Abstract
For many years, membrane potential (Vm) and input resistance have been used to characterize the electrophysiological nature of a seal (barrier) that forms at the cut end of a transected axon or other extended cytoplasmic structure. Data from a mathematical and an analog model of a transected axon and other theoretical considerations show that steady-state values of Vm and input resistance measured from any cable-like structure provide a very equivocal assessment of the electrical barrier (seal) at the cut end. Extracellular assessments of injury currents almost certainly provide a better electrophysiological measure of the status of plasma membrane sealing because measurements of these currents do not depend on the cable properties of extended cytoplasmic processes after transection.
Full text
PDF

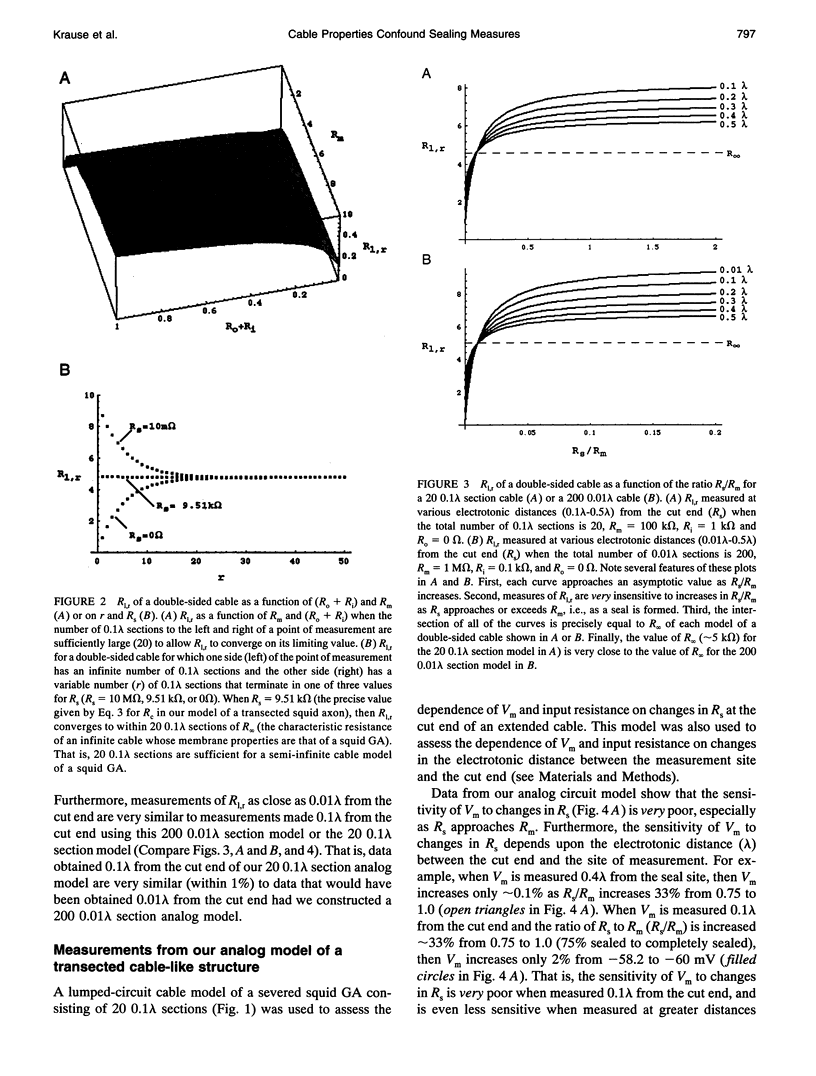
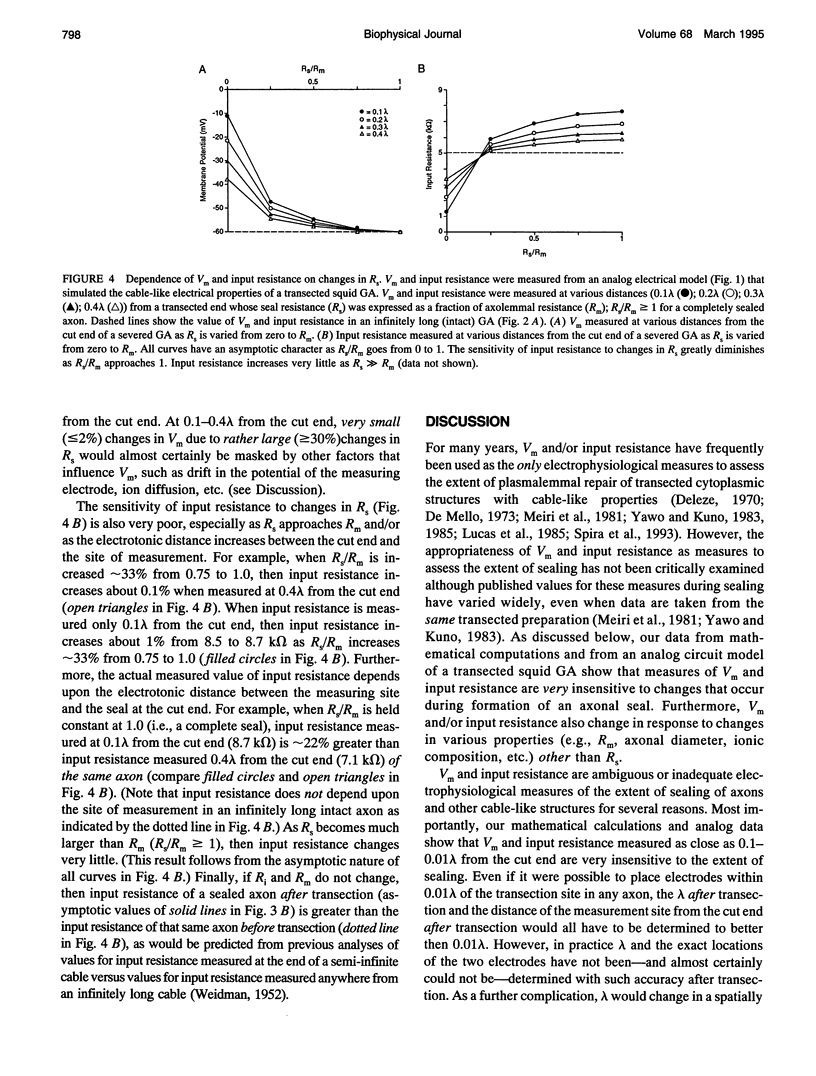

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Mello W. C. Membrane sealing in frog skeletal-muscle fibers. Proc Natl Acad Sci U S A. 1973 Apr;70(4):982–984. doi: 10.1073/pnas.70.4.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Délèze J. The recovery of resting potential and input resistance in sheep heart injured by knife or laser. J Physiol. 1970 Jul;208(3):547–562. doi: 10.1113/jphysiol.1970.sp009136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman H. M., Tewari K. P., Stein P. G. Injury-induced vesiculation and membrane redistribution in squid giant axon. Biochim Biophys Acta. 1990 Apr 30;1023(3):421–435. doi: 10.1016/0005-2736(90)90135-b. [DOI] [PubMed] [Google Scholar]
- Gallant P. E. Effects of the external ions and metabolic poisoning on the constriction of the squid giant axon after axotomy. J Neurosci. 1988 May;8(5):1479–1484. doi: 10.1523/JNEUROSCI.08-05-01479.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. F., Nuccitelli R. An ultrasensitive vibrating probe for measuring steady extracellular currents. J Cell Biol. 1974 Nov;63(2 Pt 1):614–628. doi: 10.1083/jcb.63.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause T. L., Fishman H. M., Ballinger M. L., Bittner G. D. Extent and mechanism of sealing in transected giant axons of squid and earthworms. J Neurosci. 1994 Nov;14(11 Pt 1):6638–6651. doi: 10.1523/JNEUROSCI.14-11-06638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. H., Gross G. W., Emery D. G., Gardner C. R. Neuronal survival or death after dendrite transection close to the perikaryon: correlation with electrophysiologic, morphologic, and ultrastructural changes. Cent Nerv Syst Trauma. 1985 Winter;2(4):231–255. doi: 10.1089/cns.1985.2.231. [DOI] [PubMed] [Google Scholar]
- Meiri H., Spira M. E., Parnas I. Membrane conductance and action potential of a regenerating axonal tip. Science. 1981 Feb 13;211(4483):709–712. doi: 10.1126/science.7455707. [DOI] [PubMed] [Google Scholar]
- Rall W. Time constants and electrotonic length of membrane cylinders and neurons. Biophys J. 1969 Dec;9(12):1483–1508. doi: 10.1016/S0006-3495(69)86467-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira M. E., Benbassat D., Dormann A. Resealing of the proximal and distal cut ends of transected axons: electrophysiological and ultrastructural analysis. J Neurobiol. 1993 Mar;24(3):300–316. doi: 10.1002/neu.480240304. [DOI] [PubMed] [Google Scholar]
- WEIDMANN S. The electrical constants of Purkinje fibres. J Physiol. 1952 Nov;118(3):348–360. doi: 10.1113/jphysiol.1952.sp004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawo H., Kuno M. Calcium dependence of membrane sealing at the cut end of the cockroach giant axon. J Neurosci. 1985 Jun;5(6):1626–1632. doi: 10.1523/JNEUROSCI.05-06-01626.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawo H., Kuno M. How a nerve fiber repairs its cut end: involvement of phospholipase A2. Science. 1983 Dec 23;222(4630):1351–1353. doi: 10.1126/science.6658457. [DOI] [PubMed] [Google Scholar]


