Abstract
Quantitative description of the thermodynamic consequences of macromolecular crowding (excluded volume nonideality) is an important component of analyses of the thermodynamics and kinetics of noncovalent interactions of biopolymers in vivo and in concentrated polymer solutions in vitro. By analyzing previously published thermodynamic data, we have investigated extensively the comparative applicability of two forms of scaled particle theory (SPT). In both forms, macromolecules are treated as hard spheres, but MSPT, introduced by Ross and Minton, treats the solvent as a structureless continuum, whereas bulk water molecules are included explicitly as hard spheres in BSPT, an approach developed by Berg. Here we use both MSPT and BSPT to calculate the excluded volume component of the macromolecular activity coefficient of hemoglobin (Hb) at concentrations up to 509 mg/ml by fitting osmotic pressure data for Hb and sedimentation equilibrium data for Hb and sickle-cell Hb (HbS). Both forms of SPT also are used here to analyze the effects of other globular proteins (BSA and Hb) on the solubility of HbS. In applying MSPT and BSPT to analyze macromolecular crowding, the extent of hydration delta Hb (in gH2O/gprotein) is introduced as an adjustable parameter to specify the effective (hard sphere) radius of hydrated Hb. In our nonlinear least-squares fittings based on BSPT, the hard sphere radius of bulk water molecules is either fixed at 1.375 A or floated. Although both forms of SPT yield good fittings (with different values of delta Hb) at Hb concentrations up to 350 mg/ml, only BSPT gives good fittings of all available Hb osmotic pressure data as well as of the sedimentation equilibrium and solubility data. Only BSPT predicts values for delta Hb (approximately 0.5-0.6 g/g) in the range obtained for Hb from hydrodynamic measurements (approximately 0.36-0.78 g/g). These findings indicate the applicability, at least in the context of BSPT, of a simple two-state classification of water (bulk water and water of macromolecular hydration) as a basis for interpreting excluded volume nonideality in concentrated solutions of globular proteins.
Full text
PDF




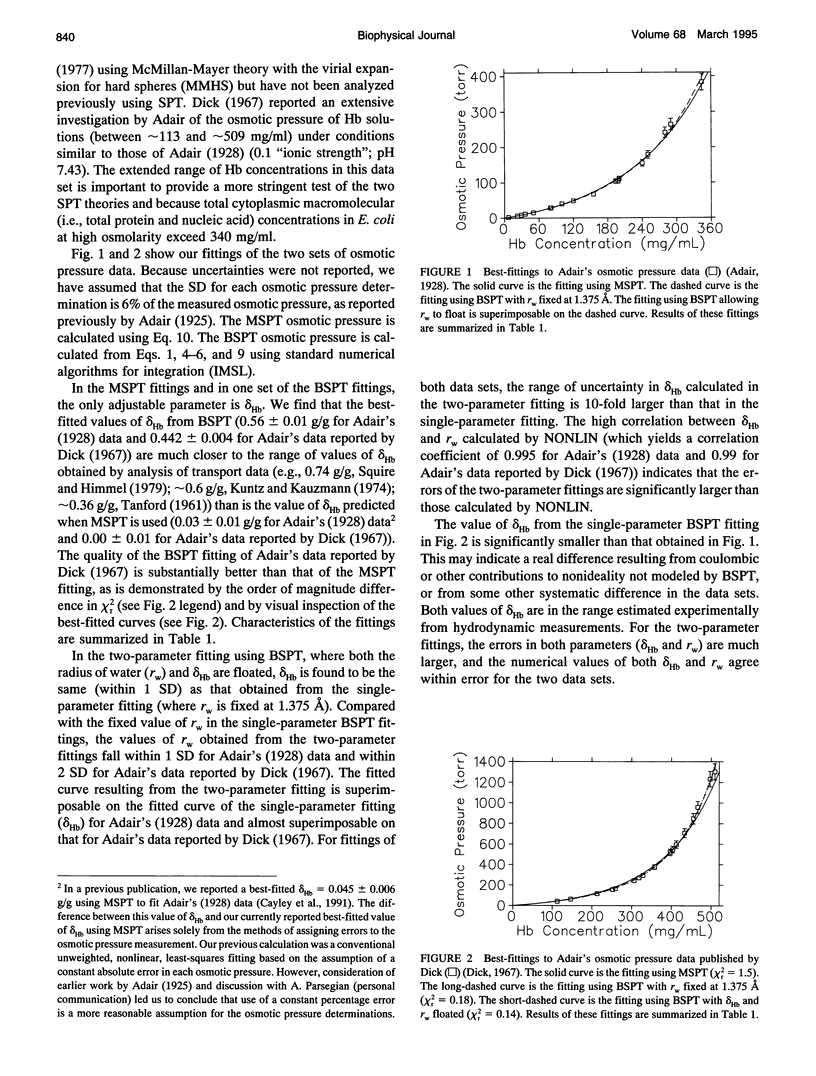
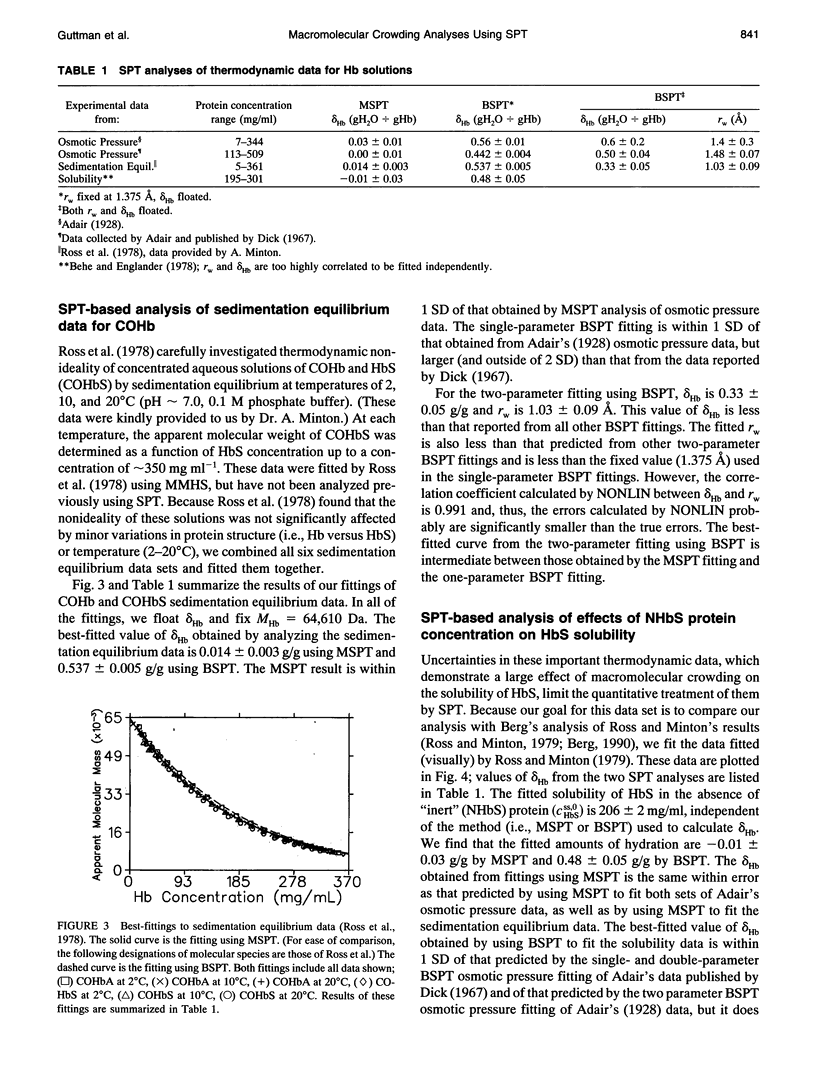
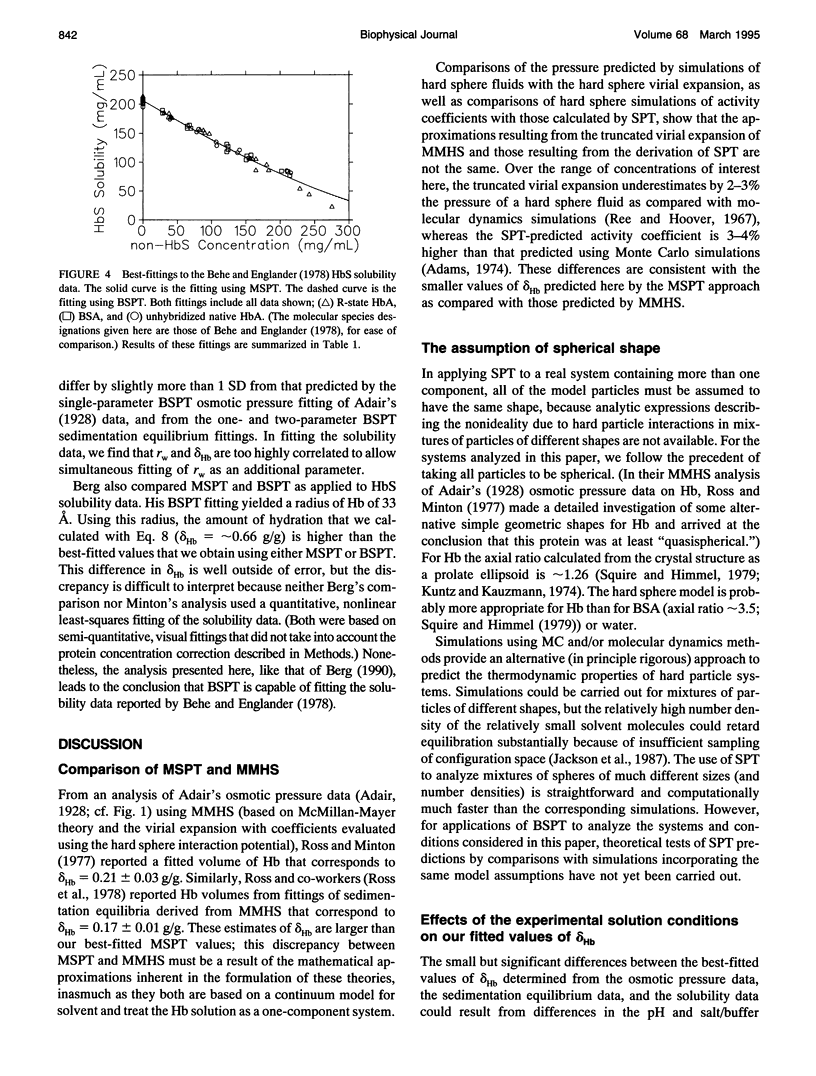

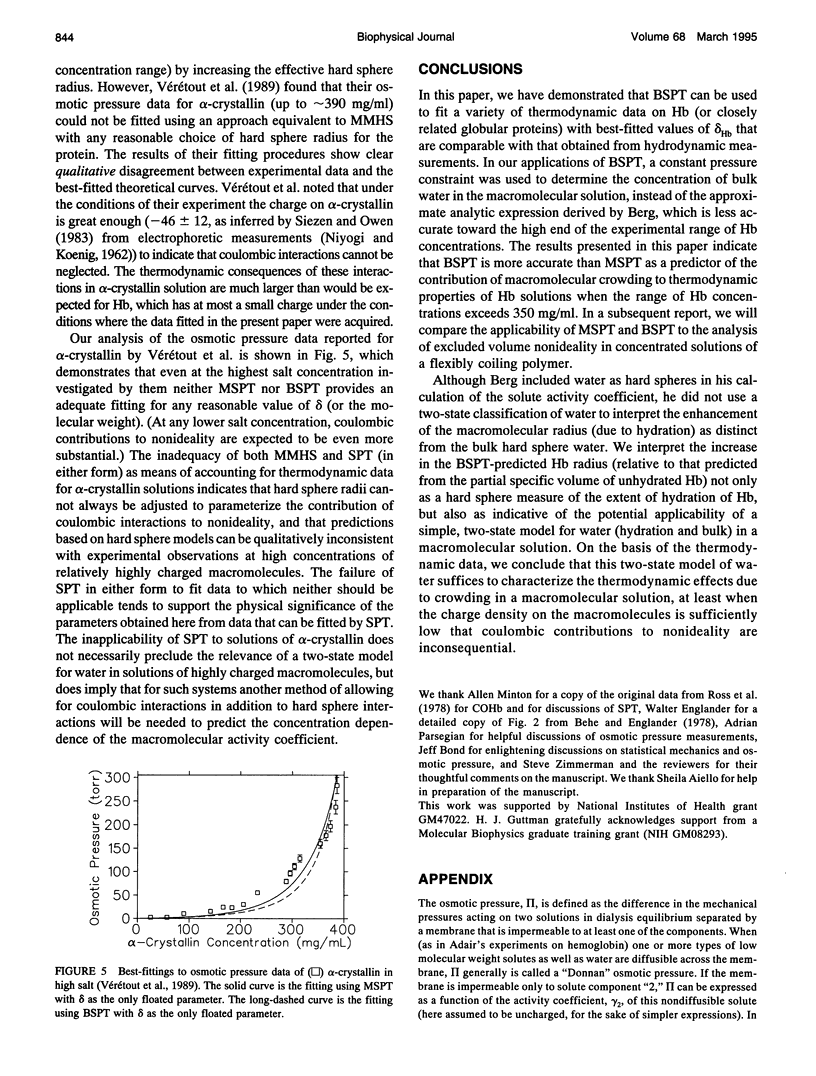


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behe M. J., Englander S. W. Sickle hemoglobin gelation. Reaction order and critical nucleus size. Biophys J. 1978 Jul;23(1):129–145. doi: 10.1016/S0006-3495(78)85438-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg O. G. The influence of macromolecular crowding on thermodynamic activity: solubility and dimerization constants for spherical and dumbbell-shaped molecules in a hard-sphere mixture. Biopolymers. 1990;30(11-12):1027–1037. doi: 10.1002/bip.360301104. [DOI] [PubMed] [Google Scholar]
- Cayley S., Lewis B. A., Guttman H. J., Record M. T., Jr Characterization of the cytoplasm of Escherichia coli K-12 as a function of external osmolarity. Implications for protein-DNA interactions in vivo. J Mol Biol. 1991 Nov 20;222(2):281–300. doi: 10.1016/0022-2836(91)90212-o. [DOI] [PubMed] [Google Scholar]
- Fulton A. B. How crowded is the cytoplasm? Cell. 1982 Sep;30(2):345–347. doi: 10.1016/0092-8674(82)90231-8. [DOI] [PubMed] [Google Scholar]
- Krueger S., Chen S. H., Hofrichter J., Nossal R. Small angle neutron scattering studies of HbA in concentrated solutions. Biophys J. 1990 Sep;58(3):745–757. doi: 10.1016/S0006-3495(90)82417-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz I. D., Jr, Kauzmann W. Hydration of proteins and polypeptides. Adv Protein Chem. 1974;28:239–345. doi: 10.1016/s0065-3233(08)60232-6. [DOI] [PubMed] [Google Scholar]
- Leikin S., Rau D. C., Parsegian V. A. Direct measurement of forces between self-assembled proteins: temperature-dependent exponential forces between collagen triple helices. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):276–280. doi: 10.1073/pnas.91.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton A. P. The effect of volume occupancy upon the thermodynamic activity of proteins: some biochemical consequences. Mol Cell Biochem. 1983;55(2):119–140. doi: 10.1007/BF00673707. [DOI] [PubMed] [Google Scholar]
- Minton A. P. Thermodynamic nonideality and the dependence of partition coefficient upon solute concentration in exclusion chromatography. Application to self-associating and non-self-associating solutes. Application to hemoglobin. Biophys Chem. 1980 Dec;12(3-4):271–277. doi: 10.1016/0301-4622(80)80004-4. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Briehl R. W., Minton A. P. Temperature dependence of nonideality in concentrated solutions of hemoglobin. Biopolymers. 1978 Sep;17(9):2285–2288. doi: 10.1002/bip.1978.360170920. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Minton A. P. Analysis of non-ideal behavior in concentrated hemoglobin solutions. J Mol Biol. 1977 May 25;112(3):437–452. doi: 10.1016/s0022-2836(77)80191-5. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Minton A. P. The effect of non-aggregating proteins upon the gelation of sickle cell hemoglobin: model calculations and data analysis. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1308–1314. doi: 10.1016/0006-291x(79)91123-9. [DOI] [PubMed] [Google Scholar]
- Squire P. G., Himmel M. E. Hydrodynamics and protein hydration. Arch Biochem Biophys. 1979 Aug;196(1):165–177. doi: 10.1016/0003-9861(79)90563-0. [DOI] [PubMed] [Google Scholar]
- Vérétout F., Delaye M., Tardieu A. Molecular basis of eye lens transparency. Osmotic pressure and X-ray analysis of alpha-crystallin solutions. J Mol Biol. 1989 Feb 20;205(4):713–728. doi: 10.1016/0022-2836(89)90316-1. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Trach S. O. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J Mol Biol. 1991 Dec 5;222(3):599–620. doi: 10.1016/0022-2836(91)90499-v. [DOI] [PubMed] [Google Scholar]


