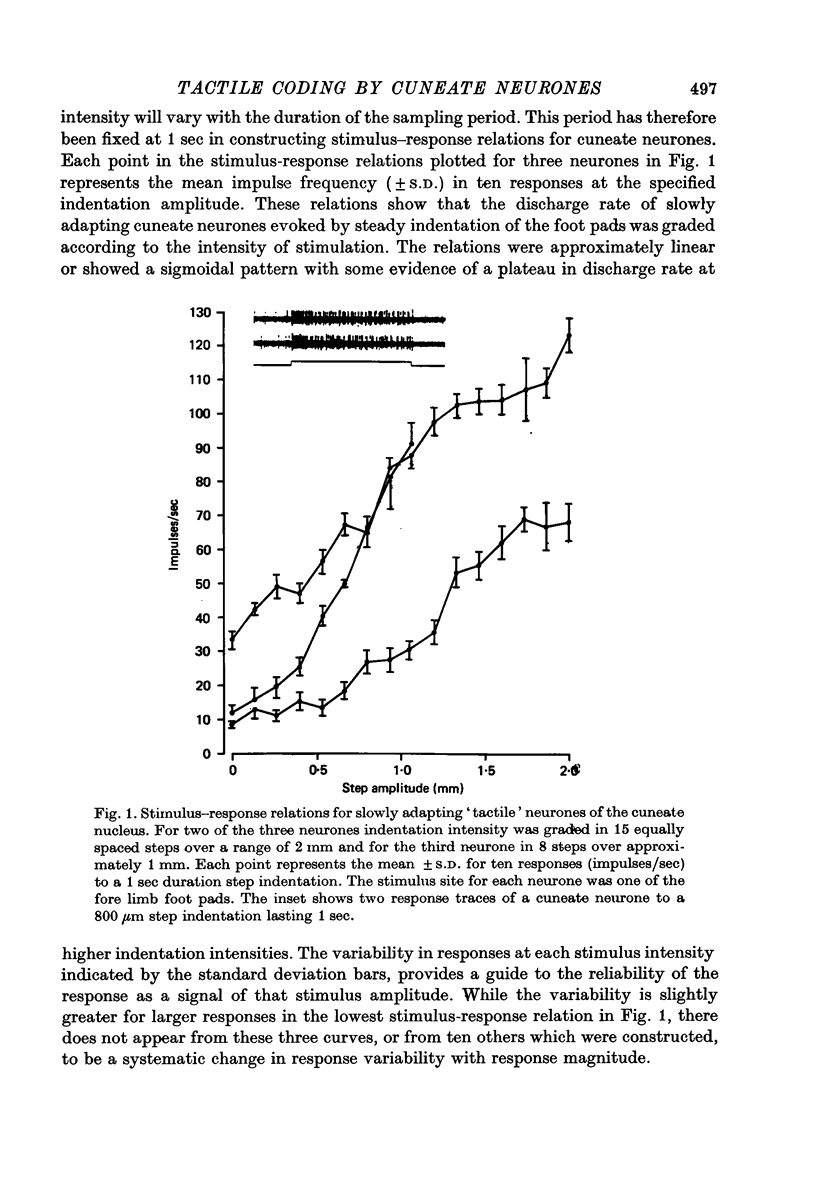
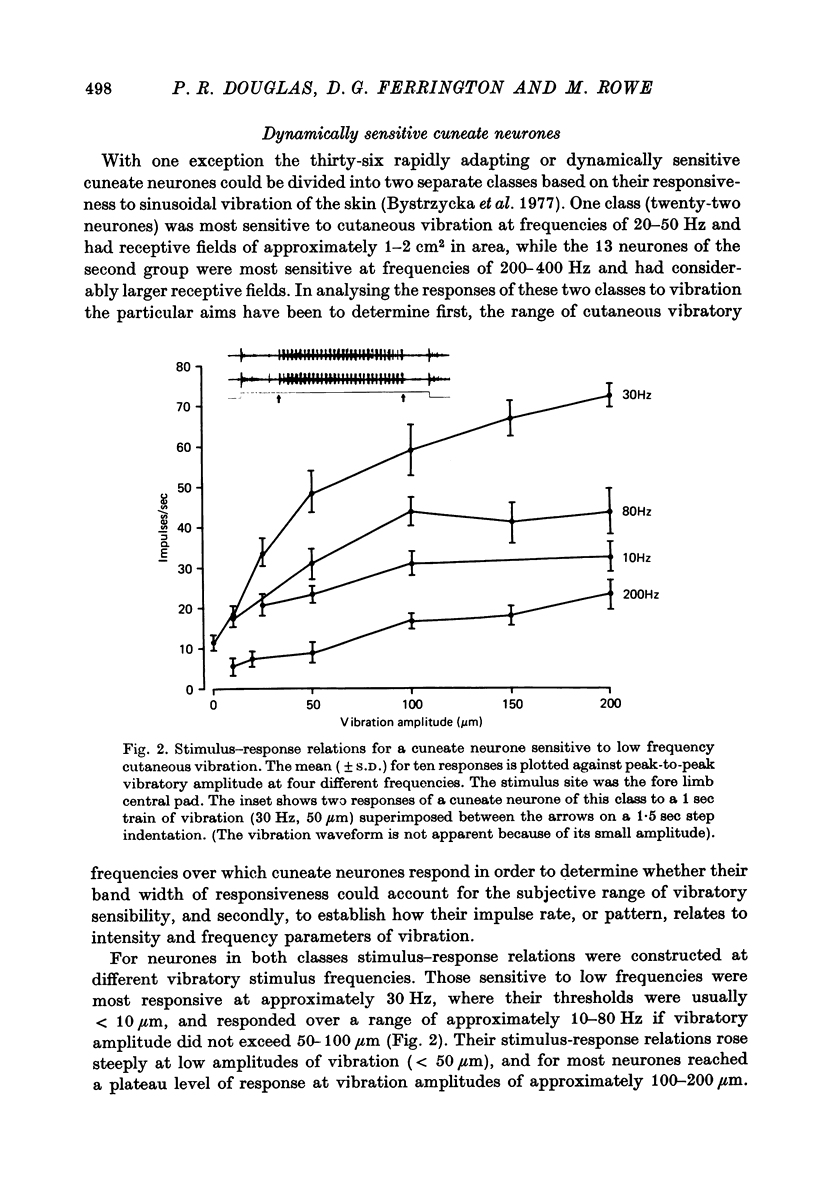
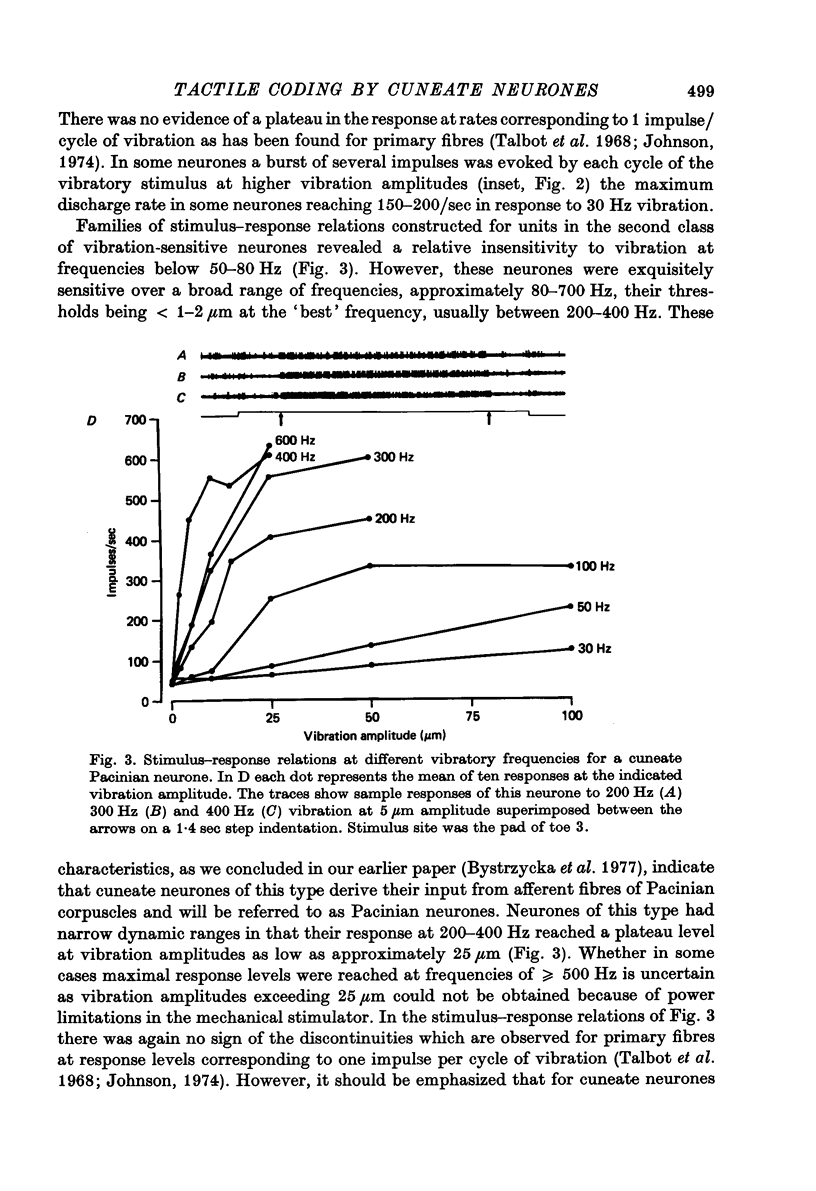
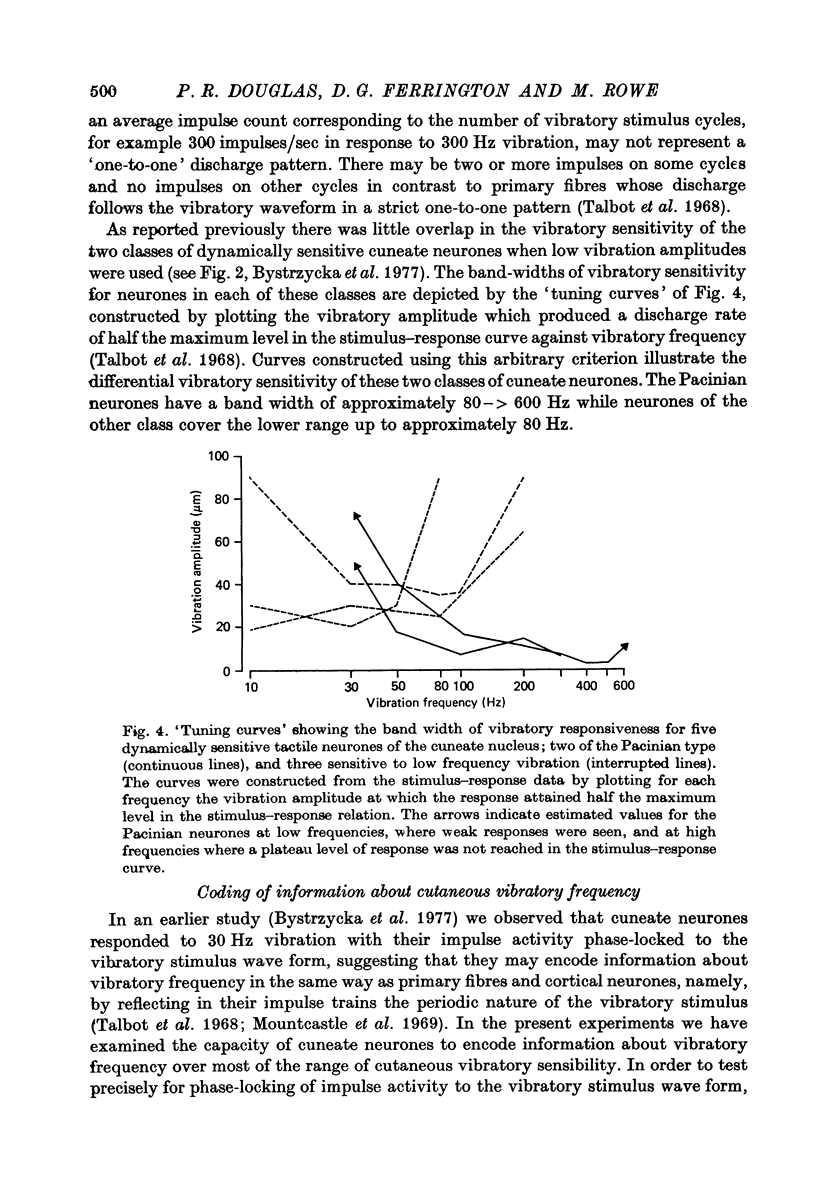
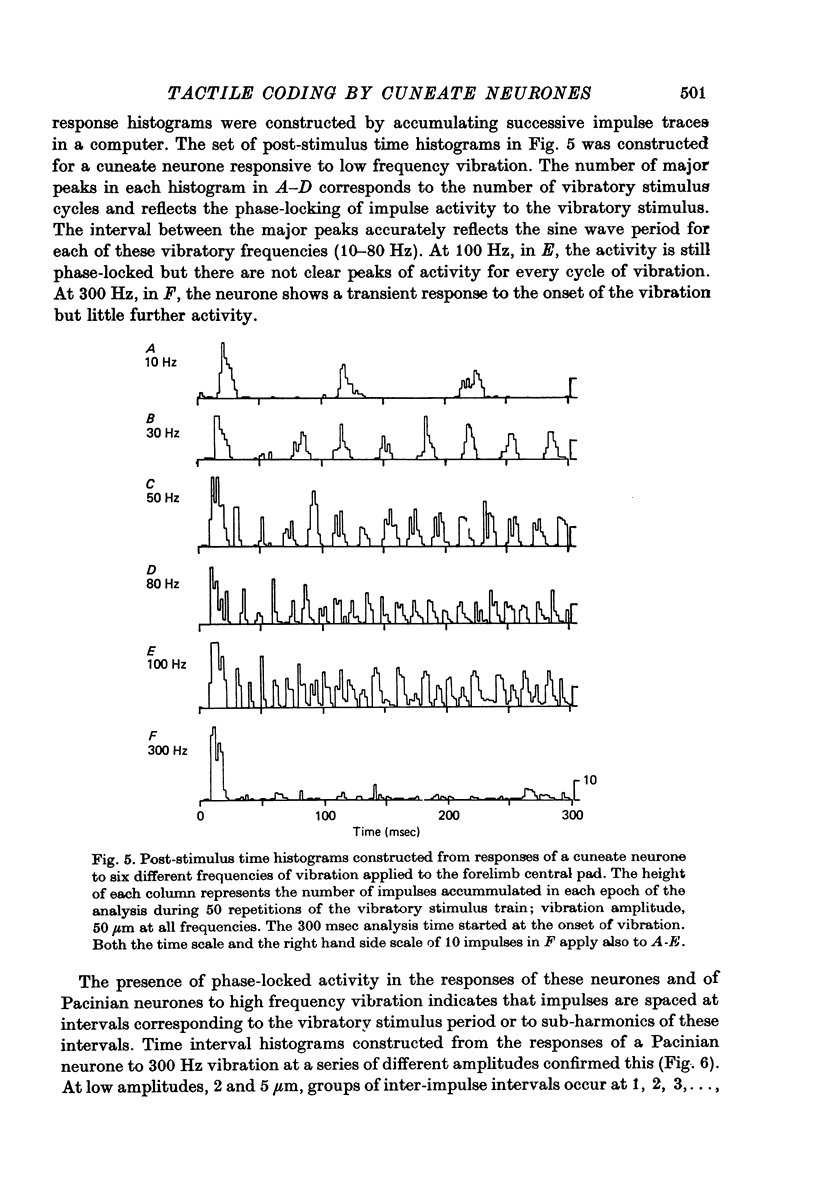
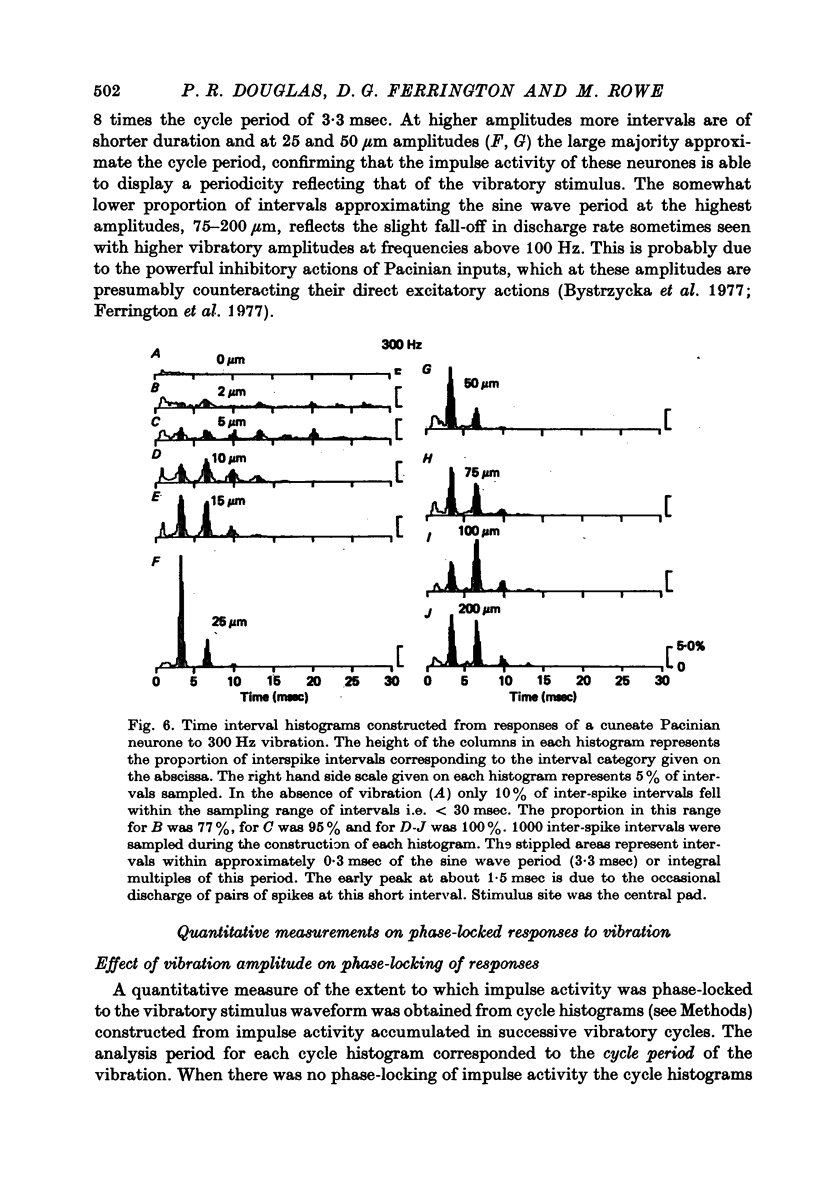
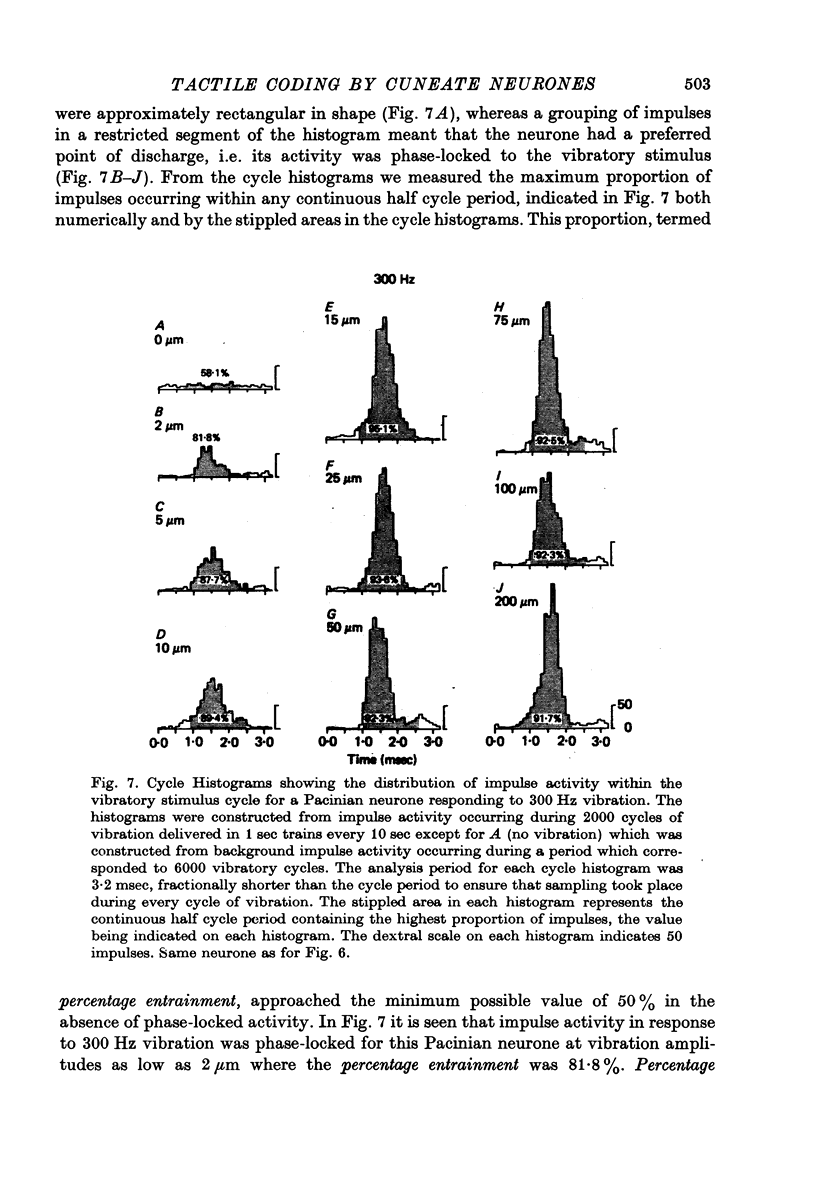
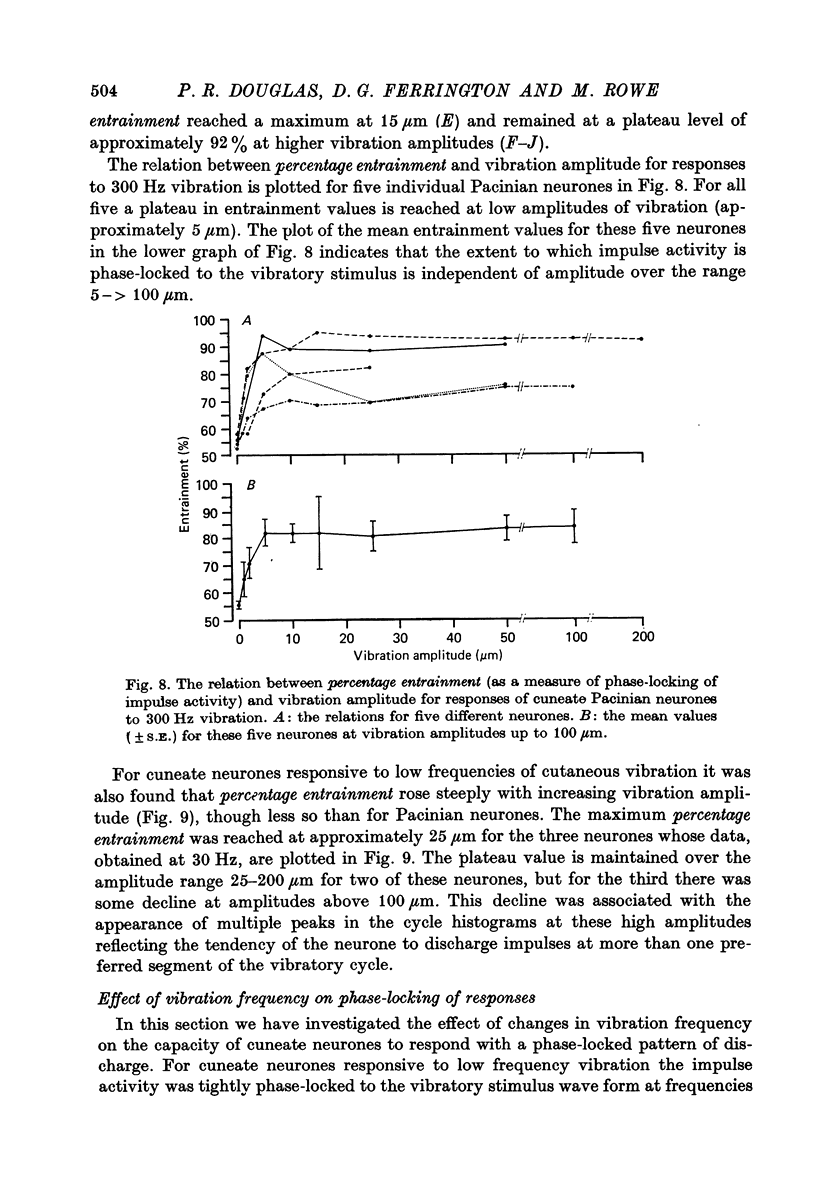
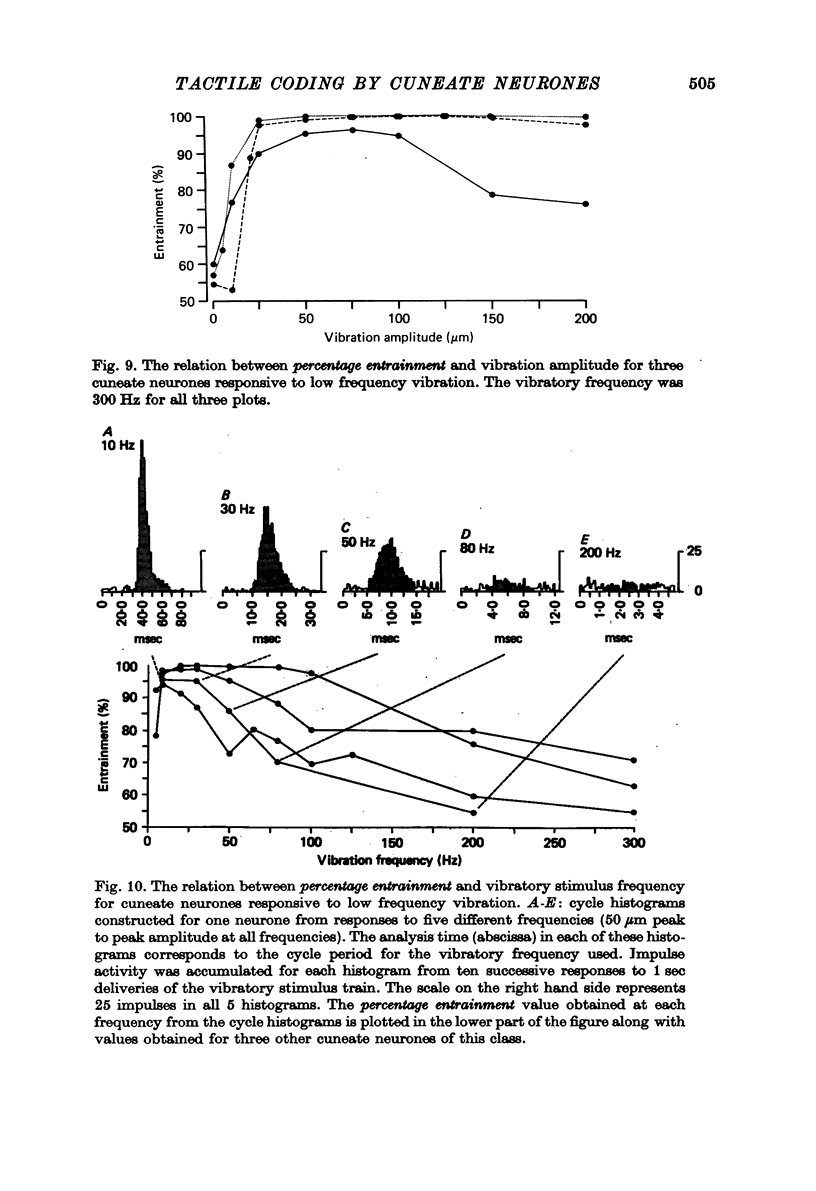
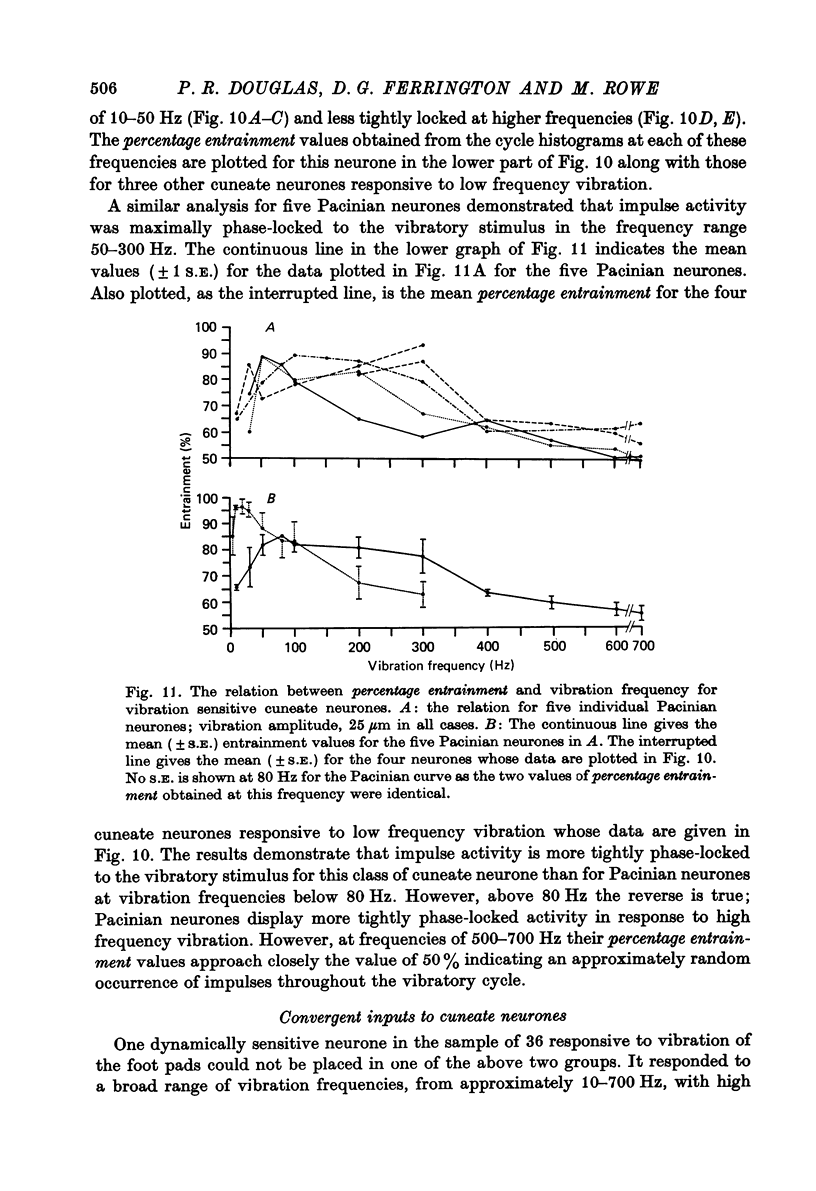
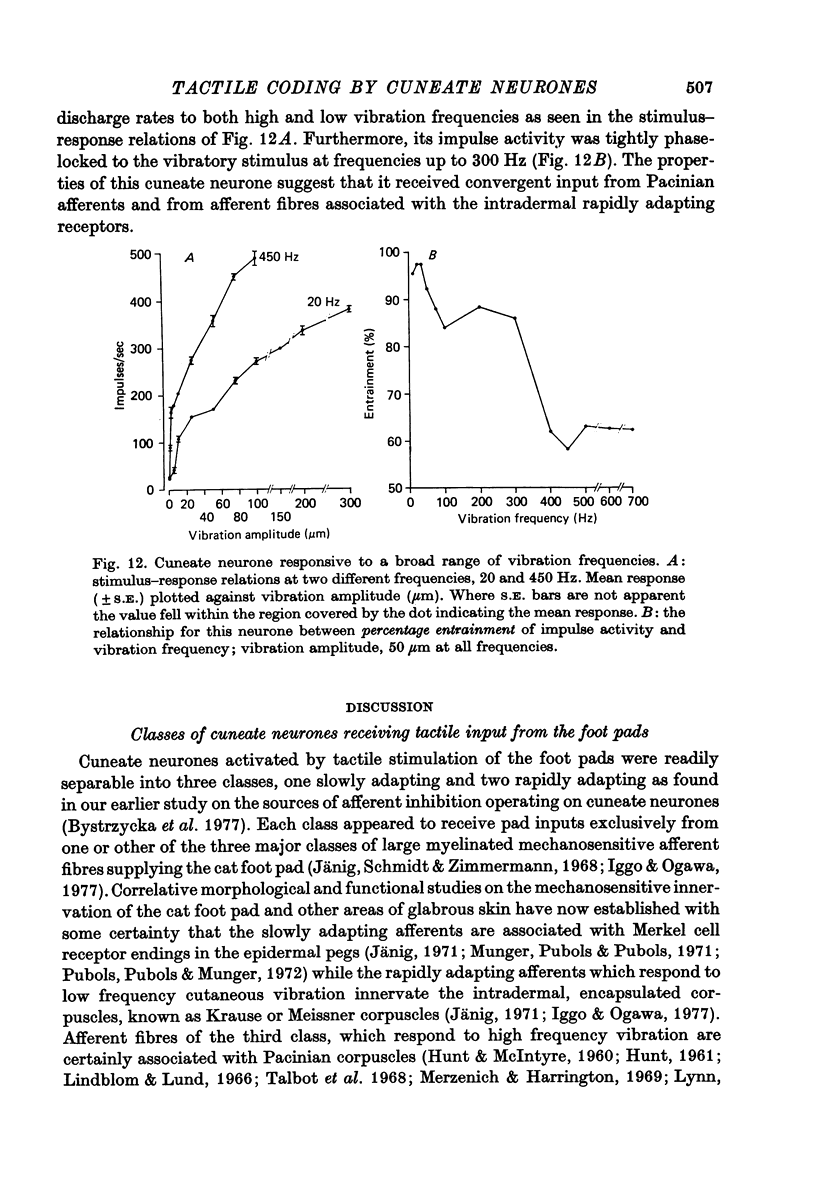
1. The responses of cuneate neurones to controlled tactile stimulation of the foot pads were examined in unanaesthetized, decerebrate cats. The neurones were divided into three functional classes; one sensitive to steady tactile stimuli, and two dynamically sensitive classes which could be readily differentiated by their responsiveness to cutaneous vibration. Each class appeared to receive an exclusive input from only one of the three known groups of tactile receptors associated with the foot pads, namely the Pacinian corpuscles, the Merkel endings and the intradermal, encapsulated endings known as Krause or Meissner corpuscles. 2. Cuneate neurones responsive to steady indentation of the skin displayed approximately linear or sigmoidal stimulus-response relations over indentation ranges up to approximately 1.5--2 mm. Response variability at a fixed stimulus intensity was relatively low and showed little systematic change over the full range of the stimulus-response curves. 3. One class of dynamically sensitive cuneate neurones responded to cutaneous vibration over a range of approximately 5-80 Hz with maximal responsiveness around 30 Hz. The other class, the Pacinian neurones, responded over a range of approximately 80- greater than 600 Hz with maximal responsiveness at 200-400 Hz. The thresholds and combined band width of vibratory sensitivity of these populations were comparable with known subjective thresholds and range of cutaneous vibratory sensibility. 4. Responses of cuneate neurones were phase-locked to the vibratory stimulus suggesting that information about vibration frequency could be coded by the patterns of impulse activity. Quantitative measures indicated that maximal phase-locking occurred in responses to vibration frequencies of 10-50 Hz with a progressive decline at higher frequencies. Above 400 Hz, impulse activity occurred almost randomly throughout the vibratory stimulus cycle and therefore carried little further signal of vibratory frequency. The decline, with increasing frequency, in the ability of cuneate neurones to signal information about vibratory frequency parallels the known subjective capacities for frequency discrimination. 5. A switch-over occurred at approximately 80 Hz in the population of cuneate neurones able to provide the more reliable signal of vibratory frequency; above 80 Hz, the Pacinian neurones; below 80 Hz, the neurones receiving intradermal, rapidly adapting receptor input from the pads. 6. The observed properties of cuneate neurones are compatible with a role in signalling information which could contribute to subjective tactile abilities.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP P. O., BURKE W., DAVIS R. Single-unit recording from antidromically activated optic radiation neurones. J Physiol. 1962 Aug;162:432–450. doi: 10.1113/jphysiol.1962.sp006943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOSHES B., PADBERG F. Studies on the cervical spinal cord of man; sensory pattern after interruption of the posterior columns. Neurology. 1953 Feb;3(2):90–101. doi: 10.1212/wnl.3.2.90. [DOI] [PubMed] [Google Scholar]
- Berkley K. J. Different targets of different neurons in nucleus gracilis of the cat. J Comp Neurol. 1975 Oct 1;163(3):285–303. doi: 10.1002/cne.901630304. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Franz D. N. Responses of spinocervical tract neurones to natural stimulation of identified cutaneous receptors. Exp Brain Res. 1969;7(3):231–249. doi: 10.1007/BF00239031. [DOI] [PubMed] [Google Scholar]
- Brown A. G. Subcortical mechanisms concerned in somatic sensation. Br Med Bull. 1977 May;33(2):121–128. doi: 10.1093/oxfordjournals.bmb.a071411. [DOI] [PubMed] [Google Scholar]
- Bystrzycka E., NAil B. S., Rowe M. Inhibition of cuneate neurones: its afferent source and influence on dynamically sensitive "tactile" neurones. J Physiol. 1977 Jun;268(1):251–270. doi: 10.1113/jphysiol.1977.sp011856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOK A. W., BROWDER E. J. FUNCTION OF POSTERIOR COLUMNS IN MAN. Arch Neurol. 1965 Jan;12:72–79. doi: 10.1001/archneur.1965.00460250076009. [DOI] [PubMed] [Google Scholar]
- Carmody J., Rowe M. Inhibition within the trigeminal nucleus induced by afferent inputs and its influence on stimulus coding by mechanosensitive neurones. J Physiol. 1974 Nov;243(1):195–210. doi: 10.1113/jphysiol.1974.sp010749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith I., Rowe M. J., Sessle B. J. "Tactile" stimulus intensity: information transmission by relay neurons in different trigeminal nuclei. Science. 1968 May 17;160(3829):791–794. doi: 10.1126/science.160.3829.791. [DOI] [PubMed] [Google Scholar]
- Ferrington D. G., Nail B. S., Rowe M. Human tactile detection thresholds: modification by inputs from specific tactile receptor classes. J Physiol. 1977 Nov;272(2):415–433. doi: 10.1113/jphysiol.1977.sp012052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON G., JUKES M. G. DUAL ORGANIZATION OF THE EXTEROCEPTIVE COMPONENTS OF THE CAT'S GRACILE NUCLEUS. J Physiol. 1964 Sep;173:263–290. doi: 10.1113/jphysiol.1964.sp007456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON G., PAINE C. H. Functional organization in nucleus gracilis of the cat. J Physiol. 1960 Sep;153:331–349. doi: 10.1113/jphysiol.1960.sp006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON G., SEED W. A. An investigation of nucleus gracilis of the cat by antidromic stimulation. J Physiol. 1961 Mar;155:589–601. doi: 10.1113/jphysiol.1961.sp006649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff G. D. Differential discrimination of frequency of cutaneous mechanical vibration. J Exp Psychol. 1967 Jun;74(2):294–299. doi: 10.1037/h0024561. [DOI] [PubMed] [Google Scholar]
- HUNT C. C. On the nature of vibration receptors in the hind limb of the cat. J Physiol. 1961 Jan;155:175–186. doi: 10.1113/jphysiol.1961.sp006621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A., Ogawa H. Correlative physiological and morphological studies of rapidly adapting mechanoreceptors in cat's glabrous skin. J Physiol. 1977 Apr;266(2):275–296. doi: 10.1113/jphysiol.1977.sp011768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. O. Reconstruction of population response to a vibratory stimulus in quickly adapting mechanoreceptive afferent fiber population innervating glabrous skin of the monkey. J Neurophysiol. 1974 Jan;37(1):48–72. doi: 10.1152/jn.1974.37.1.48. [DOI] [PubMed] [Google Scholar]
- Jänig W. Morphology of rapidly and slowly adapting mechanoreceptors in the hairless skin of the cat's hind foot. Brain Res. 1971 May 7;28(2):217–231. doi: 10.1016/0006-8993(71)90656-1. [DOI] [PubMed] [Google Scholar]
- Jänig W., Schmidt R. F., Zimmermann M. Single unit responses and the total afferent outflow from the cat's foot pad upon mechanical stimulation. Exp Brain Res. 1968;6(2):100–115. doi: 10.1007/BF00239165. [DOI] [PubMed] [Google Scholar]
- KIANG N. Y., WATANABE T., THOMAS E. C., CLARK L. F. Stimulus coding in the cat's auditory nerve. Preliminary report. Ann Otol Rhinol Laryngol. 1962 Dec;71:1009–1026. doi: 10.1177/000348946207100415. [DOI] [PubMed] [Google Scholar]
- KRUGER L., SIMINOFF R., WITKOVSKY P. Single neuron analysis of dorsal column nuclei and spinal nucleus of trigeminal in cat. J Neurophysiol. 1961 Jul;24:333–349. doi: 10.1152/jn.1961.24.4.333. [DOI] [PubMed] [Google Scholar]
- Knibestöl M. Stimulus-response functions of slowly adapting mechanoreceptors in the human glabrous skin area. J Physiol. 1975 Feb;245(1):63–80. doi: 10.1113/jphysiol.1975.sp010835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger L., Kenton B. Quantitative neural and psychophysical data for cutaneous mechanoreceptor function. Brain Res. 1973 Jan 15;49(1):1–24. doi: 10.1016/0006-8993(73)90398-3. [DOI] [PubMed] [Google Scholar]
- LaMotte R. H., Mountcastle V. B. Capacities of humans and monkeys to discriminate vibratory stimuli of different frequency and amplitude: a correlation between neural events and psychological measurements. J Neurophysiol. 1975 May;38(3):539–559. doi: 10.1152/jn.1975.38.3.539. [DOI] [PubMed] [Google Scholar]
- Lindblom U., Lund L. The discharge from vibration-sensitive receptors in the monkey foot. Exp Neurol. 1966 Aug;15(4):401–417. doi: 10.1016/0014-4886(66)90138-5. [DOI] [PubMed] [Google Scholar]
- McIntyre A. K., Holman M. E., Veale J. L. Cortical responses to impulses from single Pacinian corpuscles in the cat's hind limb. Exp Brain Res. 1967;4(3):243–255. doi: 10.1007/BF00248025. [DOI] [PubMed] [Google Scholar]
- Mountcastle V. B., LaMotte R. H., Carli G. Detection thresholds for stimuli in humans and monkeys: comparison with threshold events in mechanoreceptive afferent nerve fibers innervating the monkey hand. J Neurophysiol. 1972 Jan;35(1):122–136. doi: 10.1152/jn.1972.35.1.122. [DOI] [PubMed] [Google Scholar]
- Mountcastle V. B., Talbot W. H., Sakata H., Hyvärinen J. Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol. 1969 May;32(3):452–484. doi: 10.1152/jn.1969.32.3.452. [DOI] [PubMed] [Google Scholar]
- Munger B. L., Pubols L. M., Pubols B. H. The Merkel rete papilla--a slowly adapting sensory receptor in mammalian glabrous skin. Brain Res. 1971 Jun 4;29(1):47–61. doi: 10.1016/0006-8993(71)90416-1. [DOI] [PubMed] [Google Scholar]
- PERL E. R., WHITLOCK D. G., GENTRY J. R. Cutaneous projection to second-order neurons of the dorsal column system. J Neurophysiol. 1962 May;25:337–358. doi: 10.1152/jn.1962.25.3.337. [DOI] [PubMed] [Google Scholar]
- Pubols B. H., Pubols L. M. Coding of mechanical stimulus velocity and indentation depth by squirrel monkey and raccoon glabrous skin mechanoreceptors. J Neurophysiol. 1976 Jul;39(4):773–787. doi: 10.1152/jn.1976.39.4.773. [DOI] [PubMed] [Google Scholar]
- Pubols L. M., Pubols B. H., Jr, Munger B. L. Functional properties of mechanoreceptors in glabrous skin of the raccoon's forepaw. Exp Neurol. 1971 May;31(2):165–182. doi: 10.1016/0014-4886(71)90185-3. [DOI] [PubMed] [Google Scholar]
- Rose J. E., Brugge J. F., Anderson D. J., Hind J. E. Phase-locked response to low-frequency tones in single auditory nerve fibers of the squirrel monkey. J Neurophysiol. 1967 Jul;30(4):769–793. doi: 10.1152/jn.1967.30.4.769. [DOI] [PubMed] [Google Scholar]
- Rowe M. J., Carmody J. J. Afferent inhibition over the response range of secondary trigeminal neurones. Brain Res. 1970 Mar 3;18(2):371–374. doi: 10.1016/0006-8993(70)90338-0. [DOI] [PubMed] [Google Scholar]
- STEVENS S. S. Tactile vibration: dynamics of sensory intensity. J Exp Psychol. 1959 Apr;57(4):210–218. doi: 10.1037/h0042828. [DOI] [PubMed] [Google Scholar]
- Schwartz A. S., Eidelberg E., Marchok P., Azulay A. Tactile discrimination in the monkey after section of the dorsal funiculus and lateral lemniscus. Exp Neurol. 1972 Dec;37(3):582–596. doi: 10.1016/0014-4886(72)90101-x. [DOI] [PubMed] [Google Scholar]
- Schwartzman R. J., Bogdonoff M. D. Behavioral and anatomical analysis of vibration sensibility. Exp Neurol. 1968 Jan;20(1):43–51. doi: 10.1016/0014-4886(68)90123-4. [DOI] [PubMed] [Google Scholar]
- Talbot W. H., Darian-Smith I., Kornhuber H. H., Mountcastle V. B. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol. 1968 Mar;31(2):301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]
- Uddenberg N. Differential localization in dorsal funiculus of fibres originating from different receptors. Exp Brain Res. 1968;4(4):367–376. doi: 10.1007/BF00235701. [DOI] [PubMed] [Google Scholar]
- WINTER D. L. N. GRACILIS OF CAT. FUNCTIONAL ORGANIZATION AND CORTICOFUGAL EFFECTS. J Neurophysiol. 1965 Jan;28:48–70. doi: 10.1152/jn.1965.28.1.48. [DOI] [PubMed] [Google Scholar]
- Wall P. D. The sensory and motor role of impulses travelling in the dorsal columns towards cerebral cortex. Brain. 1970;93(3):505–524. doi: 10.1093/brain/93.3.505. [DOI] [PubMed] [Google Scholar]
- Willis W. D., Maunz R. A., Foreman R. D., Coulter J. D. Static and dynamic responses of spinothalamic tract neurons to mechanical stimuli. J Neurophysiol. 1975 May;38(3):587–600. doi: 10.1152/jn.1975.38.3.587. [DOI] [PubMed] [Google Scholar]


