Abstract
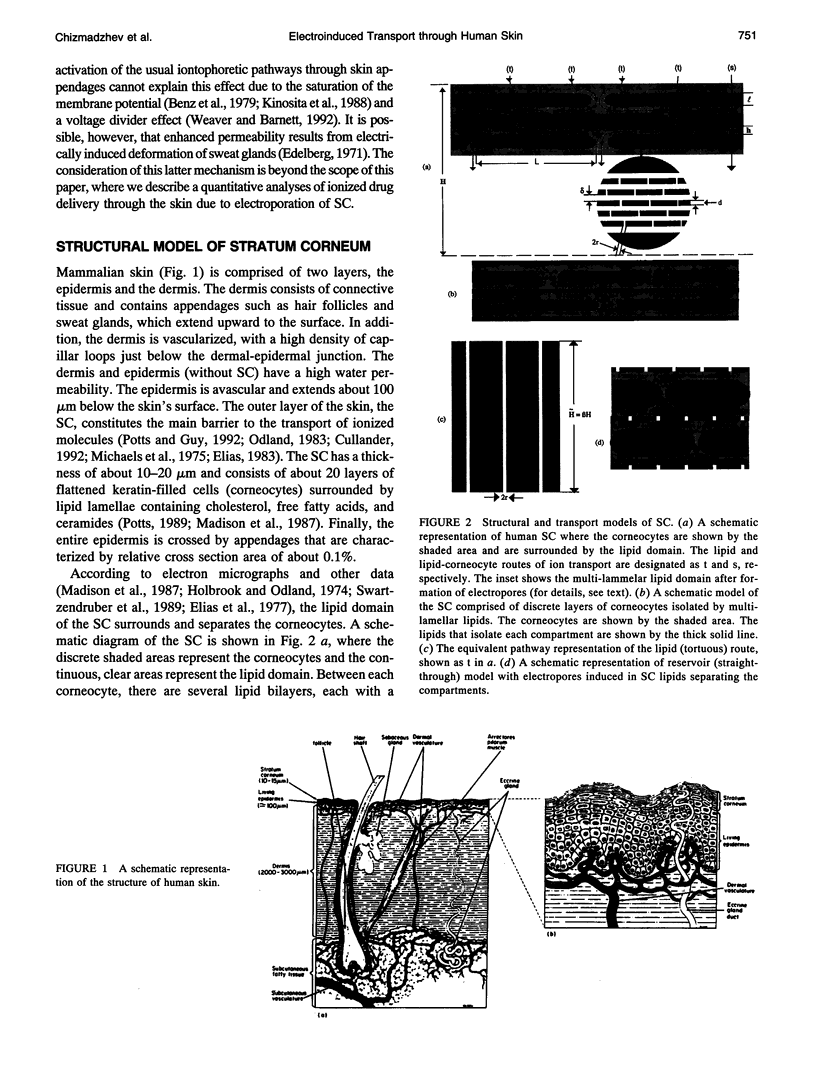
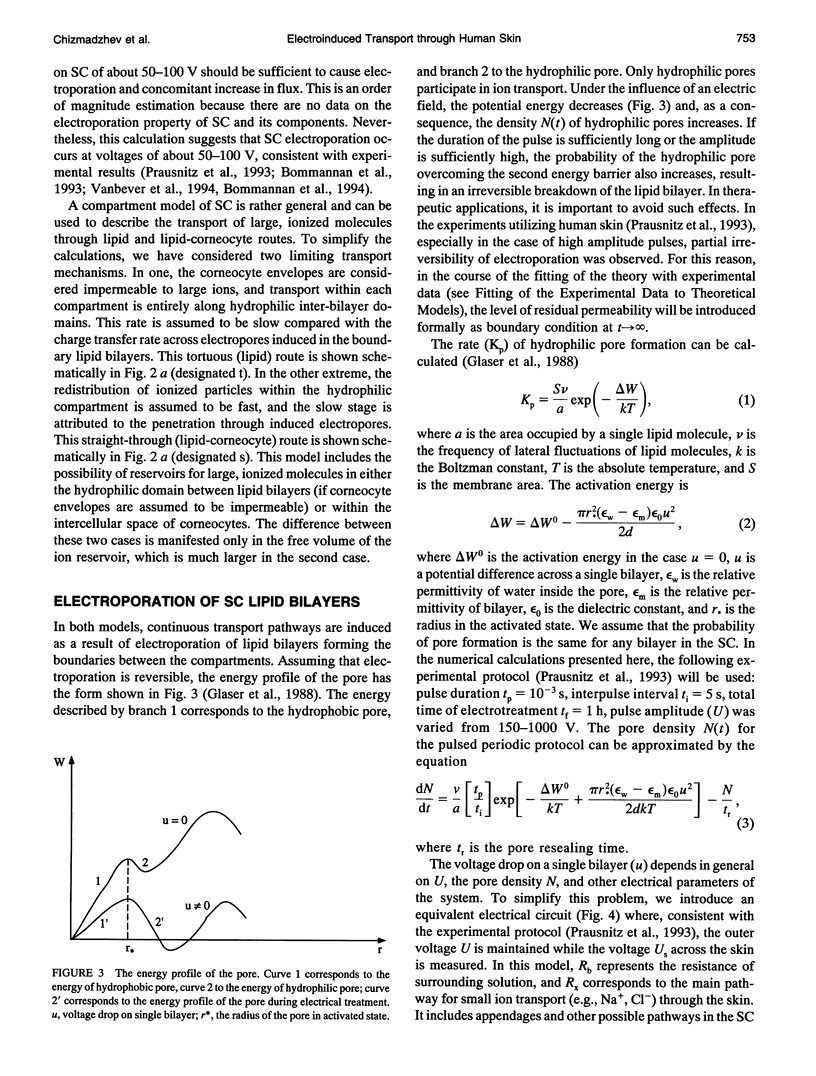
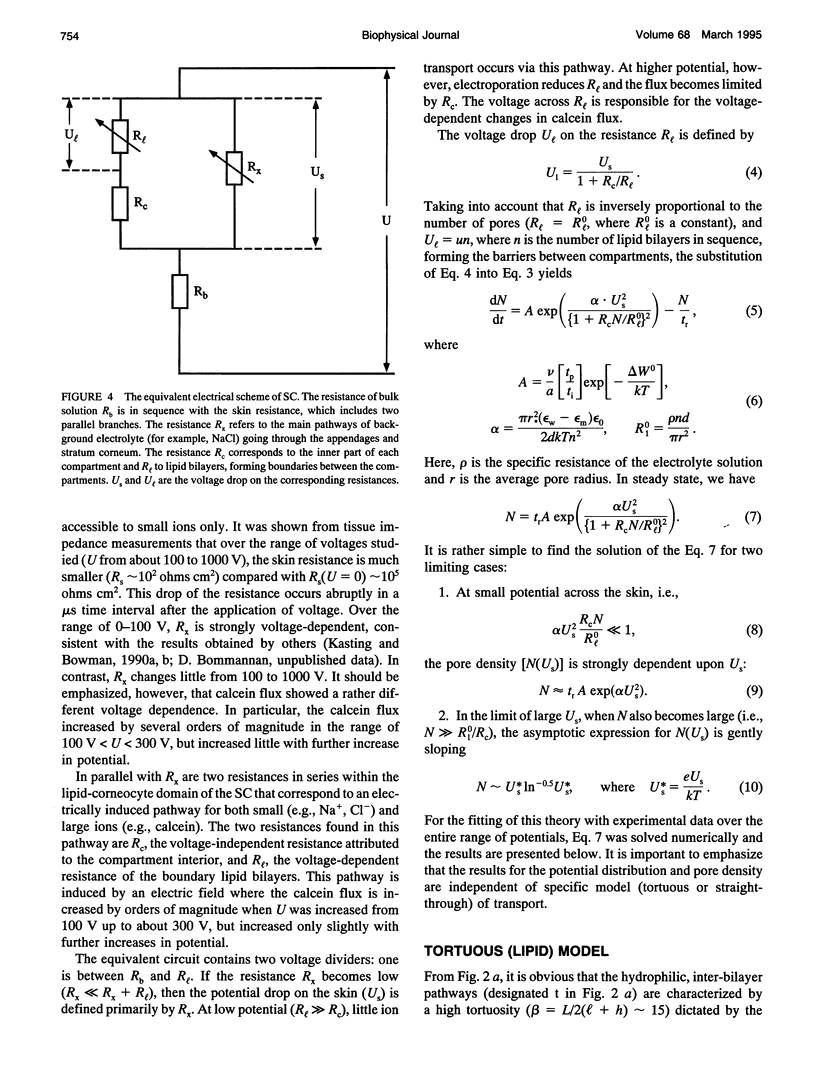
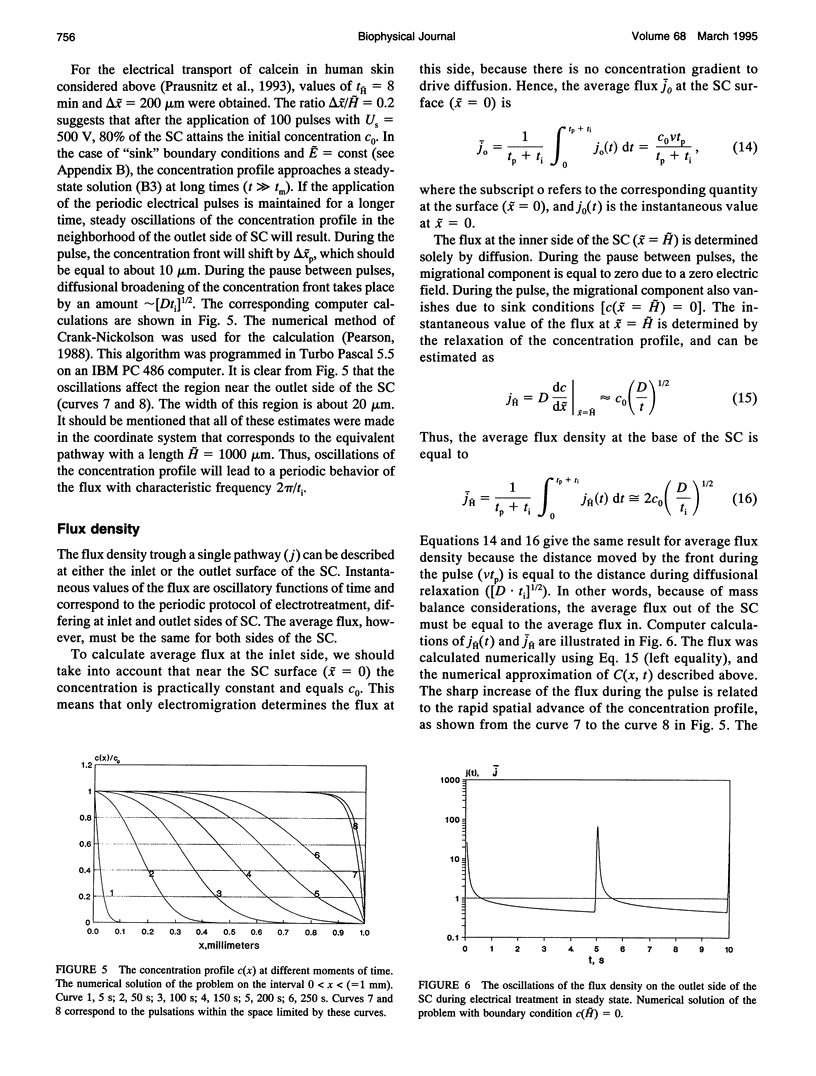
A theoretical model for electroporation of multilamellar lipid system due to a series of large electrical pulses is presented and then used to predict the functional dependence of the transport of charged molecules. Previously, electroporation has been considered only for single bilayer systems such as artificial planar bilayer membranes and cell membranes. The former have been extensively studied with respect to electrical and mechanical behavior, and the latter with respect to molecular transport. Recent experimental results for both molecular transport and electrical resistance changes in the stratum corneum (SC) suggest that electroporation also occurs in the multilamellar lipid membranes of the SC. In addition, there is the possibility that other skin structures (the "appendages") also experience electroporation. A compartment model is introduced to describe the transport of charged species across the SC, and the predicted dependence is compared with available data. In this model, the SC is assumed to contain many hydrophilic compartments in series separated by boundary bilayers, so that these compartments become connected only upon electroporation. Two limiting cases for the transport of charged molecules are considered: (1) transport along tortuous inter-bilayer pathways in each compartment, followed by transport across individual boundary bilayers due to electroporation, and (2) transport along straight-through pathways in the boundary bilayers with fast mixing in each compartment, which includes the interior space of corneocytes. Both models were fitted to the experimental data. The large electropore radius (rt approximately 200 A) and porated fractional area (ft approximately 10(-3) obtained from the fitting for the tortuous model relative to the more reasonable values obtained for the straight-through model (rs approximately 4 A, fs approximately 10(-6) suggest that the latter is a more realistic description of electroinduced transport of ionized species through the skin.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Beckers F., Zimmermann U. Reversible electrical breakdown of lipid bilayer membranes: a charge-pulse relaxation study. J Membr Biol. 1979 Jul 16;48(2):181–204. doi: 10.1007/BF01872858. [DOI] [PubMed] [Google Scholar]
- Bommannan D. B., Tamada J., Leung L., Potts R. O. Effect of electroporation on transdermal iontophoretic delivery of luteinizing hormone releasing hormone (LHRH) in vitro. Pharm Res. 1994 Dec;11(12):1809–1814. doi: 10.1023/a:1018983804635. [DOI] [PubMed] [Google Scholar]
- Cullander C., Guy R. H. Sites of iontophoretic current flow into the skin: identification and characterization with the vibrating probe electrode. J Invest Dermatol. 1991 Jul;97(1):55–64. doi: 10.1111/1523-1747.ep12478060. [DOI] [PubMed] [Google Scholar]
- Elias P. M. Epidermal lipids, barrier function, and desquamation. J Invest Dermatol. 1983 Jun;80 (Suppl):44s–49s. [PubMed] [Google Scholar]
- Elias P. M., McNutt N. S., Friend D. S. Membrane alterations during cornification of mammalian squamous epithelia: a freeze-fracture, tracer, and thin-section study. Anat Rec. 1977 Dec;189(4):577–594. doi: 10.1002/ar.1091890404. [DOI] [PubMed] [Google Scholar]
- Freeman S. A., Wang M. A., Weaver J. C. Theory of electroporation of planar bilayer membranes: predictions of the aqueous area, change in capacitance, and pore-pore separation. Biophys J. 1994 Jul;67(1):42–56. doi: 10.1016/S0006-3495(94)80453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R. W., Leikin S. L., Chernomordik L. V., Pastushenko V. F., Sokirko A. I. Reversible electrical breakdown of lipid bilayers: formation and evolution of pores. Biochim Biophys Acta. 1988 May 24;940(2):275–287. doi: 10.1016/0005-2736(88)90202-7. [DOI] [PubMed] [Google Scholar]
- Grimnes S. Dielectric breakdown of human skin in vivo. Med Biol Eng Comput. 1983 May;21(3):379–381. doi: 10.1007/BF02478510. [DOI] [PubMed] [Google Scholar]
- Holbrook K. A., Odland G. F. Regional differences in the thickness (cell layers) of the human stratum corneum: an ultrastructural analysis. J Invest Dermatol. 1974 Apr;62(4):415–422. doi: 10.1111/1523-1747.ep12701670. [DOI] [PubMed] [Google Scholar]
- Kasting G. B., Bowman L. A. DC electrical properties of frozen, excised human skin. Pharm Res. 1990 Feb;7(2):134–143. doi: 10.1023/a:1015820600672. [DOI] [PubMed] [Google Scholar]
- Kasting G. B., Bowman L. A. Electrical analysis of fresh, excised human skin: a comparison with frozen skin. Pharm Res. 1990 Nov;7(11):1141–1146. doi: 10.1023/a:1015928225089. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Ashikawa I., Saita N., Yoshimura H., Itoh H., Nagayama K., Ikegami A. Electroporation of cell membrane visualized under a pulsed-laser fluorescence microscope. Biophys J. 1988 Jun;53(6):1015–1019. doi: 10.1016/S0006-3495(88)83181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. Y. Formation and resealing of pores of controlled sizes in human erythrocyte membrane. Nature. 1977 Aug 4;268(5619):438–441. doi: 10.1038/268438a0. [DOI] [PubMed] [Google Scholar]
- Klenchin V. A., Sukharev S. I., Serov S. M., Chernomordik L. V., Chizmadzhev YuA Electrically induced DNA uptake by cells is a fast process involving DNA electrophoresis. Biophys J. 1991 Oct;60(4):804–811. doi: 10.1016/S0006-3495(91)82115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison K. C., Swartzendruber D. C., Wertz P. W., Downing D. T. Presence of intact intercellular lipid lamellae in the upper layers of the stratum corneum. J Invest Dermatol. 1987 Jun;88(6):714–718. doi: 10.1111/1523-1747.ep12470386. [DOI] [PubMed] [Google Scholar]
- Mir L. M., Orlowski S., Belehradek J., Jr, Paoletti C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur J Cancer. 1991;27(1):68–72. doi: 10.1016/0277-5379(91)90064-k. [DOI] [PubMed] [Google Scholar]
- Monteiro-Riviere N. A., Inman A. O., Riviere J. E. Identification of the pathway of iontophoretic drug delivery: light and ultrastructural studies using mercuric chloride in pigs. Pharm Res. 1994 Feb;11(2):251–256. doi: 10.1023/a:1018907508501. [DOI] [PubMed] [Google Scholar]
- Neumann E., Rosenheck K. Permeability changes induced by electric impulses in vesicular membranes. J Membr Biol. 1972 Dec 29;10(3):279–290. doi: 10.1007/BF01867861. [DOI] [PubMed] [Google Scholar]
- Orlowski S., Mir L. M. Cell electropermeabilization: a new tool for biochemical and pharmacological studies. Biochim Biophys Acta. 1993 Jun 8;1154(1):51–63. doi: 10.1016/0304-4157(93)90016-h. [DOI] [PubMed] [Google Scholar]
- Potts R. O., Francoeur M. L. The influence of stratum corneum morphology on water permeability. J Invest Dermatol. 1991 Apr;96(4):495–499. doi: 10.1111/1523-1747.ep12470197. [DOI] [PubMed] [Google Scholar]
- Prausnitz M. R., Bose V. G., Langer R., Weaver J. C. Electroporation of mammalian skin: a mechanism to enhance transdermal drug delivery. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10504–10508. doi: 10.1073/pnas.90.22.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prausnitz M. R., Lau B. S., Milano C. D., Conner S., Langer R., Weaver J. C. A quantitative study of electroporation showing a plateau in net molecular transport. Biophys J. 1993 Jul;65(1):414–422. doi: 10.1016/S0006-3495(93)81081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott E. R., Laplaza A. I., White H. S., Phipps J. B. Transport of ionic species in skin: contribution of pores to the overall skin conductance. Pharm Res. 1993 Dec;10(12):1699–1709. doi: 10.1023/a:1018909811672. [DOI] [PubMed] [Google Scholar]
- Sukharev S. I., Klenchin V. A., Serov S. M., Chernomordik L. V., Chizmadzhev YuA Electroporation and electrophoretic DNA transfer into cells. The effect of DNA interaction with electropores. Biophys J. 1992 Nov;63(5):1320–1327. doi: 10.1016/S0006-3495(92)81709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzendruber D. C., Wertz P. W., Kitko D. J., Madison K. C., Downing D. T. Molecular models of the intercellular lipid lamellae in mammalian stratum corneum. J Invest Dermatol. 1989 Feb;92(2):251–257. doi: 10.1111/1523-1747.ep12276794. [DOI] [PubMed] [Google Scholar]
- Swartzendruber D. C., Wertz P. W., Madison K. C., Downing D. T. Evidence that the corneocyte has a chemically bound lipid envelope. J Invest Dermatol. 1987 Jun;88(6):709–713. doi: 10.1111/1523-1747.ep12470383. [DOI] [PubMed] [Google Scholar]
- Titomirov A. V., Sukharev S., Kistanova E. In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. Biochim Biophys Acta. 1991 Jan 17;1088(1):131–134. doi: 10.1016/0167-4781(91)90162-f. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. Electroporation of cell membranes. Biophys J. 1991 Aug;60(2):297–306. doi: 10.1016/S0006-3495(91)82054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver J. C. Electroporation: a general phenomenon for manipulating cells and tissues. J Cell Biochem. 1993 Apr;51(4):426–435. doi: 10.1002/jcb.2400510407. [DOI] [PubMed] [Google Scholar]
- Xie T. D., Tsong T. Y. Study of mechanisms of electric field-induced DNA transfection. II. Transfection by low-amplitude, low-frequency alternating electric fields. Biophys J. 1990 Oct;58(4):897–903. doi: 10.1016/S0006-3495(90)82434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]



