Abstract
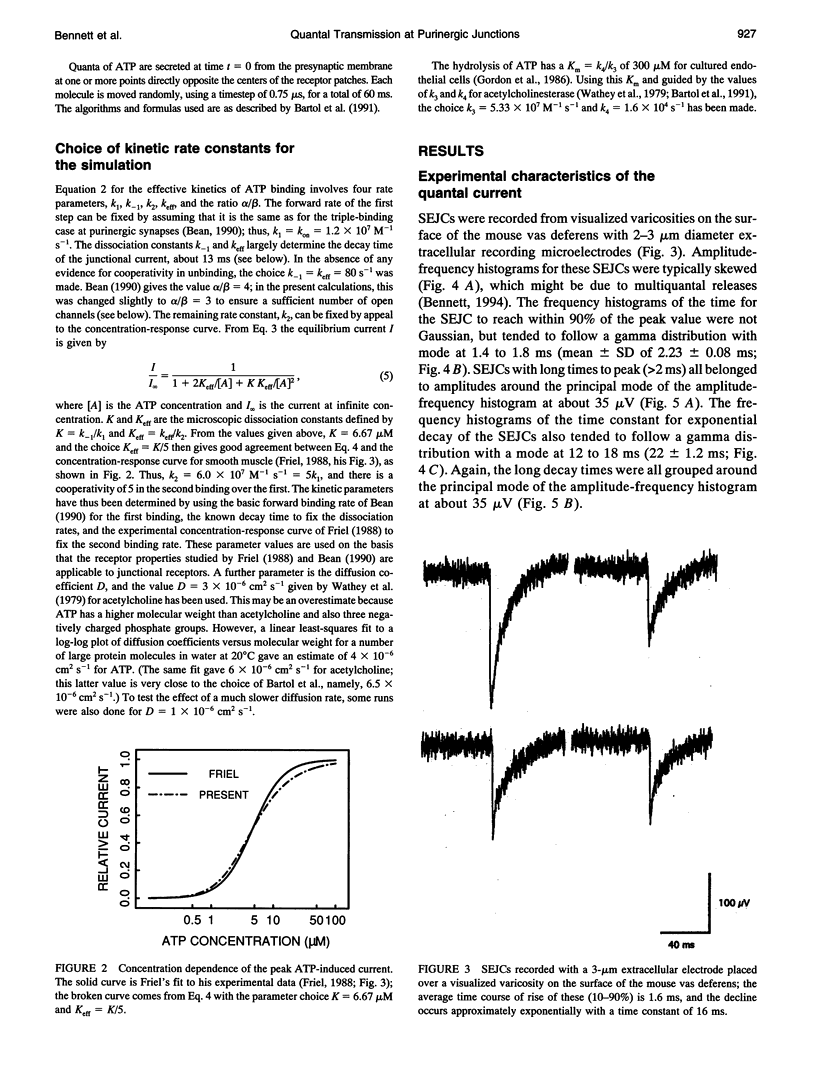
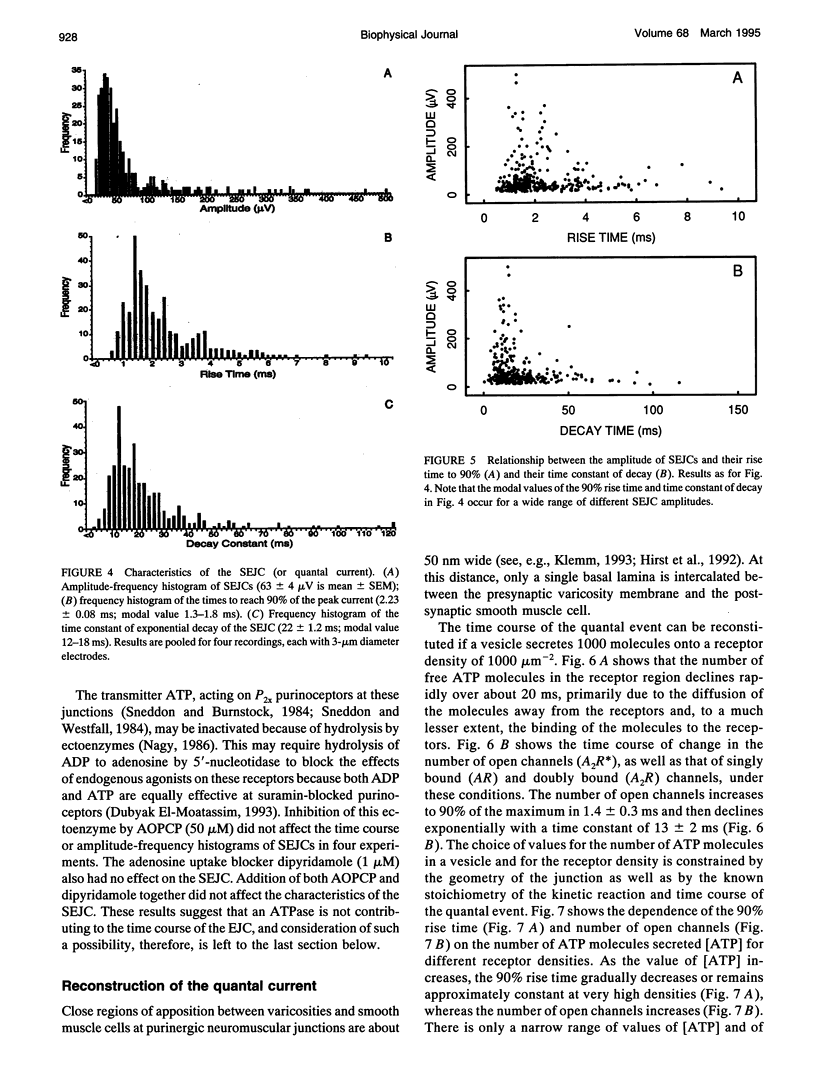
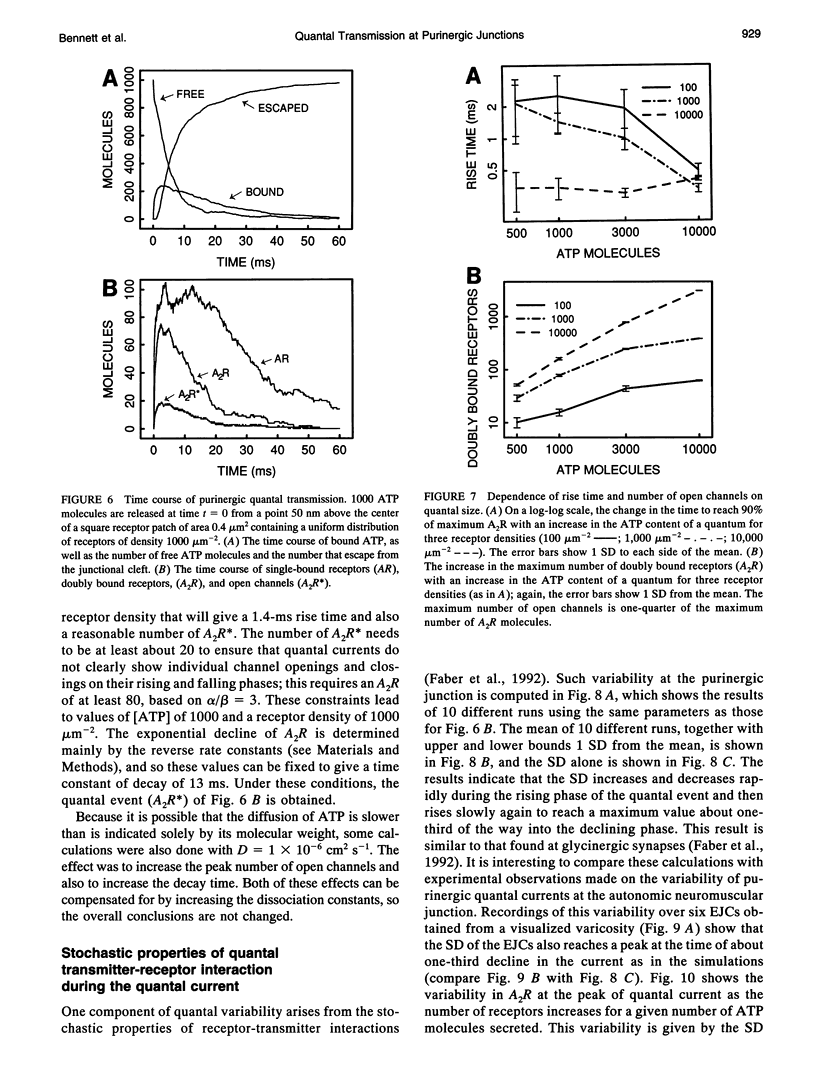
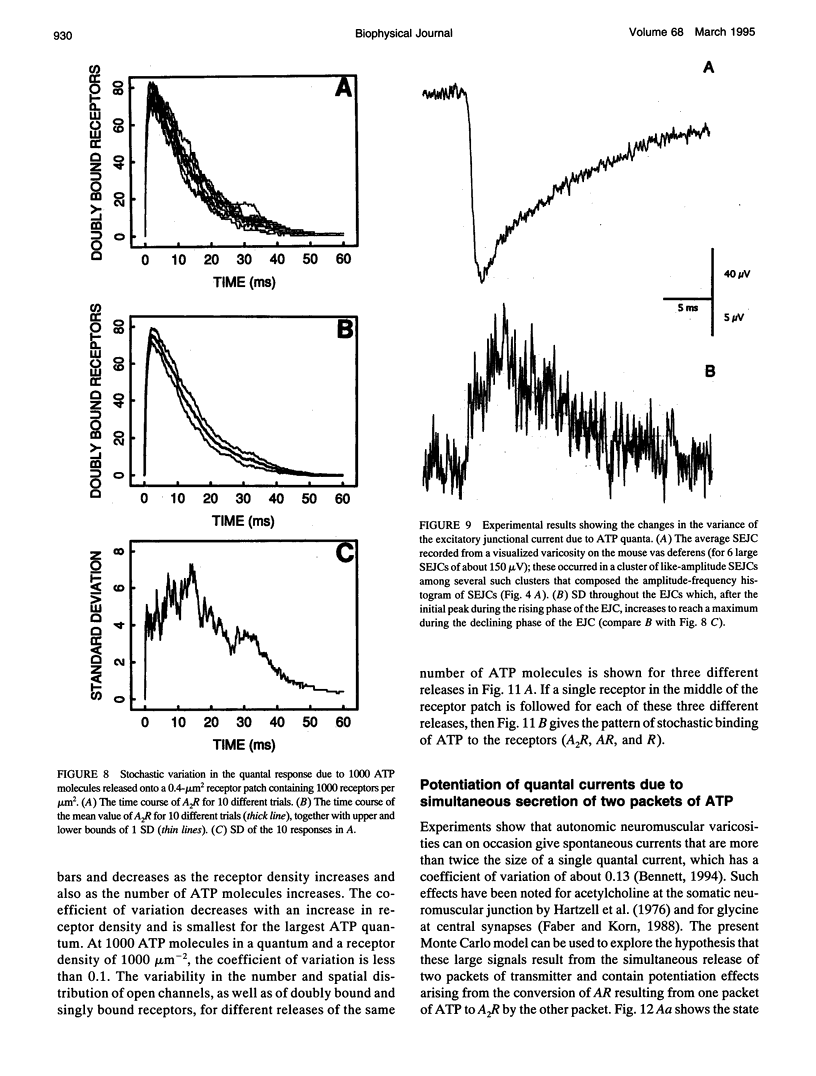
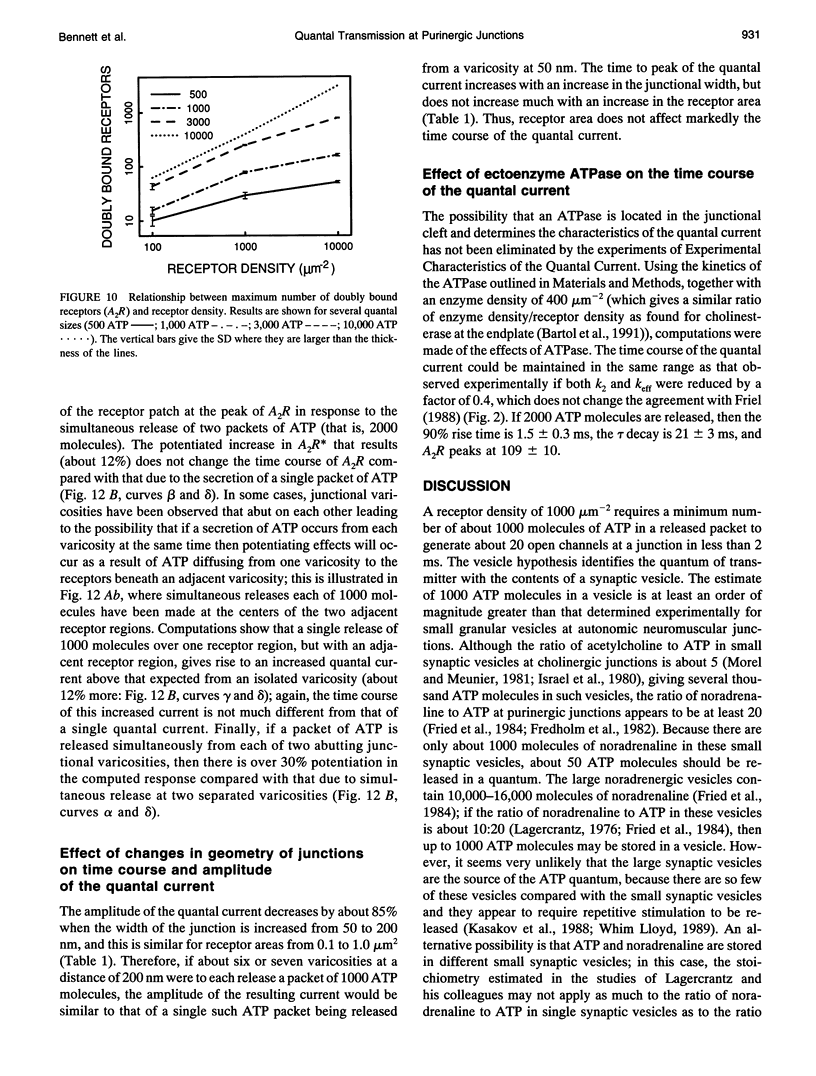
The time course of most quantal currents recorded with a small diameter electrode placed over visualized varicosities of sympathetic nerve terminals that secrete ATP was determined: these had a time to reach 90% of peak of 1.3-1.8 ms and a time constant of decay of 12-18 ms; they were unaffected by blocking ectoenzymes or the uptake of adenosine. Monte Carlo methods were used to analyze the stochastic interaction between ATP, released in a packet from a varicosity, and the underlying patch of purinoceptors, to reconstitute the time course of the quantal current. This leads to certain restrictions on the possible number of ATP molecules in a quantum (about 1000) and the density of purinoceptors at the junctions (about 1000 microns-1), given the known geometry of the junction and the kinetics of ATP action. The observed quantal current has a relatively small variability (coefficient of variation < 0.1), and this stochastic property is reproduced for a given quantum of ATP. Potentiation effects (of about 12%) occur if two quanta are released from the same varicosity because the receptor patch is not saturated even by the release of two quanta. The simulations show that quantal currents have a characteristically distinct shape for varicosities with different junctional cleft widths (50-200 nm). Finally, incorporation of an ectoenzyme with the known kinetics of ATPase into the junctional cleft allows for a quantal current of the observed time course, provided the number of ATP molecules in a quantum is increased over the number in the absence of the ATPase.
Full text
PDF
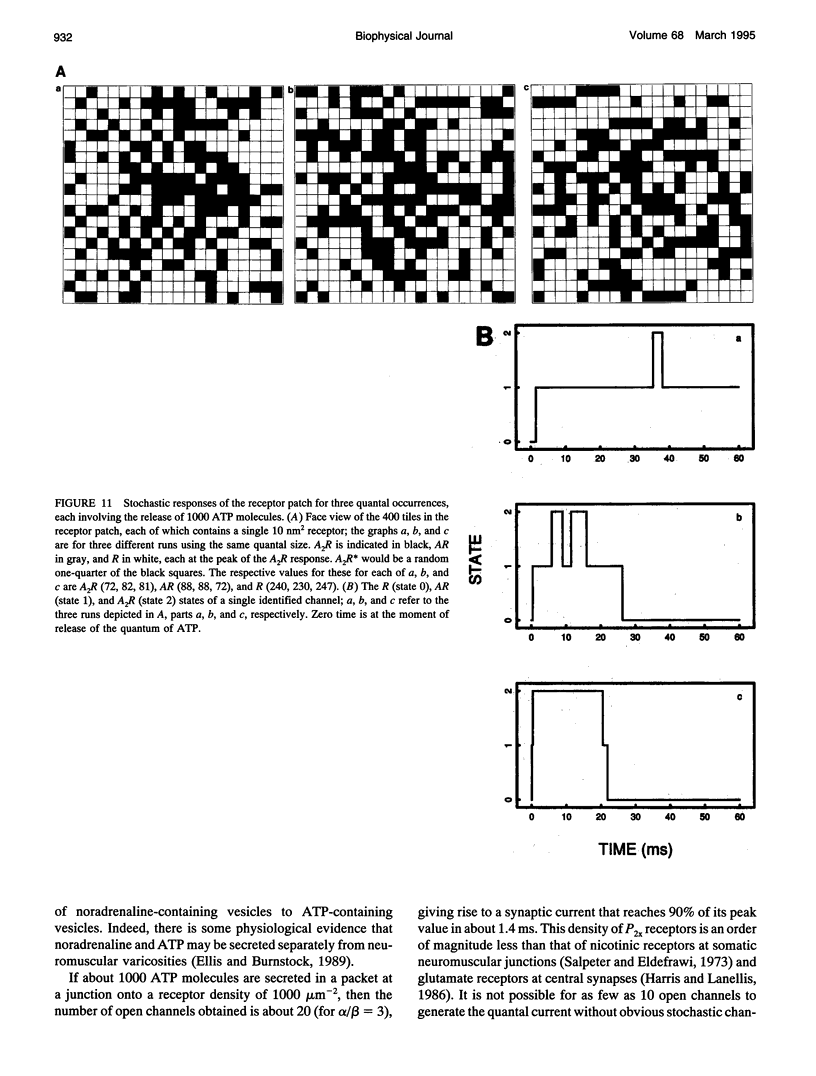
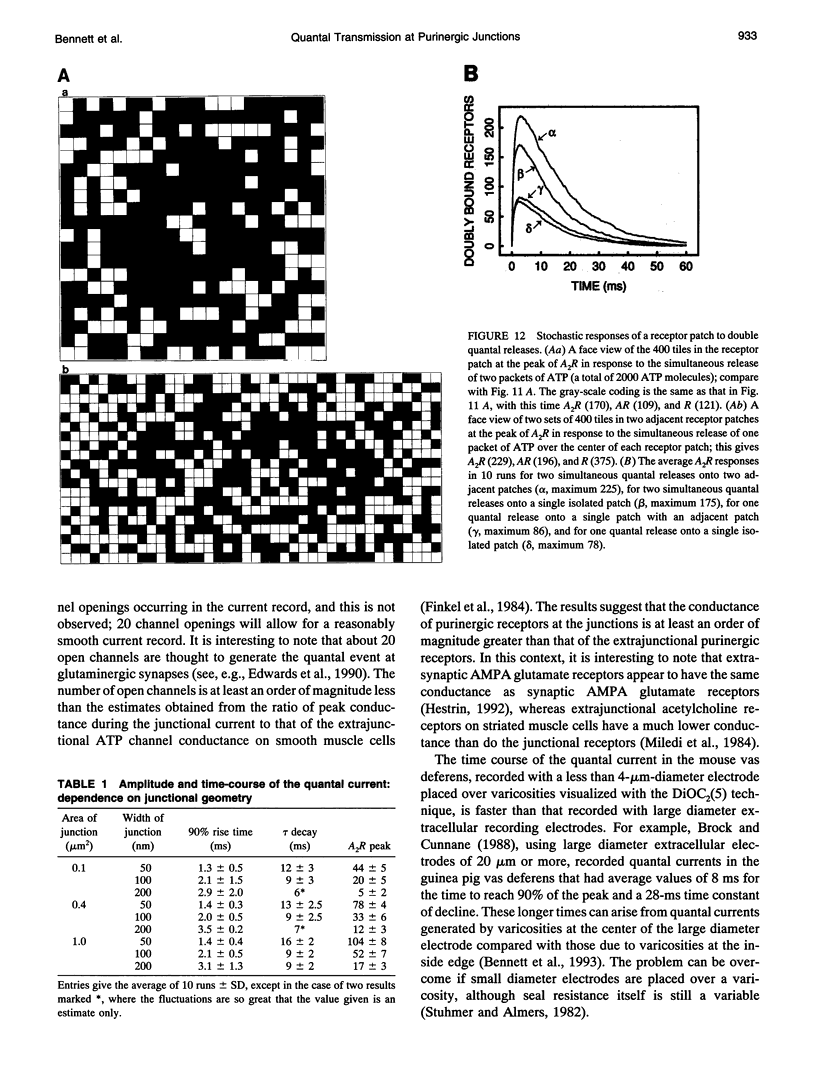


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrand P., Stjärne L. On the secretory activity of single varicosities in the sympathetic nerves innervating the rat tail artery. J Physiol. 1989 Feb;409:207–220. doi: 10.1113/jphysiol.1989.sp017493. [DOI] [PMC free article] [PubMed] [Google Scholar]
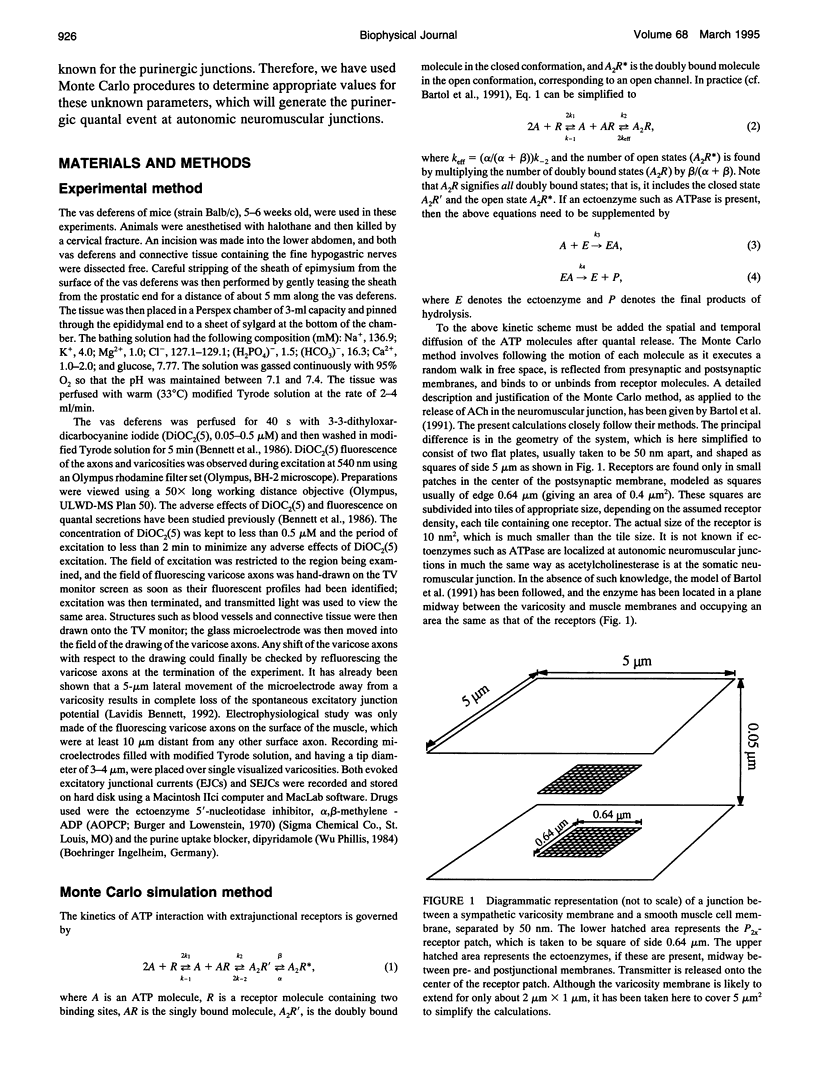
- Bartol T. M., Jr, Land B. R., Salpeter E. E., Salpeter M. M. Monte Carlo simulation of miniature endplate current generation in the vertebrate neuromuscular junction. Biophys J. 1991 Jun;59(6):1290–1307. doi: 10.1016/S0006-3495(91)82344-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. ATP-activated channels in rat and bullfrog sensory neurons: concentration dependence and kinetics. J Neurosci. 1990 Jan;10(1):1–10. doi: 10.1523/JNEUROSCI.10-01-00001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P., Williams C. A., Ceelen P. W. ATP-activated channels in rat and bullfrog sensory neurons: current-voltage relation and single-channel behavior. J Neurosci. 1990 Jan;10(1):11–19. doi: 10.1523/JNEUROSCI.10-01-00011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Gibson W. G., Poznanski R. R. Extracellular current flow and potential during quantal transmission from varicosities in a smooth muscle syncytium. Philos Trans R Soc Lond B Biol Sci. 1993 Oct 29;342(1300):89–99. doi: 10.1098/rstb.1993.0140. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Jones P., Lavidis N. A. The probability of quantal secretion along visualized terminal branches at amphibian (Bufo marinus) neuromuscular synapses. J Physiol. 1986 Oct;379:257–274. doi: 10.1113/jphysiol.1986.sp016252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R. Quantal secretion from single visualized synaptic varicosities of sympathetic nerve terminals. Adv Second Messenger Phosphoprotein Res. 1994;29:399–423. doi: 10.1016/s1040-7952(06)80028-5. [DOI] [PubMed] [Google Scholar]
- Blakeley A. G., Dunn P. M., Petersen S. A. Properties of excitatory junction potentials and currents in smooth muscle cells of the mouse vas deferens. J Auton Nerv Syst. 1989 Jun;27(1):47–56. doi: 10.1016/0165-1838(89)90128-8. [DOI] [PubMed] [Google Scholar]
- Brock J. A., Cunnane T. C. Electrical activity at the sympathetic neuroeffector junction in the guinea-pig vas deferens. J Physiol. 1988 May;399:607–632. doi: 10.1113/jphysiol.1988.sp017099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger R. M., Lowenstein J. M. Preparation and properties of 5'-nucleotidase from smooth muscle of small intestine. J Biol Chem. 1970 Dec 10;245(23):6274–6280. [PubMed] [Google Scholar]
- Burnstock G. Review lecture. Neurotransmitters and trophic factors in the autonomic nervous system. J Physiol. 1981;313:1–35. doi: 10.1113/jphysiol.1981.sp013648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabala L. D., Gurney A. M., Lester H. A. Dose-response of acetylcholine receptor channels opened by a flash-activated agonist in voltage-clamped rat myoballs. J Physiol. 1986 Feb;371:407–433. doi: 10.1113/jphysiol.1986.sp015983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Ogden D. C. Activation of ion channels in the frog end-plate by high concentrations of acetylcholine. J Physiol. 1988 Jan;395:131–159. doi: 10.1113/jphysiol.1988.sp016912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnane T. C., Manchanda R. Electrophysiological analysis of the inactivation of sympathetic transmitter in the guinea-pig vas deferens. J Physiol. 1988 Oct;404:349–364. doi: 10.1113/jphysiol.1988.sp017293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnane T. C., Stjärne L. Transmitter secretion from individual varicosities of guinea-pig and mouse vas deferens: highly intermittent and monoquantal. Neuroscience. 1984 Sep;13(1):1–20. doi: 10.1016/0306-4522(84)90255-0. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Steinbach J. H., Stevens C. F. An analysis of the dose-response relationship at voltage-clamped frog neuromuscular junctions. J Physiol. 1978 Aug;281:421–444. doi: 10.1113/jphysiol.1978.sp012431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak G. R., el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993 Sep;265(3 Pt 1):C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Gibb A. J. ATP--a fast neurotransmitter. FEBS Lett. 1993 Jun 28;325(1-2):86–89. doi: 10.1016/0014-5793(93)81419-z. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Gibb A. J., Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992 Sep 10;359(6391):144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990 Nov;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. L., Burnstock G. Angiotensin neuromodulation of adrenergic and purinergic co-transmission in the guinea-pig vas deferens. Br J Pharmacol. 1989 Aug;97(4):1157–1164. doi: 10.1111/j.1476-5381.1989.tb12574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. J., Derkach V., Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992 Jun 11;357(6378):503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Faber D. S., Korn H. Synergism at central synapses due to lateral diffusion of transmitter. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8708–8712. doi: 10.1073/pnas.85.22.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber D. S., Young W. S., Legendre P., Korn H. Intrinsic quantal variability due to stochastic properties of receptor-transmitter interactions. Science. 1992 Nov 27;258(5087):1494–1498. doi: 10.1126/science.1279813. [DOI] [PubMed] [Google Scholar]
- Finkel A. S., Hirst G. D., Van Helden D. F. Some properties of excitatory junction currents recorded from submucosal arterioles of guinea-pig ileum. J Physiol. 1984 Jun;351:87–98. doi: 10.1113/jphysiol.1984.sp015234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B., Fried G., Hedqvist P. Origin of adenosine released from rat vas deferens by nerve stimulation. Eur J Pharmacol. 1982 Apr 23;79(3-4):233–243. doi: 10.1016/0014-2999(82)90629-x. [DOI] [PubMed] [Google Scholar]
- Friel D. D. An ATP-sensitive conductance in single smooth muscle cells from the rat vas deferens. J Physiol. 1988 Jul;401:361–380. doi: 10.1113/jphysiol.1988.sp017167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel D. D., Bean B. P. Two ATP-activated conductances in bullfrog atrial cells. J Gen Physiol. 1988 Jan;91(1):1–27. doi: 10.1085/jgp.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girod R., Corrèges P., Jacquet J., Dunant Y. Space and time characteristics of transmitter release at the nerve-electroplaque junction of Torpedo. J Physiol. 1993 Nov;471:129–157. doi: 10.1113/jphysiol.1993.sp019894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E. L., Pearson J. D., Slakey L. L. The hydrolysis of extracellular adenine nucleotides by cultured endothelial cells from pig aorta. Feed-forward inhibition of adenosine production at the cell surface. J Biol Chem. 1986 Nov 25;261(33):15496–15507. [PubMed] [Google Scholar]
- Harris K. M., Landis D. M. Membrane structure at synaptic junctions in area CA1 of the rat hippocampus. Neuroscience. 1986 Nov;19(3):857–872. doi: 10.1016/0306-4522(86)90304-0. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Yoshikami D. Post-synaptic potentiation: interaction between quanta of acetylcholine at the skeletal neuromuscular synapse. J Physiol. 1975 Oct;251(2):427–463. doi: 10.1113/jphysiol.1975.sp011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S. Activation and desensitization of glutamate-activated channels mediating fast excitatory synaptic currents in the visual cortex. Neuron. 1992 Nov;9(5):991–999. doi: 10.1016/0896-6273(92)90250-h. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Bramich N. J., Edwards F. R., Klemm M. Transmission at autonomic neuroeffector junctions. Trends Neurosci. 1992 Feb;15(2):40–46. doi: 10.1016/0166-2236(92)90024-3. [DOI] [PubMed] [Google Scholar]
- Israël M., Manaranche R., Marsal J., Meunier F. M., Morel N., Frachon P., Lesbats B. ATP-dependent calcium uptake by cholinergic synaptic vesicles isolated from Torpedo electric organ. J Membr Biol. 1980 May 23;54(2):115–126. doi: 10.1007/BF01940565. [DOI] [PubMed] [Google Scholar]
- Kasakov L., Ellis J., Kirkpatrick K., Milner P., Burnstock G. Direct evidence for concomitant release of noradrenaline, adenosine 5'-triphosphate and neuropeptide Y from sympathetic nerve supplying the guinea-pig vas deferens. J Auton Nerv Syst. 1988 Feb;22(1):75–82. doi: 10.1016/0165-1838(88)90156-7. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The number of transmitter molecules in a quantum: an estimate from iontophoretic application of acetylcholine at the neuromuscular synapse. J Physiol. 1975 Oct;251(2):465–482. doi: 10.1113/jphysiol.1975.sp011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagercrantz H. On the composition and function of large dense cored vesicles in sympathetic nerves. Neuroscience. 1976;1(2):81–92. doi: 10.1016/0306-4522(76)90002-6. [DOI] [PubMed] [Google Scholar]
- Land B. R., Harris W. V., Salpeter E. E., Salpeter M. M. Diffusion and binding constants for acetylcholine derived from the falling phase of miniature endplate currents. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1594–1598. doi: 10.1073/pnas.81.5.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavidis N. A., Bennett M. R. Probabilistic secretion of quanta from visualized sympathetic nerve varicosities in mouse vas deferens. J Physiol. 1992 Aug;454:9–26. doi: 10.1113/jphysiol.1992.sp019252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Reiser G., Uchitel O. D. Characteristics of membrane channels induced by acetylcholine at frog muscle-tendon junctions. J Physiol. 1984 May;350:269–277. doi: 10.1113/jphysiol.1984.sp015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel N., Meunier F. M. Simultaneous release of acetylcholine and ATP from stimulated cholinergic synaptosomes. J Neurochem. 1981 May;36(5):1766–1773. doi: 10.1111/j.1471-4159.1981.tb00429.x. [DOI] [PubMed] [Google Scholar]
- Morishita H., Furukawa T. Low calcium and calcium antagonists potentiate the contraction of guinea-pig vas deferens induced by ATP: a permissive role for P2-purinoceptors. Eur J Pharmacol. 1989 May 30;164(3):507–513. doi: 10.1016/0014-2999(89)90258-6. [DOI] [PubMed] [Google Scholar]
- Parnas H., Flashner M., Spira M. E. Sequential model to describe the nicotinic synaptic current. Biophys J. 1989 May;55(5):875–884. doi: 10.1016/S0006-3495(89)82886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman S. Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiol Rev. 1990 Jan;70(1):165–198. doi: 10.1152/physrev.1990.70.1.165. [DOI] [PubMed] [Google Scholar]
- Salpeter M. M., Elderfrawi M. E. Sizes of end plate compartments, densities of acetylcholine receptor and other quantitative aspects of neuromuscular transmission. J Histochem Cytochem. 1973 Sep;21(9):769–778. doi: 10.1177/21.9.769. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M., Gerzanich V., Vanner S. M. ATP mediates excitatory synaptic transmission in mammalian neurones. Br J Pharmacol. 1992 Aug;106(4):762–763. doi: 10.1111/j.1476-5381.1992.tb14408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. Inhibition of excitatory junction potentials in guinea-pig vas deferens by alpha, beta-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur J Pharmacol. 1984 Apr 13;100(1):85–90. doi: 10.1016/0014-2999(84)90318-2. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol. 1984 Feb;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer W., Almers W. Photobleaching through glass micropipettes: sodium channels without lateral mobility in the sarcolemma of frog skeletal muscle. Proc Natl Acad Sci U S A. 1982 Feb;79(3):946–950. doi: 10.1073/pnas.79.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathey J. C., Nass M. M., Lester H. A. Numerical reconstruction of the quantal event at nicotinic synapses. Biophys J. 1979 Jul;27(1):145–164. doi: 10.1016/S0006-3495(79)85208-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb T. E., Simon J., Krishek B. J., Bateson A. N., Smart T. G., King B. F., Burnstock G., Barnard E. A. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993 Jun 14;324(2):219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- Whim M. D., Lloyd P. E. Frequency-dependent release of peptide cotransmitters from identified cholinergic motor neurons in Aplysia. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9034–9038. doi: 10.1073/pnas.86.22.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]