Abstract
Fluorescence recovery after photobleaching has been a popular technique to quantify the lateral mobility of membrane components. A variety of analysis methods have been used to determine the lateral diffusional mobility, D. However, many of these methods suffer from the drawbacks that they are not able to discern two-component diffusion (i.e., three-point fit), cannot solve for two components (linearization procedures), and do not perform well at low signal-to-noise. To overcome these limitations, we have adopted the approach of fitting fluorescence recovery after photobleaching curves by the full series solution using a Marquardt algorithm. Using simulated data of one or two diffusing components, determinations of the accuracy and reliability of the method with regard to extraction of diffusion parameters and the differentiation of one- versus two-component recovery curves were made under a variety of conditions comparable with those found in actual experimental situations. The performance of the method was also examined in experiments on artificial liposomes and fibroblast membranes labeled with fluorescent lipid and/or protein components. Our results indicate that: 1) the method was capable of extracting one- and two-component D values over a large range of conditions; 2) the D of a one-component recovery can be measured to within 10% with a small signal (100 prebleach photon counts per channel); 3) a two-component recovery requires more than 100-fold greater signal level than a one-component recovery for the same error; and 4) for two-component fits, multiple recovery curves may be needed to provide adequate signal to achieve the desired level of confidence in the fitted parameters and in the differentiation of one- and two-component diffusion.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida P. F., Vaz W. L., Thompson T. E. Lateral diffusion and percolation in two-phase, two-component lipid bilayers. Topology of the solid-phase domains in-plane and across the lipid bilayer. Biochemistry. 1992 Aug 11;31(31):7198–7210. doi: 10.1021/bi00146a024. [DOI] [PubMed] [Google Scholar]
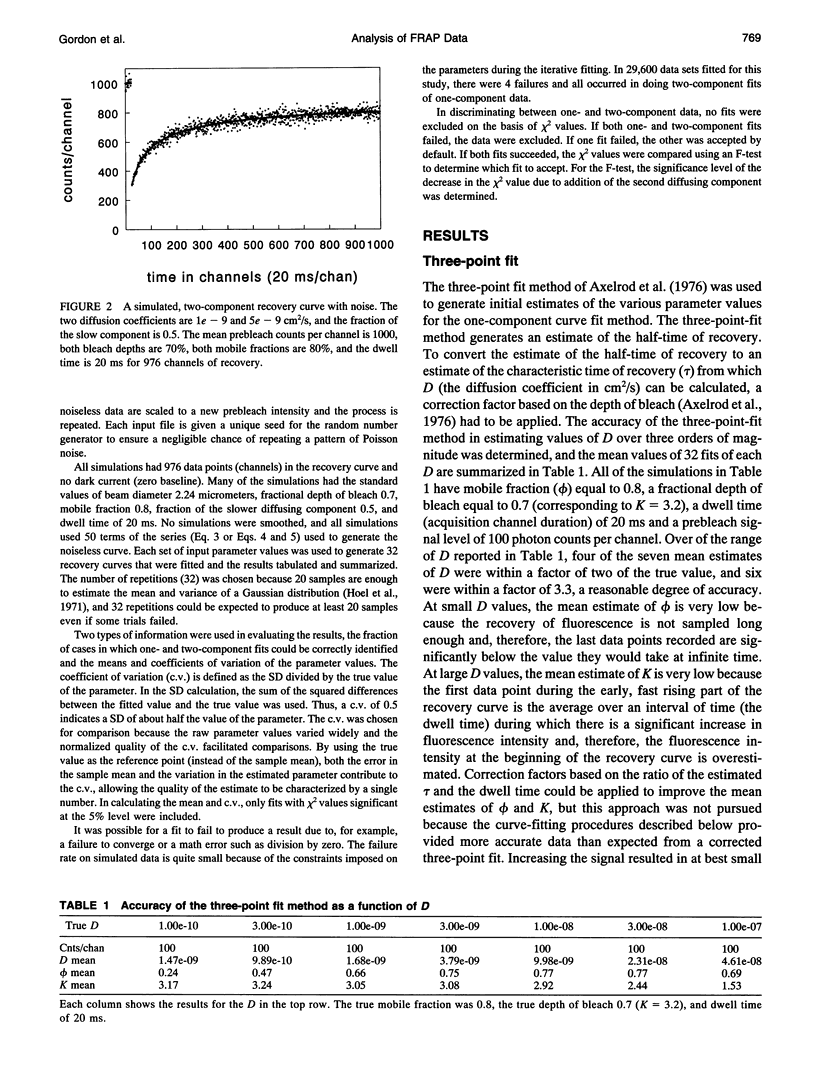
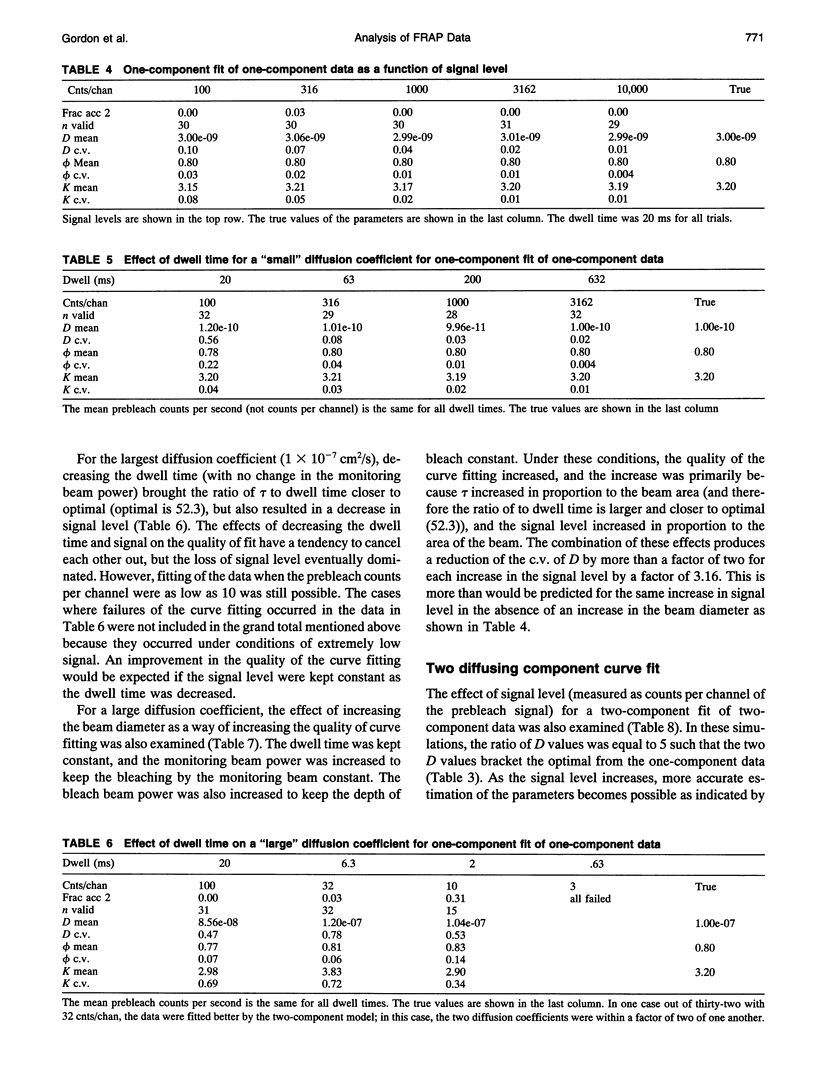
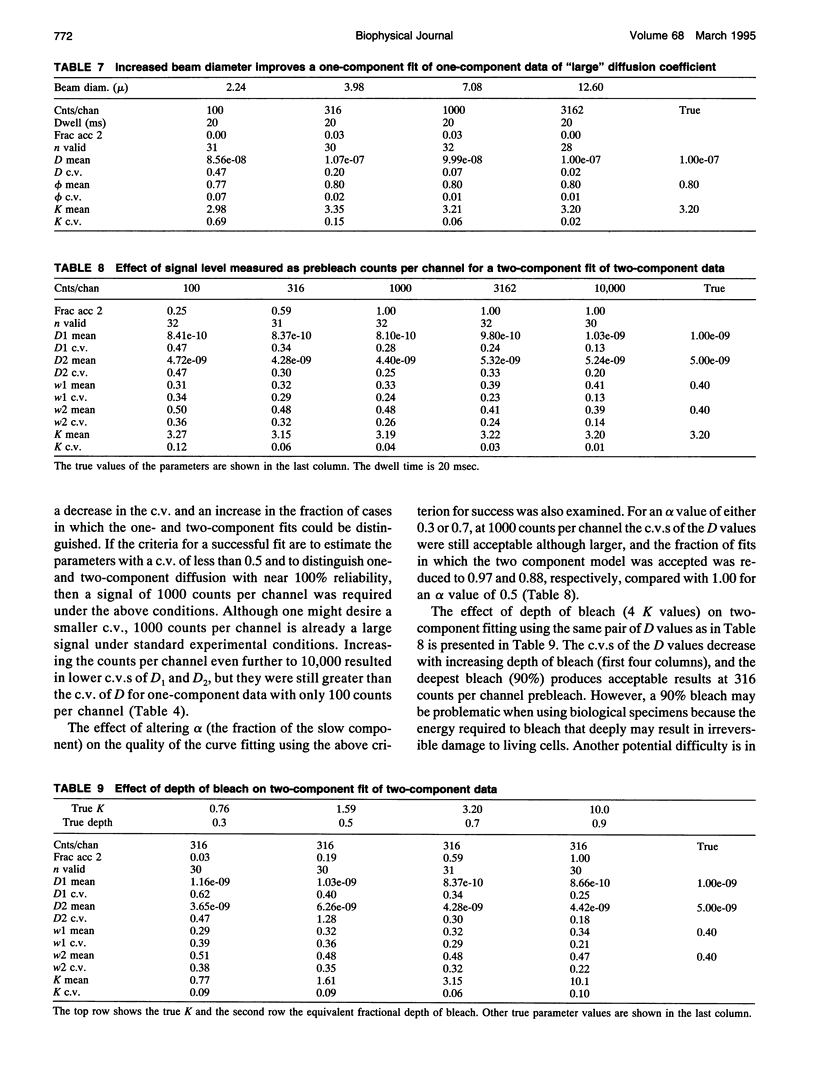
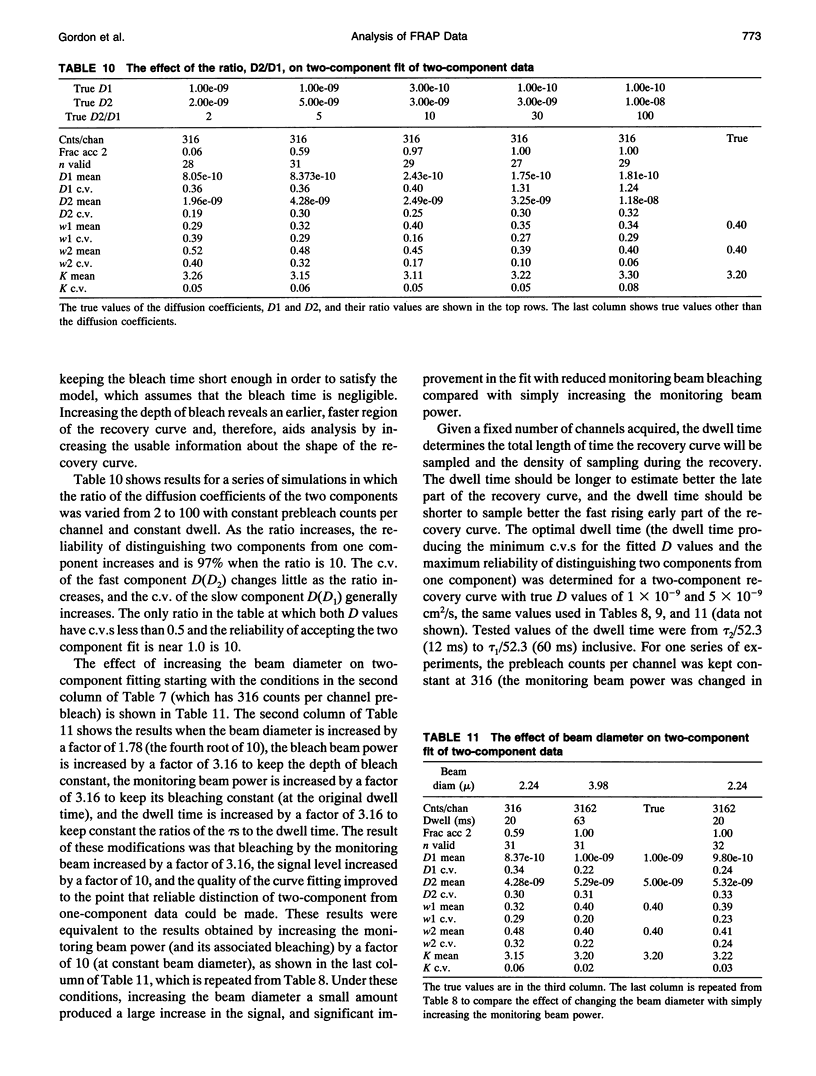
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barisas B. G., Leuther M. D. Fluorescence photobleaching recovery measurement of protein absolute diffusion constants. Biophys Chem. 1979 Sep;10(2):221–229. doi: 10.1016/0301-4622(79)85044-9. [DOI] [PubMed] [Google Scholar]
- Chazotte B., Hackenbrock C. R. The multicollisional, obstructed, long-range diffusional nature of mitochondrial electron transport. J Biol Chem. 1988 Oct 5;263(28):14359–14367. [PubMed] [Google Scholar]
- Chazotte B., Wu E. S., Höchli M., Hackenbrock C. R. Calcium-mediated fusion to produce ultra large osmotically active mitochondrial inner membranes of controlled protein density. Biochim Biophys Acta. 1985 Aug 8;818(1):87–95. doi: 10.1016/0005-2736(85)90142-7. [DOI] [PubMed] [Google Scholar]
- Elson E. L., Qian H. Interpretation of fluorescence correlation spectroscopy and photobleaching recovery in terms of molecular interactions. Methods Cell Biol. 1989;30:307–332. doi: 10.1016/s0091-679x(08)60984-x. [DOI] [PubMed] [Google Scholar]
- Florine-Casteel K., Lemasters J. J., Herman B. Lipid order in hepatocyte plasma membrane blebs during ATP depletion measured by digitized video fluorescence polarization microscopy. FASEB J. 1991 Apr;5(7):2078–2084. doi: 10.1096/fasebj.5.7.2010060. [DOI] [PubMed] [Google Scholar]
- Florine-Casteel K. Phospholipid order in gel- and fluid-phase cell-size liposomes measured by digitized video fluorescence polarization microscopy. Biophys J. 1990 Jun;57(6):1199–1215. doi: 10.1016/S0006-3495(90)82639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. L., Axelrod D. Anomalously slow mobility of fluorescent lipid probes in the plasma membrane of the yeast Saccharomyces cerevisiae. J Membr Biol. 1993 Jan;131(2):115–127. doi: 10.1007/BF02791320. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Ishihara A., Inman R. Lateral diffusion of proteins in membranes. Annu Rev Physiol. 1987;49:163–175. doi: 10.1146/annurev.ph.49.030187.001115. [DOI] [PubMed] [Google Scholar]
- Jacobson K., O'Dell D., August J. T. Lateral diffusion of an 80,000-dalton glycoprotein in the plasma membrane of murine fibroblasts: relationships to cell structure and function. J Cell Biol. 1984 Nov;99(5):1624–1633. doi: 10.1083/jcb.99.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. L., Faunt L. M. Parameter estimation by least-squares methods. Methods Enzymol. 1992;210:1–37. doi: 10.1016/0076-6879(92)10003-v. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Wolf D. E. Selectivity of fluorescent lipid analogues for lipid domains. Biochemistry. 1980 Dec 23;19(26):6199–6203. doi: 10.1021/bi00567a039. [DOI] [PubMed] [Google Scholar]
- Mueller P., Chien T. F., Rudy B. Formation and properties of cell-size lipid bilayer vesicles. Biophys J. 1983 Dec;44(3):375–381. doi: 10.1016/S0006-3495(83)84311-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F. Long tail kinetics in biophysics? Biophys J. 1992 Aug;63(2):366–370. doi: 10.1016/S0006-3495(92)81602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R. Fluorescence microphotolysis to measure nucleocytoplasmic transport and intracellular mobility. Biochim Biophys Acta. 1986 Dec 22;864(3-4):305–359. doi: 10.1016/0304-4157(86)90003-1. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P. Glycoprotein motility and dynamic domains in fluid plasma membranes. Annu Rev Biophys Biomol Struct. 1993;22:417–431. doi: 10.1146/annurev.bb.22.060193.002221. [DOI] [PubMed] [Google Scholar]
- Soumpasis D. M. Theoretical analysis of fluorescence photobleaching recovery experiments. Biophys J. 1983 Jan;41(1):95–97. doi: 10.1016/S0006-3495(83)84410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz W. L., Melo E. C., Thompson T. E. Translational diffusion and fluid domain connectivity in a two-component, two-phase phospholipid bilayer. Biophys J. 1989 Nov;56(5):869–876. doi: 10.1016/S0006-3495(89)82733-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D. E. Designing, building, and using a fluorescence recovery after photobleaching instrument. Methods Cell Biol. 1989;30:271–306. doi: 10.1016/s0091-679x(08)60983-8. [DOI] [PubMed] [Google Scholar]
- Wolf D. E., Voglmayr J. K. Diffusion and regionalization in membranes of maturing ram spermatozoa. J Cell Biol. 1984 May;98(5):1678–1684. doi: 10.1083/jcb.98.5.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yguerabide J., Schmidt J. A., Yguerabide E. E. Lateral mobility in membranes as detected by fluorescence recovery after photobleaching. Biophys J. 1982 Oct;40(1):69–75. doi: 10.1016/S0006-3495(82)84459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zoelen E. J., Tertoolen L. G., de Laat S. W. Simple computer method for evaluation of lateral diffusion coefficients from fluorescence photobleaching recovery kinetics. Biophys J. 1983 Apr;42(1):103–108. doi: 10.1016/S0006-3495(83)84374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


