Abstract
1. Following the intracellular recording of bipolar and horizontal cell responses, each unit was injected with horseradish peroxidase and a histochemical staining used to identify it at the level of the light and electron microscopes.
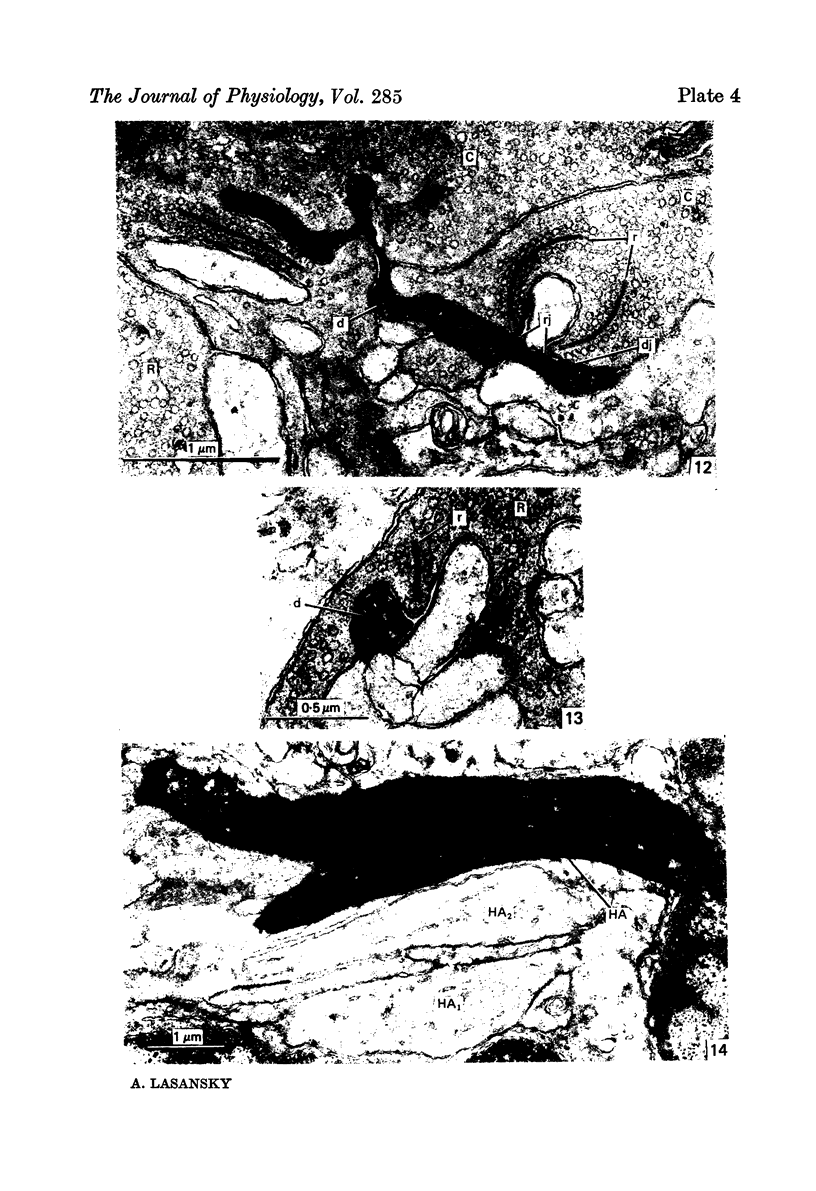
2. Centre-depolarizing bipolar cells made contact with rods and cones at basal and ribbon junctions, the latter being fewer. Centre-hyperpolarizing bipolar cells made the same types of contacts with the receptors, but ribbon junctions predominated.
3. It appears, therefore, that there is no fixed relationship between the sign of synaptic transmission from receptors to bipolar cells and the junctional features revealed by present methods for electron microscopy of tissue sections. Accordingly, the reason for the existence of more than one type of contact between receptors and bipolar cells remains to be determined.
4. Light microscopy of the peroxidase-injected horizontal cells gave further support to the notion that type B responses are recorded from the cell body, and type A responses from the axon terminal, of a single type of horizontal cell. Electron microscopy showed that the processes (dendrites) originating from the cell body make ribbon and distal junctions with rods and cones, just as shown before for the axon terminals.
5. As a result of these observations, it is possible to exclude one of two alternative hypotheses previously proposed to account for the properties of the surround responses recorded from the horizontal cell bodies.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott B. B., Kolb H. The connections between bipolar cells and photoreceptors in the retina of the domestic cat. J Comp Neurol. 1973 Mar 1;148(1):91–114. doi: 10.1002/cne.901480106. [DOI] [PubMed] [Google Scholar]
- Boycott B. B., Kolb H. The horizontal cells of the rhesus monkey retina. J Comp Neurol. 1973 Mar 1;148(1):115–139. doi: 10.1002/cne.901480107. [DOI] [PubMed] [Google Scholar]
- Famiglietti E. V., Jr, Kaneko A., Tachibana M. Neuronal architecture of on and off pathways to ganglion cells in carp retina. Science. 1977 Dec 23;198(4323):1267–1269. doi: 10.1126/science.73223. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hárosi F. I. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975 Sep;66(3):357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Hashimoto H. Electrophysiological study of single neurons in the inner nuclear layer of the carp retina. Vision Res. 1969 Jan;9(1):37–55. doi: 10.1016/0042-6989(69)90030-3. [DOI] [PubMed] [Google Scholar]
- Karnovsky M. J. The ultrastructural basis of capillary permeability studied with peroxidase as a tracer. J Cell Biol. 1967 Oct;35(1):213–236. doi: 10.1083/jcb.35.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. The connections between horizontal cells and photoreceptors in the retina of the cat: electron microscopy of Golgi preparations. J Comp Neurol. 1974 May 1;155(1):1–14. doi: 10.1002/cne.901550102. [DOI] [PubMed] [Google Scholar]
- Lasansky A. Basal junctions at synaptic endings of turtle visual cells. J Cell Biol. 1969 Feb;40(2):577–581. doi: 10.1083/jcb.40.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A. Cell junctions at the outer synaptic layer of the retina. Invest Ophthalmol. 1972 May;11(5):265–275. [PubMed] [Google Scholar]
- Lasansky A., Marchiafava P. L. Light-induced resistance changes in retinal rods and cones of the tiger salamander. J Physiol. 1974 Jan;236(1):171–191. doi: 10.1113/jphysiol.1974.sp010429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A. Organization of the outer synaptic layer in the retina of the larval tiger salamander. Philos Trans R Soc Lond B Biol Sci. 1973;265(872):471–489. doi: 10.1098/rstb.1973.0033. [DOI] [PubMed] [Google Scholar]
- Lasansky A., Vallerga S. Horizontal cell responses in the retina of the larval tiger salamander. J Physiol. 1975 Sep;251(1):145–165. doi: 10.1113/jphysiol.1975.sp011085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payton B. W., Bennett M. V., Pappas G. D. Permeability and structure of junctional membranes at an electrotonic synapse. Science. 1969 Dec 26;166(3913):1641–1643. doi: 10.1126/science.166.3913.1641. [DOI] [PubMed] [Google Scholar]
- Raviola E., Gilula N. B. Intramembrane organization of specialized contacts in the outer plexiform layer of the retina. A freeze-fracture study in monkeys and rabbits. J Cell Biol. 1975 Apr;65(1):192–222. doi: 10.1083/jcb.65.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson I., Rose B., Loewenstein W. R. Size limit of molecules permeating the junctional membrane channels. Science. 1977 Jan 21;195(4275):294–296. doi: 10.1126/science.831276. [DOI] [PubMed] [Google Scholar]
- Snow P. J., Rose P. K., Brown A. G. Tracing axons and axon collaterals of spinal neurons using intracellular injection of horseradish peroxidase. Science. 1976 Jan 23;191(4224):312–313. doi: 10.1126/science.54936. [DOI] [PubMed] [Google Scholar]
- Stell W. K. Correlation of retinal cytoarchitecture and ultrastructure in Golgi preparations. Anat Rec. 1965 Dec;153(4):389–397. doi: 10.1002/ar.1091530409. [DOI] [PubMed] [Google Scholar]
- Stell W. K. Horizontal cell axons and axon terminals in goldfish retina. J Comp Neurol. 1975 Feb 15;159(4):503–520. doi: 10.1002/cne.901590405. [DOI] [PubMed] [Google Scholar]
- Stell W. K., Ishida A. T., Lightfoot D. O. Structural basis for on-and off-center responses in retinal bipolar cells. Science. 1977 Dec 23;198(4323):1269–1271. doi: 10.1126/science.201028. [DOI] [PubMed] [Google Scholar]