Abstract
Streptococcus suis capsular type 2 is an important etiological agent of swine meningitis, and it is also a zoonotic agent. Since one hypothesis of the pathogenesis of S. suis infection is that bacteria enter the bloodstream and invade the meninges and other tissues in close association with mononuclear phagocytes, the objective of the present study was to evaluate the capacity of S. suis type 2 to adhere to macrophages. An enzyme-linked immunosorbent assay technique was standardized to simply and accurately measure the rate of bacterial attachment to phagocytic cells. Results were confirmed by plate counting. Adhesion was dependent on bacterial concentration and incubation time and was not affected by cytochalasin pretreatment of macrophages. Inhibition studies showed that the sialic acid moiety of the S. suis capsule would be, at least in part, responsible for bacterial recognition by macrophages. Serum preopsonization of bacteria increased adhesion levels. Complement would be partially implicated in the serum-enhanced binding of S. suis to cells. Adhesion varied among different S. suis type 2 isolates. However, high bacterial concentrations of several isolates were cytotoxic for cells, and these cytotoxic effects correlated with suilysin production. Indeed, hemolytic strain supernatants, as well as purified suilysin, reproduced cytotoxic effects observed with live bacteria, and these effects were inhibited by cholesterol pretreatment. Bacterial adhesion and cytotoxicity were confirmed by scanning and transmission electron microscopy. We hypothesize that attachment of bacteria to phagocytes could play an important role in the pathogenesis of S. suis infection by allowing bacterial dissemination and causing a bacteremia and/or septicemia. This interaction could also be related to the activation of the host inflammatory response observed during meningitis.
Streptococcus suis is not only one of the most important swine pathogens worldwide, but it is also a zoonotic agent. Among the serotypes described, type 2 is the serotype most frequently associated with disease (21). The most important clinical feature associated with S. suis is meningitis; however, other pathologies have also been described (22). Knowledge of virulence factors and of the pathogenesis of S. suis infection is still limited. S. suis is transmitted via the respiratory route and remains localized in the palatine tonsils. Some animals will only be healthy carriers and will never develop disease, whereas others will, sooner or later, develop bacteremia, sometimes septicemia, and finally meningitis. Hence, in these cases, bacteria should travel and persist throughout the bloodstream and reach the central nervous system (17). An early theory suggested uptake of bacteria by monocytes, intracellular survival, and invasion of the central nervous system by the theorized “Trojan horse” mechanism (53). This bacterial uptake could take place directly at the tonsils by macrophages or by monocytes once bacteria are in the bloodstream. However, most studies carried out during the last decade suggest that bacteria may also use (an)other mechanism(s) to disseminate themselves (17). In fact, S. suis is a well-encapsulated bacterium, and it has been proposed that the capsular polysaccharide (CPS) confers antiphagocytic properties to it (6, 40, 42). The CPS of S. suis serotype 2 is composed of five sugars: glucose, galactose, N-acetylglucosamine, rhamnose, and sialic acid (N-acetylneuraminic acid) (25), this latter component being related to virulence for other bacterial agents of meningitis (29, 52). The CPS is so far the only proven critical virulence factor, based on studies with nonencapsulated isogenic mutants. The absence of CPS correlated with increased hydrophobicity and phagocytosis using murine and porcine phagocytes. In addition, unencapsulated mutants were shown to be avirulent and cleared from circulation rapidly in both mouse and pig models of infection (6, 42). Even though the CPS seems to be a major virulence factor, most avirulent strains are encapsulated, indicating that other important virulence factors are essential.
S. suis also produces a hemolysin (suilysin), a thiol-activated toxin, which may have a role in virulence (18, 24). This protein belongs to the family of antigenically related cholesterol-binding toxins that form transmembrane pores and possess a “multi-hit” mechanism of action (18). While most European strains are suilysin positive, variable production of this protein has been observed with North American strains (16, 43). Suilysin production is associated to the presence of the 136-kDa muramidase-released protein (MRP) and the 110-kDa extracellular factor (EF) protein, suggested as virulence markers in most European strains (49). This phenotype is normally absent in virulent North American strains (16).
Thus, the attributes responsible for S. suis bloodstream survival and dissemination leading to meningeal invasion are still not clear. Inflammation is a hallmark of S. suis infection, and in this regard, S. suis activation of cytokine release by phagocytes has been reported elsewhere (38, 39). This activation was shown to be phagocytosis independent (38). It could be hypothesized that surface adherence to phagocytes with impaired uptake could be a key step for a successful infection, as suggested for Haemophilus influenzae type b, another important meningeal pathogen. The type b capsule is an antiphagocytic factor, and only mice infected with encapsulated bacteria, which are largely bound but not ingested by macrophages, are bacteremic (30).
Even though mononuclear phagocytes have been implicated as playing a central role in the pathogenesis of the meningitis, the interactions of S. suis type 2 with phagocytic cells are still controversial. Furthermore, the surface adhesion of S. suis to these cells has never been addressed. Since the murine model of infection has been widely used to evaluate the virulence of S. suis strains (4), our objective was to evaluate the capacity of S. suis type 2 to adhere to murine macrophages. In addition, different bacterial pretreatments were carried out to preliminarily characterize this interaction.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The S. suis capsular type 2 suilysin-positive, virulent strain 31533, originally isolated from a case of porcine meningitis (27) and previously used for evaluation of cytokine induction studies (38, 39), served as the reference strain in this study. In selected experiments, the S. suis capsular type 2 suilysin-negative, virulent strain 89-1591 was also used (4). S. suis isolates used in comparative studies are listed in Table 1. These isolates had previously been tested in the murine model of infection (4, 34). Bacteria were maintained as stock cultures in 50% glycerol-Todd-Hewitt broth (THB; Difco Laboratories, Detroit, Mich.) at −80°C. Bacteria were grown overnight onto bovine blood agar plates at 37°C, and isolated colonies were used as inocula for THB, being incubated for 18 h at 37°C. Working cultures for adhesion and cytotoxicity studies were made by inoculating 400 μl of these cultures in 10 ml of THB at 37°C with agitation until they reached the mid-log phase (4 to 6 h of incubation time; optical density at 540 nm [OD540] of 0.4 to 0.5). Bacteria were washed twice in phosphate-buffered saline (PBS), pH 7.4, and were appropriately diluted (Results). An accurate determination of the number of CFU per milliliter in the final suspension was made by plating onto THB agar.
TABLE 1.
Comparative studies with different S. suis capsular type 2 isolates
| Strain | Origin | Geographic origin | Phenotypea
|
Adhesionb (no. of CFU/well) | Cytotoxicityc (%) | ||
|---|---|---|---|---|---|---|---|
| Sly | MRP | EF | |||||
| 166 | Diseased pig | France | + | + | + | 1.3 × 104 (±0.5)d | 43.5 (±4) |
| S735 | Diseased pig | The Netherlands | + | + | * | 4.7 × 103 (±1.1) | 38.5 (±2) |
| 31533 | Diseased pig | France | + | + | + | 2.5 × 103 (±0.8) | 40.6 (±5) |
| 24 | Diseased pig | France | + | + | + | 2.0 × 103 (±0.6) | 42.8 (±3) |
| 95-8242 | Diseased pig | Canada | + | + | + | 1.4 × 103 (±0.3) | 31.5 (±6) |
| Reims | Human | France | + | + | + | 9.1 × 102 (±2.0)e | 20.5 (±5) |
| 4.1 × 103f | 36.2f | ||||||
| JL590 | Diseased pig | Mexico | − | * | − | 1.9 × 104 (±0.7)d | 2.5 (±2) |
| AAH4 | Diseased pig | United States | − | + | − | 1.6 × 104 (±0.5)d | 8.0 (±5) |
| TD10 | Pig, healthy carrier | United Kingdom | − | − | − | 1.0 × 104 (±0.3)d | 1.0 (±2) |
| 89-1591 | Diseased pig | Canada | − | − | − | 4.5 × 103 (±1.1) | 0.0 (±0) |
| 89-999 | Diseased pig | Canada | − | − | − | 4.4 × 103 (±1.4) | 0.0 (±0) |
| 94-623 | Pig, healthy carrier | France | − | * | − | 4.2 × 103 (±1.0) | 2.0 (±2) |
| 90-1330 | Pig, healthy carrier | Canada | − | + | − | 4.2 × 103 (±1.2) | 0.0 (±0) |
| 94-3037 | Human | Canada | − | − | − | 1.8 × 103 (±0.5)e | 8.0 (±3) |
| 8.1 × 103f | 2.7f | ||||||
*, higher-molecular-weight variants of EF protein or MRP; Sly, suilysin.
Adhesion assay was performed at an infection ratio of 10 bacteria/cell and for 30 min of incubation time. Data are expressed as means (± standard deviations) of adhered bacteria (n = 4).
Cytotoxicity assay was performed by methylene blue staining of remaining macrophages after 3 h of bacterium-cell contact at an infection ratio of 100 bacteria/cell. Data are expressed as the percentage of cytotoxicity (± standard deviations) in infected wells with respect to control wells with macrophages alone (n = 4).
Significantly higher adhesion levels than found for all other strains within the same group by Tukey-Kramer post hoc test analysis (P < 0.001).
Significantly lower adhesion levels than found for all other strains within the same group by Tukey-Kramer post hoc test analysis (P < 0.001).
Group average.
Cell line and cell culture.
The J774.A1 murine (BALB/c) macrophage-like cell line (TIB 67; American Type Culture Collection, Rockville, Md.) was grown at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% heat-inactivated fetal bovine serum (hiFBS) (Gibco, Burlington, Vt.). This cell line has been previously used in S. suis phagocytosis and cytokine stimulation studies (38, 40) and has been shown to present features similar to those of normal peritoneal macrophages (40).
Assay of adhesion to J774 macrophages.
The adhesion assay was adapted from Athamna and Ofek (3) and Sloan and Pistole (41). For adhesion assays, 48-h cultures of J774 cells were scraped, washed once with PBS, and resuspended at 106 cells/ml in DMEM-10% hiFBS (unless specified otherwise). Plates of 96 wells (Falcon; Becton Dickinson, Bedford, Mass.) were precoated overnight at 4°C with 1% bovine serum albumin (BSA; Sigma-Aldrich, Oakville, Ontario, Canada) solution in PBS and washed once with PBS before use. BSA-coated plates showed a lower nonspecific (background) adhesion of bacteria to the plastic surface than did untreated plates (data not shown). J774 suspension (100 μl) was distributed in precoated plates and was incubated 3 h at 37°C and 5% CO2 to allow macrophage adhesion to plates. Nonadherent cells were removed by one washing with PBS just before addition of bacteria. Bacterial dilutions at different concentrations (Results) in DMEM-10% hiFBS (unless specified otherwise) were added to the macrophage-containing plates. Plates were centrifuged at 130 × g for 10 min to enhance the contact of bacteria with the surface of cells, and were then incubated at 37°C and 5% CO2. At different time intervals (Results), plates were washed four times with PBS to remove nonadherent bacteria. The plates were air dried and fixed overnight with 50 μl of 100% methanol. Empty wells without cells served as the control for background adhesion of bacteria to the plastic surface. To confirm that bacteria were extracellularly bound, cytochalasin C (from Metarrhizium anisopliae; Sigma-Aldrich) treatment of macrophages was performed as previously described (2 μg/ml, 30 min before and during the test). This cytochalasin C concentration was shown to effectively block the uptake of a highly phagocytosed nonencapsulated strain of S. suis (38). Bacteria that adhered to macrophages or control wells were quantified by enzyme-linked immunosorbent assay (ELISA).
ELISA.
To each well, containing methanol-fixed cells, was added 200 μl of 5% bovine serum albumin (BSA) solution in PBS to block nonspecific binding of antibodies and 1 μg of human immunoglobulin G Fc fragment (Jackson ImmunoResearch Laboratory, West Grove, Pa.)/ml to block the Fc receptors on the macrophages. After incubation for 1 h at 37°C, plates were washed three times with PBS-0.05% Tween 20 (PBS-T; Sigma), followed by the addition of 100 μl of type-specific anti-S. suis serotype 2 rabbit serum per well, diluted 1/4,000 in PBS-T for 1 h at 37°C, produced as previously described (23). Plates were washed three times with PBS-T, followed by the addition of 100 μl of horseradish peroxidase-labeled anti-rabbit immunoglobulin G (Jackson ImmunoResearch)/well, diluted 1/8,000 in PBS-T for 1 h at 37°C. After three washes with PBS-T, 100 μl of the substrate 3,3′,5,5′-tetramethylbenzidine (TM Blue; Intergen, St. Milford, Mass.) was added to each well, and the blueness was allowed to develop at room temperature. The enzyme reaction was stopped with the addition of 50 μl of 1 N H2SO4/well. The absorbance was read at 450 nm with an ELISA plate reader (UVmax; Molecular Devices, Menlo Park, Calif.). Blank wells and control wells with macrophages only were included to ensure that the eukaryotic cells did not react with ELISA antibodies and substrate.
A standard curve served to derive the number of bacteria (expressed as number of CFU/well) adhered to macrophages. For this purpose, 100 μl of known bacterial concentrations (from 107 to 101 CFU/well) in distilled water was allowed to dry overnight in the wells of a microtiter plate, followed by fixation with methanol. The ELISA was performed on the immobilized bacteria as described above. Loss of bacteria during washes was considered negligible, as previously reported (3, 41). The ELISA values in OD450 units were plotted as a function of the number of bacteria in each well. A linear relationship between the number of immobilized bacteria and the ELISA values was obtained over the range of 103 to 105 CFU/well. The curve obtained was used to calculate the number of bacteria adhering to macrophages from the ELISA values obtained in each experiment.
Adhesion inhibition assays.
For bacterial pretreatment studies, washed organisms (grown as mentioned above) were exposed to various modifying agents: proteinase K (Boehringer-Mannheim, Laval, Quebec, Canada) at 0.5 or 1 mg/ml, trypsin (Gibco) at 0.5 or 1 mg/ml, or pronase (Boehringer-Mannheim) at 50 or 100 μg/ml in PBS for 1 h at 37°C. Sialidase (from Clostridium perfringens; Boehringer-Mannheim) treatment was performed as previously described (8), at 0.5, 1, 2, or 5 U/ml in buffer sodium acetate (50 mM sodium acetate, 0.9% NaCl, 0.1% CaCl2, pH 5.5) for 3 h at 37°C with agitation. Treated bacteria were then washed three times with PBS and finally resuspended at different concentrations in DMEM-10% hiFBS for the adhesion assay. Bacterial viability and concentration after each treatment were evaluated by plating on THB agar. In some experiments, bacteria were killed by heat treatment at 60°C for 45 min (minimal condition required to kill S. suis) (38) and at 100°C for 30 min or by treatment with 0.2% formaldehyde for 1 h at 37°C with agitation, washed, and resuspended as described above. The killed cultures were subcultured on blood agar plates at 37°C for 48 h to confirm the absence of viable organisms. The different treatments did not affect bacterial recognition by the anti-S. suis serum in the ELISA (data not shown).
In competitive binding studies, J774 macrophages were pretreated with the monosaccharides d-galactose, d-glucose, and N-acetyl-d-glucosamine (1, 10, and 100 mM); d-mannose and l-rhamnose (1, 10, 100, and 500 mM); and N-acetylneuraminic acid (1, 10, 100, and 500 μg/ml) and the conjugate 6′-N-acetyl-neuramyl-N-acetyl-lactosamine [α-Neu-5Ac-(2→6)-β-d-Gal-(1→4)-d-GlcNAc; 0.1, 1, 10, 100, and 500 μg/ml]. Different concentrations of sugars (Sigma-Aldrich) in PBS were added individually to macrophages 1 h at 37°C prior to the addition of bacterial dilution at 106 CFU/well in DMEM-10% hiFBS for the adhesion assay. J774 macrophages were also pretreated for 90 min at 37°C with different dilutions in PBS of purified S. suis CPS (at 0.5 or 1 mg/ml), purified S. suis cell wall (at 0.5, 1, or 2.5 mg/ml) or purified Streptococcus faecalis lipoteichoic acid (LTA; Sigma-Aldrich; from 0.5 to 2,000 μg/ml). Purified CPS and cell wall of S. suis type 2 were prepared as previously described (13, 38). Since the anti-S. suis serum used for ELISA recognized these bacterial components, the adhesion assay was performed in these cases by the standard technique of counting the number of CFU recovered from wells as previously described (10). The adhesion of S. suis to macrophages in the absence of any treatment served as a control for all of these studies (100% adhesion). Results were expressed as the percentage of inhibition respect to the control.
Effect of bacterial preopsonization on adhesion.
Washed S. suis (grown as mentioned above) was preopsonized 30 min with agitation at 37°C with FBS (Gibco) or complement from mouse serum (C'MS; hemolytic titer of 1.0 CH50 [volume of serum that will result in 50% hemolysis of red blood cells] unit/ml; Sigma-Aldrich) at different concentrations (0.5, 1, 5, 10, 20, and 50% [vol/vol]) in DMEM. In certain experiments, FBS or C'MS was inactivated by heating at 56°C for 30 min to destroy the complement. Opsonized bacteria were added at 106 CFU/well to macrophage plates prepared as mentioned above. As a control, bacteria were preincubated 30 min with 5% BSA and 2% dextrose in DMEM. In fact, bacteria in DMEM alone presented high background levels of adhesion to the plastic surface, as already reported (46). In our hands, a combination of BSA and dextrose considerably reduced this background (not shown).
Cytotoxicity assays.
The cytotoxic effect of bacteria was evaluated in parallel with the adhesion assay by lactate dehydrogenase (LDH) measurement using a miniaturized version of the Sigma colorimetric assay as previously described (10). The percentage of cytotoxicity was calculated as [(sample OD414 − OD0%)/(OD100% − OD0%)] × 100, wherein OD0% represents the OD414 of noninfected cells and OD100% represents the OD414 of water-lysed cells. In some experiments, for comparative purposes, plates were stained to quantify the number of remained macrophages by the selective staining of phagocyte nuclei with methylene blue as described elsewhere (41). Empty wells or wells with macrophages alone (without bacteria) served as controls to determine the percentage of cytotoxicity in bacterial infected wells.
To evaluate the role of bacterial products in cytotoxicity, J774 plates were prepared as for the adhesion assay and were then incubated for 3 h at 37°C with 107 CFU of S. suis suilysin-positive strain 31533 or of the suilysin-negative strain 89-1591 (live or heat killed) per well or with 18-h culture supernatants of either strain (supernatants were recovered by centrifugation and filtration through 0.22-μm-pore-size filters and used immediately). Purifed suilysin (kindly provided by T. Jacobs, Intervet International, Boxmeer, The Netherlands) was also evaluated at different concentrations (1 to 5 μg/ml) in DMEM-10% hiFBS. The suilysin was reactivated by addition of 1% 2-mercaptoethanol to culture medium during assays (24). Inhibition of cytotoxic activity was performed by treatment of bacterial supernatants or suilysin dilution (at 5 μg/ml), with ethanol-soluble cholesterol (Sigma-Aldrich) at a final concentration of 100 μg/ml for 1 h at 37°C (10) before they were added to the macrophage plates for LDH measurement. Noninfected cells with cholesterol in culture medium were used as control.
Electron microscopy studies.
For the adhesion microscopy study, 24-well plates containing 13-mm-diameter round glass slides were used and the adhesion assay was performed as described above during an incubation period of 30 min at 37°C and 5% CO2. After four washes with PBS, cells were fixed for 1 h at room temperature with 2% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.3) and were then postfixed for 45 min at room temperature with 2% osmium tetroxide. A modified cytotoxic test, with cells in suspension instead of the macrophage plates, was carried out to facilitate microscopy studies of damage cells. J774 cell suspension (106 cells/ml) was mixed with bacterial dilutions (S. suis strain 31533 or strain 89-1591 at 108 CFU/ml) in polypropylene tubes (Sarstedt, Montréal, Quebec, Canada) and were incubated 3 h at 37°C with gentle rotation. Cells were washed once with PBS, and the pellets were embedded with 4% bacteriological agar (Difco) and kept at 4°C until solidification. Agar samples were then fixed 2 h at room temperature with 2% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.3) and were then postfixed overnight at 4°C with 2% osmium tetroxide. Specimens from both cytotoxic and adhesion assays were dehydrated in a graded series of ethanol solutions and were processed separately for transmission or scanning electron microscopy (TEM or SEM) as previously described (28).
Statistical analysis.
Each adhesion or cytotoxicity test was done at least in triplicate, and samples were run in quadruplicate in each plate. Results were derived from linear regression calculations from the standard curves and expressed as bound bacteria per well (as mean ± standard deviation of independent experiments). Differences were analyzed for significance by using Student's unpaired t test (two-tailed P value). A P that was < 0.05 was considered statistically significant. Differences between groups of strains (classified according to the phenotype; see Table 1) and differences among strains within the same group were analyzed for significance by using general linear models, followed by Tukey-Kramer post hoc tests for differences between strains. The SAS v8 software (SAS, Cary, N.C.) was used for these analyses.
RESULTS
S. suis adheres to J774 macrophages.
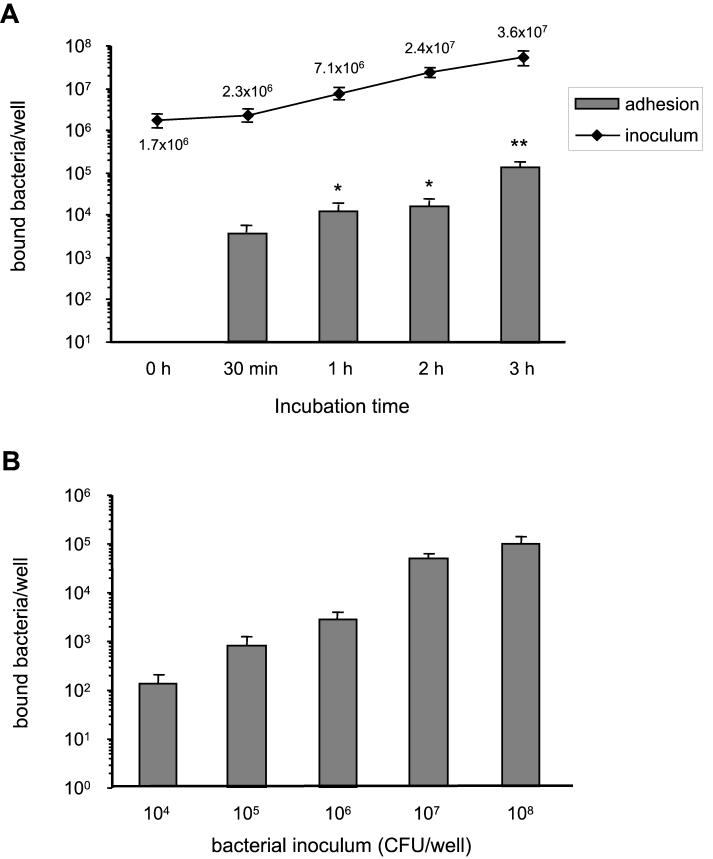
Figure 1A shows the kinetics of adhesion of S. suis strain 31533 to murine J774 macrophages, with a multiplicity of infection (MOI) of 10 bacteria/cell (∼106 CFU/well). Adhesion occurred rapidly and increased with incubation time, reaching a first plateau between 1 and 2 h (P < 0.001 with respect to 30-min adhesion levels). A second and higher increase of adhesion was observed after 3 h of bacterium-cell contact (P < 0.01 with respect to values observed at other incubation times). This increase in adhesion was not related to the inoculum multiplication during the adhesion assay, since the inoculum CFU counts remained constant between 2 and 3 h of incubation (P > 0.1). A similar kinetics of adhesion was observed with an MOI of 100 bacteria/cell (∼107 CFU/well; data not shown). It must be noted that all data were corrected from background adhesion to the plastic surface. For subsequent experiments, an incubation time of 30 min was chosen for two reasons. First, low bacterial cytotoxicity was seen at 30 min (see Fig. 4), and second, inoculum counts were also stable between 0 and 30 min of the adhesion assay (P > 0.1), while significant differences in the inoculum growth rates were observed between 30 min and 2 h of incubation (P < 0.01) (Fig. 1A). When different bacterial concentrations were evaluated under these conditions, adhesion was shown to be bacterial dose dependent (Fig. 1B). Maximal adhesion was observed at an MOI of 107 (P < 0.001), and no significant increase in adhesion levels was observed with higher bacterial concentrations. Adhesion levels with less than 104 bacteria/well were almost undetectable. Adhesion was confirmed in selected experiments by using the viable counting technique, as previously described (data not shown) (10, 40), and by SEM and TEM as shown in Fig. 2.
FIG. 1.
(A) Kinetics of S. suis adhesion to J774 macrophages with an MOI of 10 bacteria/cell (∼106 CFU/well). The kinetics of inoculum multiplication during the adhesion assay is also shown. ∗, P < 0.001 with respect to 30-min adhesion levels; ∗∗, P < 0.01 with respect to values observed at other incubation times. (B) Effect of bacterial concentration on 30-min adhesion to J774 macrophages. Data are expressed as means ± standard deviations of bound bacteria (in number of CFU recovered per well).
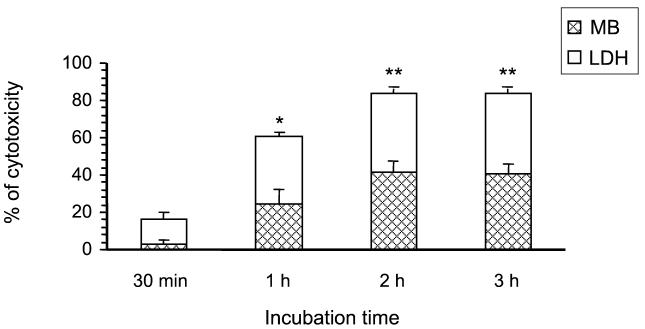
FIG. 4.
Effect of incubation time in presence of S. suis strain 31533 on J774 injury. The cytotoxic effect of bacteria was evaluated in parallel by the LDH test and by the selective stain of cell nuclei of remaining macrophages with methylene blue (MB) after different intervals of bacterium-cell contact at an MOI of 100 bacteria/cell (107 CFU/well). Data are expressed as the percentage of cytotoxicity (± standard deviations) in infected wells with respect to control wells with macrophages alone. ∗, P < 0.001 with respect to 30-min incubation time; ∗∗, P < 0.001 with respect to cytotoxic levels at 30 min and 1 h of incubation time.
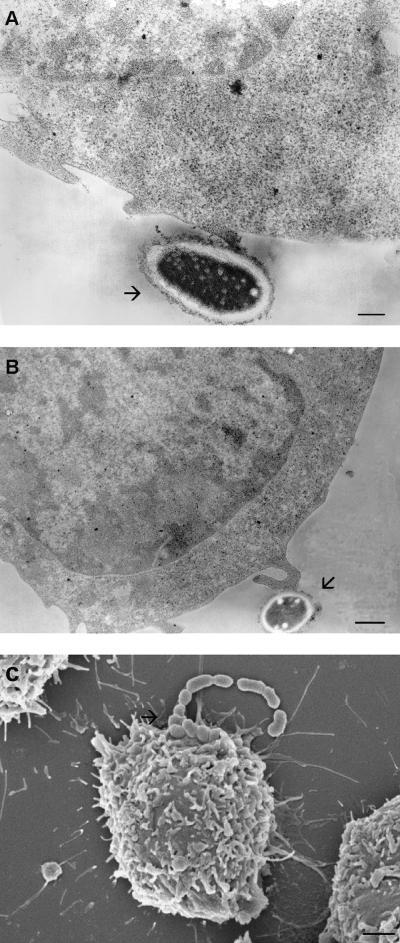
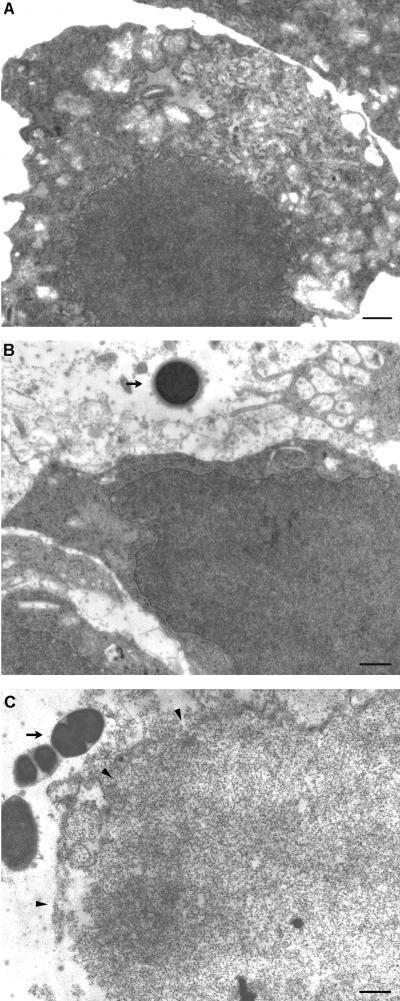
FIG. 2.
Transmission electron micrographs showing S. suis adhesion to J774 macrophages at different parts of the plasma membrane. S. suis adhered to the plain cell surface (bar, 250 nm) (A) or to the cell surface projections (bar, 0.5 μm) (B). (C) Scanning electron micrograph showing a small chain of S. suis cocci adhering to the surface of macrophages (bar, 1 μm). Arrows indicate S. suis cocci.
Even though it has been previously shown that encapsulated S. suis is practically not phagocytosed by J774 cells (40), conflicting results do exist in the literature regarding S. suis phagocytosis (17). To confirm that adhesion occurs without bacterial ingestion, cytochalasin C pretreatment of cells was performed before addition of bacteria. At an MOI of 10 bacteria/cell, cytochalasin C did not affect adhesion (3.0 × 103 and 2.8 × 103 CFU of bacteria per well bound to control cells and to cytochalasin C-treated cells, respectively; P > 0.1).
Comparative adhesion studies were done with different S. suis isolates at an MOI of 10 bacteria/cell and for 30 min of incubation time. As shown in Table 1, strains were classified into two groups according to the MRP, EF, and suilysin phenotypes (11). Most European virulent strains present the suilysin+, MRP+, and EF+ phenotype, whereas most of the North American strains are characterized as suilysin, EF, and MRP variable (11, 16, 49). The analysis of the differences between the two groups of strains revealed that strains of the latter group possess significantly higher levels of adhesion than do European phenotype strains (P < 0.0001) (Table 1). On the other hand, Tukey-Kramer post hoc tests revealed several significant differences among strains within each group (Table 1). Similar results were observed at an MOI of 100 bacteria/cell (data not shown).
Effect of bacterial pretreatments on adhesion.
In order to further characterize the interactions between S. suis and J774 macrophages, the inhibitory effect of different bacterial pretreatments was evaluated. Proteinase K, trypsin, or pronase treatment of bacteria did not significantly affect S. suis adhesion to macrophages (Table 2). Two different concentrations of proteases (Materials and Methods) in combination with three different concentrations of bacteria (107, 106, and 105 CFU/well) were evaluated, but none affected S. suis adhesion after 30 min of bacterium-cell contact. It must be noted that the different treatments did not affect bacterial viability (data not shown).
TABLE 2.
Effect of bacterial pretreatments on adhesion
| Treatmenta | Concn or incubation time | % of inhibitionb | Pc |
|---|---|---|---|
| Proteinase K | 0.5 mg/ml | 0 | >0.1 |
| 1 mg/ml | 0 | >0.1 | |
| Trypsin | 0.5 mg/ml | 0 | >0.1 |
| 1 mg/ml | 0 | >0.1 | |
| Pronase | 50 μg/ml | 0 | >0.1 |
| 100 μg/ml | 0 | >0.1 | |
| Sialidase | 0.5 U/ml | 32 (±1) | <0.03 |
| 1 U/ml | 34 (±5) | <0.03 | |
| 2 U/ml | 47 (±1) | <0.01 | |
| 100°C | 30 min | 72 (±6) | <0.0001 |
| 60°C | 45 min | 71 (±6) | <0.0001 |
| 0.2% Formaldehyde | 60 min | 74 (±11) | <0.0001 |
For bacterial pretreatments, washed organisms were exposed to proteinase, trypsin, and pronase for 1 h at 37°C. Sialidase (from C. perfringens) treatment was performed in buffer sodium acetate for 3 h at 37°C with agitation. Treated bacteria were then washed three times with PBS and were finally resuspended in DMEM-10% hiFBS for the adhesion assay.
Adhesion assay was performed at an infection ratio of 10 bacteria/cell and for 30 min of incubation time. Data are expressed as means ± standard deviations.
Statistically significant inhibition of adhesion (P < 0.05) with respect to values for bacteria without treatment (100%) as calculated by Student's unpaired t test.
Since the S. suis capsule contains sialic acid and since this sugar has been implicated in adhesion of other organisms (29), sialidase treatment was also performed. Sialidase-treated bacteria showed reduced adhesion levels compared to those found in nontreated bacteria (Table 2). Increased inhibition was observed by increasing the sialidase concentration up to 2 U/ml (P < 0.01). Higher sialidase concentrations affected bacterial viability (data not shown).
In some experiments bacteria were killed by heat treatment at 100°C for 30 min or at 60°C for 45 min or by treatment with 0.2% formaldehyde for 1 h at 37°C. Killed bacteria showed a marked reduction in adhesion levels (∼70% reduction of bacterial binding; P < 0.0001), independent of the applied treatment (Table 2).
Competitive binding studies.
J774 macrophages were pretreated with the monosaccharides galactose, glucose, N-acetyl-d-glucosamine, rhamnose, and N-acetylneuraminic acid, which are the five components of the S. suis capsule. In addition, the conjugate 6′-N-acetyl-neuramyl-N-acetyl-lactosamine was also evaluated, since it contains the link α-Neu-5Ac-(2→6)-Gal, which has been hypothesized to be present in the capsule structure (8). Since mannan-binding lectin was shown to bind to S. suis surface (48), d-mannose was also included. Even though several concentrations of sugars were tested, only N-acetylneuraminic acid, at a concentration of 100 μg/ml, showed a significant effect on S. suis adhesion after 30 min of bacterium-cell contact (47% ± 8% of inhibition; P < 0.01). Higher concentrations of sialic acid did not increase the inhibitory effect (data not shown). In addition, J774 macrophages were also pretreated with different concentrations of purified S. suis CPS or purified S. suis cell wall or purified S. faecalis LTA before addition of bacteria (at three different inoculum concentrations, 105, 106, and 107 CFU/well). None affected S. suis adhesion after 30 min of bacterium-cell contact (data not shown).
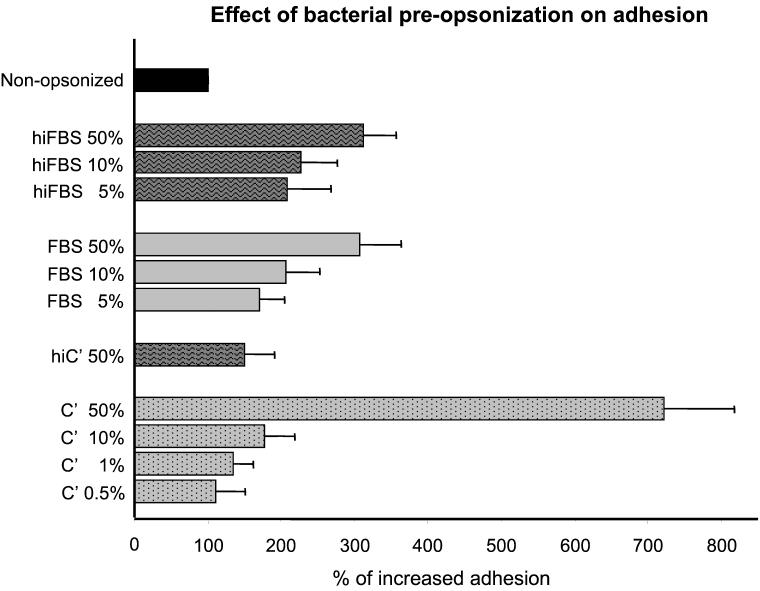
Effect of bacterial preopsonization on adhesion.
Figure 3 shows that preopsonization (30 min at 37°C) of S. suis with different concentrations of hiFBS resulted in significantly increased adhesion to J774 macrophages (P < 0.001 with respect to control bacteria that were preincubated 30 min with BSA-dextrose). A similar increase in adhesion was observed when different concentrations of normal FBS serum were used (P > 0.1 with respect to hiFBS) (Fig. 3). On the other side, when C'MS was used instead of FBS, the increase in adhesion was even higher (P < 0.001). This increase was dose dependent and was reduced by heating at 56°C for 30 min to destroy the complement (P < 0.01 with respect to C'MS) (Fig. 3).
FIG. 3.
Effect of bacterial preopsonization on 30-min adhesion to J774 macrophages. S. suis strain 31533 was preopsonized 30 min at 37°C with different concentrations of normal FBS, hiFBS, C'MS (C′), or heat-inactivated C'MS (hiC′). Preopsonized bacteria or nonopzonized control bacteria (bacteria preincubated 30 min with 5% BSA-2% dextrose in DMEM) were added at 106 CFU/well to macrophage plates. Data are expressed as means ± standard deviations of percentage of increased adhesion with respect to adhesion found with control bacteria (100% adhesion).
S. suis can damage J774 macrophages.
S. suis strain 31533 was shown to be cytotoxic to J774 cells, as evaluated by the LDH test and methylene blue stain. Cytotoxic effects were dose dependent, with maximal cytotoxic levels at 107 CFU/well and higher bacterial concentrations, after 3 h of bacterium-cell contact (data not shown, P < 0.001). An MOI of 107 bacteria/well was then chosen to study the kinetics of cytotoxicity by S. suis. Cytotoxicity was low at 30 min of incubation but increased with incubation time, reaching a plateau between 2 and 3 h of incubation (P < 0.001) (Fig. 4). The kinetics of cell damage was similar to that observed by using the methylene blue stain; however, sensitivity was higher with the LDH test (Fig. 4). This difference could be due to the release of LDH by damaged but still attached macrophages.
Suilysin-associated injury to J774 macrophages.
Table 1 shows that, while some S. suis isolates did not injure J774 cells at all, others were highly cytotoxic after 3 h of bacterium-cell contact. Interestingly, all of the cytotoxic strains produce the suilysin, as well as the MRP and EF proteins. It is known that suilysin is excreted in vitro in culture supernatants (18). Table 3 shows that addition of the culture supernatant from suilysin-positive strain 31533 to J774 cells induced cell injury, whereas the supernatant from suilysin-negative strain 89-1591 did not affect the cells. Moreover, purified suilysin reproduced the observed cytotoxic effects, reaching maximal cytotoxicity at 5 μg/ml. Inhibition studies were performed to confirm the involvement of suilysin in cell damage. First, to determine if cytotoxicity requires live bacteria, strain 31533 was heat killed and the suspension was added to J774 cells. Heat-killed strain 89-1591 was used as control. Results showed that only live suilysin-positive bacteria induced J774 injury (Table 3). Cholesterol has been demonstrated to inhibit suilysin activity (10). Thus, bacterial supernatants or purified suilysin (5 μg/ml) were treated with cholesterol (100 μg/ml for 1 h at 37°C) before addition of them to J774 cells. Table 3 shows almost complete inhibition of cytotoxicity after cholesterol treatment.
TABLE 3.
Role of bacterial products in cytotoxicity and inhibition of cytotoxic activity
| Treatment | Suilysin production | % Cytotoxicitya |
|---|---|---|
| Medium alone | 0 (±0) | |
| Strain 31533 | Yes | 73 (±16) |
| Strain 89-1591 | No | 0 (±0) |
| Strain 31533 (killed) | No | 3 (±4) |
| Strain 89-1591 (killed) | No | 8 (±7) |
| Strain 31533 (supernatant) | Yes | 72 (±7) |
| Strain 89-1591 (supernatant) | No | 0 (±0) |
| Purified suilysin | 1 μg/ml | 81 (±16) |
| 2.5 μg/ml | 90 (±9) | |
| 5 μg/ml | 100 (±1) | |
| Medium alone | + cholesterol | 2 (±2) |
| Strain 31533 (supernatant) | + cholesterol | 0 (±0) |
| Strain 89-1591 (supernatant) | + cholesterol | 0 (±0) |
| Purified suilysin (5 μg/ml) | + cholesterol | 9 (±3) |
Cytotoxicity in cultures of infected J774 cells was determined by measurement of LDH release after 3 h of bacterium-cell contact at an infection ratio of 100 bacteria/cell (107 CFU/well). Data are expressed as the percentage of cytotoxicity (± standard deviations) in infected wells with respect to control wells with macrophages alone (n = 4). Cholesterol inhibition was performed as follows 1 h at 37°C with 100 μg of ethanol-soluble cholesterol/ml.
Cytotoxic effects of S. suis to J774 macrophages were confirmed by TEM. Suilysin-negative strain 89-1591 did not affect cell integrity. After 3 h of bacterium-cell contact, characteristics of normal cells (similar to those seen in uninfected cells; Fig. 5A) are maintained, such as dense regular cytoplasmic contents and evenly distributed nuclear chromatin (Fig. 5B). In contrast, 3 h of exposure of J774 macrophages to the suilysin-positive strain 31533 resulted in cellular damage demonstrated by loss of cytoplasmic density, disruption of cytoplasmic membranes with release of cellular contents, and disappearance of the nucleus (Fig. 5C).
FIG. 5.
Transmission electron micrographs demonstrating J774 macrophage injury after infection with 108 CFU of S. suis per ml. (A) Noninfected control cells. (B) Cells incubated for 3 h with the suilysin-negative strain 89-1591. (C) Cells incubated with the suilysin-positive strain 31533. J774 integrity after 3 h of incubation with strain 89-1591 was comparable to that of noninfected control cells. Injury wasmanifested by the loss of cytoplasmic density, severe disruption of cytoplasmic membranes with release of cellular contents (arrowheads), and disappearance of the nucleus. Bars, 0.5 μm. Arrows indicate S. suis cocci.
DISCUSSION
It has been clearly demonstrated by two different research groups that encapsulated S. suis is able to resist uptake by both murine and porcine monocytes/macrophages and that the CPS is responsible for phagocytosis resistance (6, 40, 42). The present work adds evidence that S. suis is able to interact with macrophages and remains extracellular and largely bound to cells. Cytochalasin C pretreatment of cells had no effect in adhesion and thus confirmed that S. suis is not phagocytosed, as previously shown by use of the viable counting technique after infection of J774 cells (40). In addition, we have previously shown that cytochalasin pretreatment does not have an effect on tumor necrosis factor alpha or interleukin-6 production by J774 cells after S. suis stimulation (38). Electron microscopy analysis reveals attachment of S. suis to either protrusions of the cell surface or the plain plasma membrane of macrophages. Similar types of adhesion were observed in ultrastructural analysis of Brucella suis-infected monocytes; however, for this bacterial species, attachment does result in ingestion by monocytes (36). In contrast, intracellular S. suis was rarely observed by TEM analysis (data not shown).
The rapid occurrence of S. suis interactions with macrophages is similar to those reported by Albanyan et al. (1) for the association of group B Streptococcus with polymorphonuclear neutrophils (1). S. suis adhesion increases by increasing bacterial concentration in a stable, wash-resistant manner after 30 min of bacterium-cell contact. It must be noted that these microorganisms tend to bind with each other and then bind to macrophages in chains, a phenomenon that we observed by direct microscopy (data not shown) and electron microscopy (Fig. 2C). Similarly, this phenomenon has also been reported in studies of group B Streptococcus binding to polymorphonuclear neutrophils (1).
In order to preliminarily characterize S. suis components implicated in adhesion to macrophages, bacteria were subjected to several pretreatments. Despite testing of several different protease digestions, S. suis adhesion to macrophages was shown to be protease resistant. S. suis proteins that have a role as adhesins have previously been described. S. suis was found to recognize the disaccharide sequence Galα1-4Gal present in the trihexosylceramide GbO3 (20). The protease-sensitive adhesin responsible for this interaction was named the P adhesin (47). Thus, the interactions between S. suis and J774 cells seem to be different from those described in the hemagglutination tests (20). Similarly, a role of the P adhesin in S. suis adhesion to epithelial cells could not be demonstrated (28). On the other hand, heat-killed or formaldehyde-killed bacteria show a marked reduction in adhesion levels. It could be suggested that bacterial interaction with macrophages is sensitive to these treatments, or another possibility is that viable bacteria are required for binding, as already reported for other pathogens (45).
Since the S. suis capsule contains sialic acid and since this sugar has been implicated in adhesion of other organisms (29), sialidase treatment was also performed. Sialidase-treated bacteria showed reduced adhesion levels compared to those of nontreated bacteria, indicating a possible role of the capsular sialic acid moiety in attachment to macrophages. In addition, competitive binding studies with N-acetylneuraminic acid also reduced levels of adhesion and further suggest participation of this bacterial sugar in cell attachment. None of the other sugars known to be present in the bacterial surface showed an effect on S. suis adhesion, nor did the purified CPS. This result is surprising, since purified CPS contains sialic acid. One possible explanation is that the extraction method could damage the sialic acid moiety being recognized by macrophages or that the sialic acid concentration of purified CPS material was not enough to obtain a significant inhibition. Indeed, the sialic acid concentration of S. suis type 2 was shown to be ∼2 to 3.5 μg/mg of cells by the Warren-Aminoff method (9). Charland et al. (8) reported the agglutination of S. suis cells with sialic acid-binding lectins and suggested that sialic acid is the possible terminal sequence on the type 2 capsular component (7). The exact chemical structure and epitope portion of the type 2 antigen-containing sialic acid, however, still remain unkown. Since the conjugate α-Neu-5Ac-(2→6)-β-d-Gal-(1→4)-d-GlcNAc was not able to interfere with S. suis adhesion, it could be hypothesized that this type of link is either not present in the S. suis CPS or is not recognized by the J774 cells.
Components of the gram-positive bacterium cell wall, such as LTA and peptidoglycans, have been shown to be important adhesins that are recognized by various receptors on host cells (12, 19, 50). When the purified cell wall of S. suis was used in competitive binding studies, no effect on bacterial adhesion to J774 cells was observed. Purified individual subcomponents of the S. suis cell wall are not yet available, and poor information on the LTA or peptidoglycan structure is found in the literature (14, 26). To further investigate the potential role of the cell wall in S. suis adhesion to macrophages, purified S. faecalis LTA was evaluated in a competitive binding assay. Even though both S. suis LTA and S. faecalis LTA react with group D antiserum and have some structural similarities (14), S. faecalis LTA failed to inhibit the binding of S. suis to J774 cells. It has been shown that most of S. suis teichoic acid is lipid bound, located deep in the cell wall and hardly accessible to extraction (14). Thus, it is probably partially exposed at the bacterial surface or is less accessible for interacting with macrophage receptors. Even though the purified cell wall material from S. suis was shown to stimulate cytokine production by J774 cells, the presence of capsule partially masks the tumor necrosis factor alpha response and thus supports the hypothesis that S. suis LTA is probably poorly exposed at the bacterial surface, as discussed above (38).
Only a partial inhibition of binding was observed under the conditions used in the present study. These findings indicate that a number of recognition processes are involved in binding of nonopsonized bacteria to the phagocyte surface (51). Even though capsular sialic acid plays a role in S. suis adhesion to macrophages, it does not seem to be critical for virulence, since field strains of S. suis type 2 possess similar sialic acid concentrations regardless of their virulence and since blocking or enzymatic removal of this sugar does not influence the virulence and phagocytosis rates of S. suis (7-9). Since carbohydrate-specified molecular interactions may depend on extended oligosaccharide structures, in which case free monosaccharides may be poor inhibitors, a role for S. suis surface components other than sialic acid could not be excluded.
Attachment of bacteria to mammalian host cells is often mediated by sugar-lectin interactions (33). Binding of L. monocytogenes to murine macrophages was shown to be affected following bacterial treatment with neuraminidase and to be competitively inhibited by N-acetylneuraminic acid (29). Mannose and polysialic acid have been shown to facilitate the attachment of Mycobacterium tuberculosis and Neisseria meningitidis, respectively, to host cells (37, 44). S. suis was shown to bind the mannan-binding lectin (48), which recognizes mannose, N-acetylglucosamine, and glucose (33). However, neither mannose, N-acetylglucosamine, nor glucose was able to inhibit the attachment of S. suis to macrophages under the conditions used in the present study.
It has been shown that adherence to host cell surfaces can take place by two different mechanisms: an opsonin-dependent process, in which antibody and/or complement proteins become involved in the complex interaction between bacteria and host cell, and an opsonin-independent process, in which adhesins present on the bacterial cell surface directly recognize host cell receptors (1, 15, 29, 30). It has been previously shown that complement does not affect rates of S. suis phagocytosis by macrophages (5, 9). On the other hand, it was reported in the literature that complement or other serum factors could mediate adhesion without ingestion of some pathogens, such as H. influenzae type b (30, 31). In this regard, in the present study it was demonstrated that serum preopsonization of S. suis resulted in a marked increase in adhesion and that complement would be, at least in part, responsible for the increased bacterial adhesion to J774 cells, as demonstrated by using an exogenous source of mouse complement. However, other unknown serum factors would also be implicated, since heating of C'MS only partially reduced bacterial opsonization, and in addition, no differences were observed between heat-inactivated FBS and normal FBS in their ability to increase S. suis adhesion to J774 cells. Similarly, it has been reported that adhesion of N. meningitidis to human macrophages was significantly increased after opsonization with nonimmune C5-depleted serum (35), and other authors suggested that coating of meningococci with C3b and/or other serum proteins would be responsible for the increased adhesion. In the case of Streptococcus pneumoniae, interaction of bacteria with human alveolar macrophages in the absence of opsonization results in relatively poor binding, while preopsonization with complement enhances not only the binding but also the internalization and killing of pneumococci (15). Thus, the fate of S. suis after interaction with macrophages under both nonopsonic and opsonic conditions seems to be different from that of this streptococcal species (9, 15, 40).
It was demonstrated in the present study that S. suis not only adheres and resists phagocytosis but also induces cytotoxic effects to macrophages as incubation time and bacterial concentrations increase. The observed cytotoxicity correlates with the proposed mode of action of suilysin, namely, a multihit activity by accumulation of suilysin molecules at the surface of cells (18). Indeed, many lines of evidence implicate suilysin as the bacterial component responsible for in vitro macrophage cytotoxicity. First, only live suilysin-producing strains are toxic for macrophages. Second, the J774-cytotoxic component is present only in culture supernatants. It is known that suilysin is excreted during bacterial growth (18, 24). Third, the purified suilysin is toxic to J774 cells and cholesterol inhibits this effect. Cholesterol is required for the binding of the toxin to cell membranes, and free cholesterol acts as a competitive inhibitor (18). S. suis suilysin has also been shown to be cytotoxic to brain microvascular endothelial cells and epithelial cells (10, 28, 32) and thus may facilitate bacterial dissemination (17). Recently, a defined allelic replacement mutant of the sly gene, encoding the suilysin, was shown not to be toxic for J774 cells and thus further proved that the suilysin was probably the only cytolysin produced by S. suis (2).
While suilysin is implicated as an important virulence factor in European S. suis type 2 strains, the same does not seem to be the case for North American strains. In fact, unlike European strains, most virulent field strains isolated from diseased pigs or humans in North America do not produce suilysin. Similarly, most European strains produce MRP and EF proteins (named virulence markers), whereas variable production of these proteins has been observed with North American strains (16, 43). It has been suggested that the pathogenesis of the infection caused by suilysin-positive strains (European phenotype) and that of the infection caused by suilysin-negative strains (North American phenotype) are different and that different virulence factors are involved in each case (17). Indeed, in the present study, it was shown that bacteria from strains presenting the North American phenotype adhered in higher numbers than did those from strains presenting the European phenotype (Table 1). Thus, the results described herein give additional evidence that the pathogenesis of the infection may differ for S. suis strains. In particular, it is possible that suilysin-positive strains use adherence and cell injury for dissemination and evasion of the host immune system. This would be opposed to the behavior of suilysin-negative strains, which may use adherence and macrophage-dependent dissemination as a part of a complicated multistep process that leads to bacteremia and meningitis in the host (10, 17, 28). The present study demonstrates for the first time that S. suis is largely bound but not ingested by macrophages and thus remains extracellular. Further studies will be needed to characterize the molecule(s) that is responsible for adherence and bacterial phagocytosis resistance.
Acknowledgments
We gratefully acknowledge D. Montpetit from the Centre de Recherche et Développement sur les Aliments (CRDA) for the transmission and scanning electron micrographs and S. Lacouture for technical assistance. We also thank M. Jacques for critical review of the manuscript. We are also indebted to M. Kobisch (Centre National d'Études Vétérinaires et Alimentaires, Ploufragan, France), T. Alexander (Cambridge University, Cambridge, United Kingdom), L. Brasme (Centre Hospitalier Universitaire de Reims, Reims, France), B. Fenwick (Kansas University), and P. Norton (Institute for Animal Health, Compton, United Kingdom) for providing some of the S. suis type 2 strains.
This work was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) grant no. 0680154280, the Fonds pour la Formation des Chercheurs et l'Aide à la Recherche du Québec (FCAR-équipe) grant no. 99-ER-0214, and by the Canadian Research Network on Bacterial Pathogens of Swine.
Editor: E. I. Tuomanen
REFERENCES
- 1.Albanyan, E. A., J. G. Vallejo, C. W. Smith, and M. S. Edwards. 2000. Nonopsonic binding of type III group B streptococci to human neutrophils induces interleukin-8 release mediated by the p38 mitogen-activated protein kinase pathway. Infect. Immun. 68:2053-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, A. G., S. Bolitho, H. Lindsay, S. Khan, C. Bryant, P. M. Norton, P. N. Ward, J. A. Leigh, J. Morgan, H. Riches, S. Eastty, and D. Maskell. 2001. Generation and characterization of a defined mutant of Streptococcus suis lacking suilysin. Infect. Immun. 69:2732-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Athamna, A., and I. Ofek. 1988. Enzyme-linked immunosorbent assay for quantification of attachment and ingestion stages of bacterial phagocytosis. J. Clin. Microbiol. 26:62-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaudoin, M., R. Higgins, J. Harel, and M. Gottschalk. 1992. Studies on a murine model for evaluation of virulence of Streptococcus suis capsular type 2 isolates. FEMS Microbiol. Lett. 78:111-116. [DOI] [PubMed] [Google Scholar]
- 5.Brazeau, C., M. Gottschalk, S. Vincelette, and B. Martineau-Doize. 1996. In vitro phagocytosis and survival of Streptococcus suis capsular type 2 inside murine macrophages. Microbiology 142:1231-1237. [DOI] [PubMed] [Google Scholar]
- 6.Charland, N., J. Harel, M. Kobish, S. Lacasse, and M. Gottschalk. 1998. Streptococcus suis serotype 2 mutants deficient in capsular expression. Microbiology 144:325-332. [DOI] [PubMed] [Google Scholar]
- 7.Charland, N., M. Jacques, S. Lacouture, and M. Gottschalk. 1997. Characterization and protective activity of a monoclonal antibody against a capsular epitope shared by Streptococcus suis serotypes 1, 2 and 1/2. Microbiology 143:3607-3614. [DOI] [PubMed] [Google Scholar]
- 8.Charland, N., J. T. Kellens, F. Caya, and M. Gottschalk. 1995. Agglutination of Streptococcus suis by sialic acid-binding lectins. J. Clin. Microbiol. 33:2220-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charland, N., M. Kobisch, B. Martineau-Doize, M. Jacques, and M. Gottschalk. 1996. Role of capsular sialic acid in virulence and resistance to phagocytosis of Streptococcus suis capsular type 2. FEMS Immunol. Med. Microbiol. 14:195-203. [DOI] [PubMed] [Google Scholar]
- 10.Charland, N., V. Nizet, C. Rubens, K. S. Kim, S. Lacouture, and M. Gottschalk. 2000. Streptococcus suis serotype 2 interactions with human brain microvascular endothelial cells. Infect. Immun. 68:637-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatellier, S., M. Gottschalk, R. Higgins, R. Brousseau, and J. Harel. 1999. Relatedness of Streptococcus suis serotype 2 isolates from different geographic origins as evaluated by molecular fingerprinting and phenotyping. J. Clin. Microbiol. 37:362-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Courtney, H. S., J. B. Dale, and D. L. Hasty. 1997. Host cell specific adhesins of group A streptococci. Adv. Exp. Med. Biol. 418:605-606. [DOI] [PubMed] [Google Scholar]
- 13.del Campo Sepulveda, E. M., E. Altman, M. Kobisch, S. D'Allaire, and M. Gottschalk. 1996. Detection of antibodies against Streptococcus suis capsular type 2 using a purified capsular polysaccharide antigen-based indirect ELISA. Vet. Microbiol. 52:113-125. [DOI] [PubMed] [Google Scholar]
- 14.Elliott, S. D., M. McCarty, and R. C. Lancefield. 1977. Teichoic acids of group D streptococci with special reference to strains from pig meningitis (Streptococcus suis). J. Exp. Med. 145:490-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon, S. B., G. R. B. Irving, R. A. Lawson, M. E. Lee, and R. C. Read. 2000. Intracellular trafficking and killing of Streptococcus pneumoniae by human alveolar macrophages are influenced by opsonins. Infect. Immun. 68:2286-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottschalk, M., A. Lebrun, H. Wisselink, J. D. Dubreuil, H. Smith, and U. Vecht. 1998. Production of virulence-related proteins by Canadian strains of Streptococcus suis capsular type 2. Can. J. Vet. Res. 62:75-79. [PMC free article] [PubMed] [Google Scholar]
- 17.Gottschalk, M., and M. Segura. 2000. The pathogenesis of the meningitis caused by Streptococcus suis: the unresolved questions. Vet. Microbiol. 75:59-71. [DOI] [PubMed] [Google Scholar]
- 18.Gottschalk, M. G., S. Lacouture, and J. D. Dubreuil. 1995. Characterization of Streptococcus suis capsular type 2 haemolysin. Microbiology 141:189-195. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg, J. W., W. Fischer, and K. A. Joiner. 1996. Influence of lipoteichoic acid structure on recognition by the macrophage scavenger receptor. Infect. Immun. 64:3318-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haataja, S., K. Tikkanen, J. Hytonen, and J. Finne. 1996. The Gal alpha 1-4 Gal-binding adhesin of Streptococcus suis, a gram-positive meningitis-associated bacterium. Adv. Exp. Med. Biol. 408:25-34. [DOI] [PubMed] [Google Scholar]
- 21.Higgins, R., and M. Gottschalk. 2001. Distribution of Streptococcus suis capsular types in 2000. Can. Vet. J. 42:223. [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins, R., and M. Gottschalk. 1999. Streptococcal diseases, p. 563-570. In B. E. Straw, S. D'Allaire, W. L. Mengeling, and D. J. Taylor (ed.), Diseases of swine. Iowa State University, Ames.
- 23.Higgins, R., and M. Gottschalk. 1990. An update on Streptococcus suis identification. J. Vet. Diagn. Investig. 2:249-252. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs, A. A., P. L. Loeffen, A. J. van den Berg, and P. K. Storm. 1994. Identification, purification, and characterization of a thiol-activated hemolysin (suilysin) of Streptococcus suis. Infect. Immun. 62:1742-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katsumi, M., T. Saito, Y. Kataoka, T. Itoh, N. Kikuchi, and T. Hiramune. 1996. Comparative preparation methods of sialylated capsule antigen from Streptococcus suis type 2 with type specific antigenicity. J. Vet. Med. Sci. 58:947-952. [DOI] [PubMed] [Google Scholar]
- 26.Kilpper-Bälz, R., and K. H. Schleifer. 1987. Streptococcus suis sp. nov., nom. rev. Int. J. Syst. Bacteriol. 37:160-162. [Google Scholar]
- 27.Kobisch, M., M. Gottschalk, P. Morvan, R. Cariolet, G. Bénévent, and J. P. Joly. 1995. Experimental infection of SPF piglets with Streptococcus suis serotype 2. Journ. Rech. Porcine France 27:97-102. [Google Scholar]
- 28.Lalonde, M., M. Segura, S. Lacouture, and M. Gottschalk. 2000. Interactions between Streptococcus suis serotype 2 and different epithelial cell lines. Microbiology 146:1913-1921. [DOI] [PubMed] [Google Scholar]
- 29.Maganti, S., M. M. Pierce, A. Hoffmaster, and F. G. Rodgers. 1998. The role of sialic acid in opsonin-dependent and opsonin-independent adhesion of Listeria monocytogenes to murine peritoneal macrophages. Infect. Immun. 66:620-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noel, G. J., S. K. Hoiseth, and P. J. Edelson. 1992. Type b capsule inhibits ingestion of Haemophilus influenzae by murine macrophages: studies with isogenic encapsulated and unencapsulated strains. J. Infect. Dis. 166:178-182. [DOI] [PubMed] [Google Scholar]
- 31.Noel, G. J., D. M. Mosser, and P. J. Edelson. 1990. Role of complement in mouse macropahge binding of Haemophilus influenzae type b. J. Clin. Investig. 85:208-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norton, P. M., C. Rolph, P. N. Ward, R. W. Bentley, and J. A. Leigh. 1999. Epithelial invasion and cell lysis by virulent strains of Streptococcus suis is enhanced by the presence of suilysin. FEMS Immunol. Med. Microbiol. 26:25-35. [DOI] [PubMed] [Google Scholar]
- 33.Ofek, I., and N. Sharon. 1988. Lectinophagocytosis: a molecular mechanism of recognition between cell surface sugars and lectins in the phagocytosis of bacteria. Infect. Immun. 56:539-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quessy, S., J. D. Dubreuil, M. Caya, and R. Higgins. 1995. Discrimination of virulent and avirulent Streptococcus suis capsular type 2 isolates from different geographical origins. Infect. Immun. 63:1975-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Read, R. C., S. Zimmerli, V. C. Broaddus, D. A. Sanan, D. S. Stephens, and J. D. Ernst. 1996. The (α2-8)-linked polysialic acid capsule of group B Neisseria meningitidis modifies multiple steps during interaction with human macrophages. Infect. Immun. 64:3210-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rittig, M. G., M. T. Alvarez-Martinez, F. Porte, J.-P. Liautard, and B. Rouot. 2001. Intracellular survival of Brucella spp. in human monocytes involves conventional uptake but special phagosomes. Infect. Immun. 69:3995-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlesinger, L. S. 1993. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J. Immunol. 150:2920-2930. [PubMed] [Google Scholar]
- 38.Segura, M., J. Stankova, and M. Gottschalk. 1999. Heat-killed Streptococcus suis capsular type 2 strains stimulate tumor necrosis factor alpha and interleukin-6 production by murine macrophages. Infect. Immun. 67:4646-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segura, M., N. Vadeboncoeur, and M. Gottschalk. 2002. CD14-dependent and -independent cytokine and chemokine production by human THP-1 monocytes stimulated by Streptococcus suis capsular type 2. Clin. Exp. Immunol. 127:243-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segura, M. A., P. Cléroux, and M. Gottschalk. 1998. Streptococcus suis and group B Streptococcus differ in their interactions with murine macrophages. FEMS Immunol. Med. Microbiol. 21:189-195. [DOI] [PubMed] [Google Scholar]
- 41.Sloan, A. R., and T. G. Pistole. 1992. A quantitative method for measuring the adherence of group B streptococci to murine peritoneal exudate macrophages. J. Immunol. Methods 154:217-223. [DOI] [PubMed] [Google Scholar]
- 42.Smith, H. E., M. Damman, J. Van der Velde, F. Wagenaar, H. J. Wisselink, N. Stockhofe-Zurwieden, and M. A. Smits. 1999. Identification and characterization of the cps locus of Streptococcus suis serotype 2: the capsule protects against phagocytosis and is an important virulence factor. Infect. Immun. 67:1750-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staats, J., P. Brandon, G. Stewart, and M. M. Chengappa. 1999. Presence of the Streptococcus suis suilysin gene and expression of MRP and EF correlates with high virulence in Streptococcus suis type 2 isolates. Vet. Microbiol. 70:201-211. [DOI] [PubMed] [Google Scholar]
- 44.Stephens, D. S., P. A. Spellman, and J. S. Swartley. 1993. Effect of the (α2-8)-lined polysialic acid capsule on adherence of Neisseria meningitidis to human mucosal cells. J. Infect. Dis. 167:475-479. [DOI] [PubMed] [Google Scholar]
- 45.St. Geme, J. W., III, and S. Falkow. 1990. Haemophilus influenzae adheres to and enters cultured human epithelial cells. Infect. Immun. 58:4036-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamura, G. S., J. M. Kuypers, S. Smith, H. Raff, and C. E. Rubens. 1994. Adherence of group B streptococci to cultured epithelial cells: roles of environmental factors and bacterial surface components. Infect. Immun. 62:2450-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tikkanen, K., S. Haataja, C. Francois-Gerard, and J. Finne. 1995. Purification of a galactosyl-alpha 1-4-galactose-binding adhesin from the gram-positive meningitis-associated bacterium Streptococcus suis. J. Biol. Chem. 270:28874-28878. [DOI] [PubMed] [Google Scholar]
- 48.van Emmerik, L. C., E. J. Kuijper, C. A. P. Fijen, J. Dankert, and S. Thiel. 1994. Binding of mannan-binding protein to various bacterial pathogens of meningitis. Clin. Exp. Immunol. 97:411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vecht, U., H. J. Wisselink, M. L. Jellema, and H. E. Smith. 1991. Identification of two proteins associated with virulence of Streptococcus suis type 2. Infect. Immun. 59:3156-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weidemann, B., J. Schletter, R. Dziarski, S. Kusumoto, F. Stelter, E. T. Rietschel, H. D. Flad, and A. J. Ulmer. 1997. Specific binding of soluble peptidoglycan and muramyldipeptide to CD14 on human monocytes. Infect. Immun. 65:858-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weir, D. M., J. Stewart, and E. Glass. 1982. Phagocyte recognition by lectin receptors. Immunobiology 161:334-344. [DOI] [PubMed] [Google Scholar]
- 52.Wessels, M. R., C. E. Rubens, V. J. Benedi, and D. L. Kasper. 1989. Definition of a bacterial virulence factor: sialylation of the group B streptococcal capsule. Proc. Natl. Acad. Sci. USA 86:8983-8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams, A. E., and W. F. Blakemore. 1990. Pathogenesis of meningitis caused by Streptococcus suis type 2. J. Infect. Dis. 162:474-481. [DOI] [PubMed] [Google Scholar]