Abstract
Wild-type Staphylococcus aureus was observed to be capable of invading human corneal epithelial cells (HCEC) in vitro. Internalization of S. aureus required expression of fibronectin-binding proteins (FnBPs); the capacity of an FnBP-deficient isogenic strain to invade HCEC was reduced by more than 99%. The binding of S. aureus to HCEC did not require viable bacteria, since UV-killed cells were observed to adhere efficiently. Invasion of HCEC by S. aureus involved active host cell mechanisms; uptake was nearly completely eliminated by cytochalasin D and genistein. These data suggest that FnBPs play a key role in host-parasite interactions and may serve as an important adhesin or invasin in ulcerative keratitis caused by S. aureus.
Staphylococcus aureus is a leading cause of ulcerative keratitis, accounting for approximately one quarter of confirmed cases (10, 15, 21). Nevertheless, the specific mechanisms by which this organism colonizes and damages the ocular epithelium are unclear. Because of the importance of S. aureus at other anatomical sites and its role as a leading nosocomial pathogen, a great deal of effort has been focused on investigating its virulence factors and their regulation. Multiple surface adhesins expressed by this organism, including the MSCRAMMs (microbial surface components recognizing adhesive matrix molecules), have been implicated in the pathogenesis of disease at a number of anatomical sites (5, 17); however, comparatively little is known of their role in the pathogenesis of keratitis caused by S. aureus.
It is generally believed that bacterial adhesion to the corneal surface is a prerequisite for ulcerative keratitis. In previous studies, S. aureus was observed to bind ocular epithelial cells (8, 14, 18). Two of these earlier studies employed rabbit corneal epithelial cells, and none specifically addressed the underlying mechanisms of adhesion. A recent study assessed the role of S. aureus collagen-binding adhesin in a rabbit model of contact lens-associated keratitis. While this model may provide a useful tool for studying bacterial surface adhesins in keratitis, it remained somewhat unclear whether collagen-binding adhesin played a direct causal role and the results suggested the involvement of other S. aureus factors (19).
Recent research has revealed that S. aureus fibronectin-binding protein (FnBP) mediates bacterial adhesion and invasion in many cell types, including mammary epithelial cells (4), embryonic kidney cells (22), osteoblasts (1), and endothelial cells (11). The current model for FnBP-mediated adhesion and invasion proposes a fibronectin-dependent bridging between S. aureus FnBPs and host cell α5β1 integrin (11). Because of the importance of S. aureus as a cause of ulcerative keratitis, the availability of isogenic strains, and the demonstrated utility of in vitro epithelial-cell culture systems for the study of S. aureus adhesion and invasion, we were interested in determining whether the FnBP-mediated invasive properties of S. aureus extended to human corneal epithelial cells (HCEC).
Invasion assay.
HCEC were kindly provided by James Chodosh (Dean A. McGee Eye Institute, Oklahoma City, Okla.) and have been extensively characterized by Araki-Sasaki et al. (2). HCEC were cultivated in serum-free defined keratinocyte medium (DK-SFM; Gibco BRL) in 24-well plates to a confluence of >95%. All incubations were carried out at 37°C in an atmosphere of 5% CO2. Bacterial invasion assays were conducted as previously described (4, 22, 25), with the use of mid-logarithmic-phase cultures to maximize the expression of S. aureus surface proteins (5, 13, 17). Briefly, S. aureus strains were propagated to an optical density at 650 nm of approximately 0.5 in brain heart infusion broth, washed twice with DK-SFM, and applied to HCEC monolayers (approximately 108 CFU in 1 ml of DK-SFM). The actual number of bacteria applied to monolayers was determined by serial dilution and plate counting (7). Following incubation for 1 h, bacteria were removed and monolayers were washed twice with DK-SFM. In order to measure bacterial invasion, monolayers were then covered with 1.0 ml of DK-SFM containing 100 μg of gentamicin/ml and incubated for 1 h to kill residual extracellular bacteria. Monolayers were washed twice with antibiotic-free DK-SFM, lysed, and homogenized in 1 ml of lysis solution (0.25% trypsin-0.025% Triton X-100). Lysates were serially diluted and plated on brain heart infusion agar, and colonies were enumerated following overnight incubation (7).
Role of S. aureus FnBP.
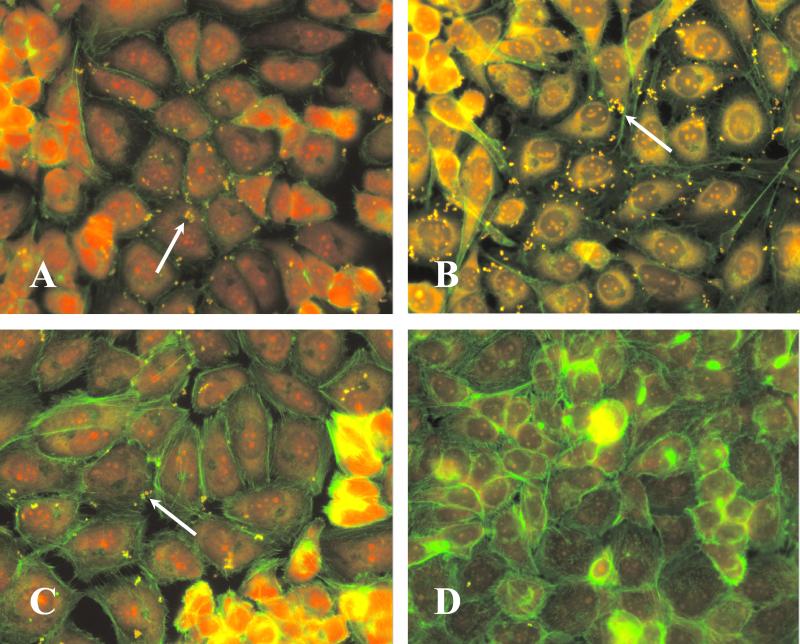
Invasion of HCEC was assessed by use of wild-type S. aureus strain 8325-4 (12) and its isogenic mutant, DU5883, which is defective in FnBP-AB (6). The contribution of global regulatory genes was assessed by use of the agr sar double mutant ALC135 (kindly provided by Ambrose Cheung, Dartmouth Medical School, Hanover, N.H.). HCEC monolayers stained with Alexa-Fluor 488 phalloidin (Molecular Probes, Eugene Oreg.) and ethidium bromide were examined by fluorescence microscopy with fluorescein isothiocyanate (FITC)-labeled (1 mg of FITC/ml in phosphate-buffered saline for 2 h) S. aureus. Qualitatively, FnBP mutants exhibited dramatically reduced binding ability compared to what was exhibited by the wild-type and agr sar double mutant strains (Fig. 1). Binding did not require living bacteria, since UV-killed and live S. aureus (wild type) demonstrated similar adhesion patterns (Fig. 1).
FIG. 1.
Adhesion of S. aureus to HCEC. HCEC monolayers were incubated for 1 h in the presence of S. aureus 8325-4 (wild type) (A), UV-killed 8325-4 (B), ALC135 (agr sar) (C), or DU5883 (fnbAB) (D). Bacteria (arrows) were labeled with FITC prior to inoculation. Monolayers were stained with Alexa-Fluor 488 phalloidin and ethidium bromide. Magnification, ×20.
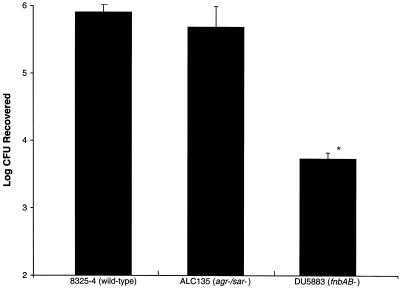
Quantitative invasion assays confirmed the results of fluorescence microscopy. Internalization of the FnBP-defective strain was reduced approximately 100-fold compared to the wild-type levels (Fig. 2). Mutations in the global regulatory loci agr and sar did not significantly affect adhesion and internalization (P > 0.05, Student's t test), a finding similar to that previously reported for the adhesion of S. aureus to human endothelial cells (16). Although the penetration of epithelial surfaces by S. aureus has generally been considered a multifactorial phenomenon, the results of the present study suggest that FnBP serves as the predominant invasion factor for corneal cells.
FIG. 2.
Invasion of HCEC by S. aureus. HCEC monolayers were incubated for 1 h in the presence of S. aureus. Monolayers were washed, and extracellular bacteria were killed by the addition of gentamicin. Monolayers were homogenized, serially diluted, and plated for enumeration of viable intracellular bacteria. Data represent mean values + standard errors of the means for duplicate experiments, each performed in triplicate. Asterisk indicates statistically significant reduction in internalization (P < 0.05, Student's t test) compared to wild-type internalization.
Role of HCEC factors.
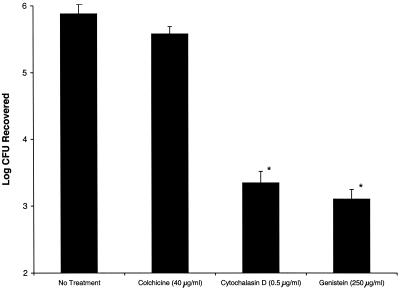
Because of our initial observations that metabolically active S. aureus was not required for adhesion and the results of previous studies using epithelial cell lines from other species and tissues (4, 9) that identified the importance of active processes in mediating uptake, we were interested in assessing whether the uptake of S. aureus by corneal cells involved similar host cell pathways. Prior to the addition of S. aureus to HCEC, monolayers were incubated for 1 h in DK-SFM containing one of several metabolic inhibitors. Inhibitors were also included in the bacterial inoculum, prepared as described above. Preincubation of HCEC in the presence of colchicine (40.0 μg/ml; Sigma) reduced the invasion of wild-type S. aureus by a measurable, yet statistically insignificant, amount (P > 0.05, Student's t test), suggesting a minimal role for microtubule polymerization during bacterial uptake. In sharp contrast, preincubation with either the actin microfilament inhibitor cytochalasin D (0.5 μg/ml; Sigma) or the tyrosine kinase inhibitor genistein (250 μg/ml; Calbiochem) reduced the internalization of S. aureus by >99.9% (P < 0.001) compared to wild-type levels (Fig. 3). These findings indicate that invasion of HCEC by S. aureus is an active process involving host cell cytoskeletal rearrangements and one or more intracellular signaling cascades.
FIG. 3.
Inhibition of S. aureus invasion. HCEC monolayers were incubated for 1 h in the presence of inhibitors. Monolayers were incubated for an additional 1 h in the presence of inhibitors and wild-type S. aureus. Monolayers were washed, and extracellular bacteria were killed by the addition of gentamicin. Monolayers were homogenized, serially diluted, and plated for enumeration of viable intracellular bacteria. Data represent mean values + standard errors of the means for duplicate experiments, each performed in triplicate. Asterisks indicate statistically significant reductions in internalization (P < 0.05, Student's t test) compared to wild-type internalization.
Taken together, the results of these studies provide a framework for a model of S. aureus-corneal epithelium interaction. In this model, S. aureus may bind to corneal epithelial cells via its cell surface FnBPs. Following ligation of the appropriate corneal epithelial cell receptor, intracellular signaling cascades become activated, leading to actin polymerization and formation of endocytic vesicles.
Similar models have been proposed for S. aureus adhesion to and invasion of bovine mammary epithelial cells (3), human embryonic kidney cells (22), and endothelial cells (11). Sinha et al. (23) described the entry of S. aureus into epithelial cells in terms of a “zipper-type” mechanism resembling professional phagocytosis and involving F-actin, as demonstrated by complete inhibition by cytochalasin D (22). They mapped the interaction between the host cell and the fibronectin-binding domain of FnBP and suggested that fibronectin acts as a bridging molecule between S. aureus FnBPs and host cell α5β1 integrin, an integrin which is known to occur in the human corneal epithelium (24). The cumulative results of multiple similar studies led Sinha et al. to exclude an efficient second S. aureus invasin, highlighting FnBP as the dominant S. aureus MSCRAMM in epithelial cell adhesion and invasion (22, 23). The findings of the present study are in good agreement with this model, as the invasion of HCEC by S. aureus was markedly reduced in the absence of FnBP or active host cell uptake processes.
To the best of our knowledge, this study represents the first identification of an S. aureus surface ligand for cells of the human cornea and the first demonstration of its importance in triggering invasion. Nevertheless, a number of questions remain unanswered. Further experiments are required to precisely quantify adhesion and elucidate the mechanism for the internalization and subcellular localization of internalized bacteria (i.e., confocal microscopy studies). Competitive inhibition studies using anti-FnBP antibodies or FnBP-derived peptides might be useful in characterizing the receptor-ligand interactions at the corneal cell surface. Since, in this study, S. aureus invasion was substantially but not completely eliminated by the absence of FnBP, there appears to be a role, albeit a minor one, for other factors. Finally, it remains to be determined to what extent these in vitro results can be extrapolated to actual cases of ulcerative keratitis. The corneal surface is bathed in a mucinous tear film, which contains multiple antimicrobial factors that collectively present a formidable barrier to bacterial adhesion and invasion. Murine models have proven useful for the investigation of keratitis caused by Pseudomonas aeruginosa (20), and rabbit models of S. aureus adhesion to the corneal surface are in the early stages of development (19). Establishment of a reliable in vivo model that incorporates quantifiable initial-adherence processes will be required to elucidate the molecular pathogenesis of ulcerative keratitis caused by S. aureus and to assess the effectiveness of therapeutic agents aimed at blocking bacterial adhesion and/or invasion.
Acknowledgments
We thank James Chodosh, Dean A. McGee Eye Institute, for providing HCEC, and Kenneth Hatter for technical assistance.
This work was supported by Public Health Service grant EY11648 from the National Eye Institute and Research to Prevent Blindness, Inc.
Editor: E. I. Tuomanen
REFERENCES
- 1.Ahmed, S., S. Meghji, R. J. Williams, B. Henderson, J. H. Brock, and S. P. Nair. 2001. Staphylococcus aureus fibronectin binding proteins are essential for internalization by osteoblasts but do not account for differences in intracellular levels of bacteria. Infect. Immun. 69:2872-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araki-Sasaki, K., Y. Ohashi, T. Sasabe, K. Hayashi, H. Watanabe, Y. Tano, and H. Handa. 1995. An SV40-immortalized human corneal epithelial cell line and its characterization. Investig. Ophthalmol. Vis. Sci. 36:614-621. [PubMed] [Google Scholar]
- 3.Dziewanowska, K., A. R. Carson, J. M. Patti, C. F. Deobald, K. W. Bayles, and G. A. Bohach. 2000. Staphylococcal fibronectin binding protein interacts with heat shock protein 60 and integrins: role in internalization by epithelial cells. Infect. Immun. 68:6321-6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dziewanowska, K., J. M. Patti, C. F. Deobald, K. W. Bayles, W. R. Trumble, and G. A. Bohach. 1999. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect. Immun. 67:4673-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster, T. J., and M. Höök. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6:484-488. [DOI] [PubMed] [Google Scholar]
- 6.Greene, C., D. McDevitt, P. Francois, P. E. Vaudaux, D. P. Lew, and T. J. Foster. 1995. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin-binding proteins and studies on the expression of fnb genes. Mol. Microbiol. 17:1143-1152. [DOI] [PubMed] [Google Scholar]
- 7.Jett, B. D., K. L. Hatter, M. M. Huycke, and M. S. Gilmore. 1997. Simplified agar plate method for quantifying viable bacteria. BioTechniques 23:648-650. [DOI] [PubMed] [Google Scholar]
- 8.Johnson, A. P., B. M. Wool, and M. K. Johnson. 1984. Adherence of Staphylococcus aureus to rabbit corneal epithelial cells. Arch. Ophthalmol. 102:1229-1231. [DOI] [PubMed] [Google Scholar]
- 9.Kahl, B. C., M. Goulian, W. Van Wamel, M. Herrmann, S. M. Simon, G. Kaplan, G. Peters, and A. L. Cheung. 2000. Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infect. Immun. 68:5385-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunimoto, D. Y., S. Sharma, M. K. Reddy, U. Gopinathan, J. Jyothi, D. Miller, and G. N. Rao. 1998. Microbial keratitis in children. Ophthalmology 105:252-257. [DOI] [PubMed] [Google Scholar]
- 11.Massey, R. C., M. N. Kantzanou, T. Fowler, N. P. Day, K. Schofield, E. R. Wann, A. R. Berendt, M. Hook, and S. J. Peacock. 2001. Fibronectin-binding protein A of Staphylococcus aureus has multiple, substituting, binding regions that mediate adherence to fibronectin and invasion of endothelial cells. Cell. Microbiol. 3:839-851. [DOI] [PubMed] [Google Scholar]
- 12.Novick, R. 1967. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33:155-166. [DOI] [PubMed] [Google Scholar]
- 13.Novick, R. P. 2000. Pathogenicity factors and their regulation, p. 392-407. In V. A. Fischetti (ed.), Gram-positive pathogens. American Society for Microbiology, Washington, D.C.
- 14.Panjwani, N., B. Clark, M. Cohen, M. Barza, and J. Baum. 1990. Differential binding of P. aeruginosa and S. aureus to corneal epithelium in culture. Investig. Ophthalmol. Vis. Sci. 31:696-701. [PubMed] [Google Scholar]
- 15.Pepose, J. S., and K. R. Wilhelmus. 1992. Divergent approaches to the management of corneal ulcers. Am. J. Ophthalmol. 114:630-632. [DOI] [PubMed] [Google Scholar]
- 16.Pöhlmann-Dietze, P., M. Ulrich, K. B. Kiser, G. Döring, J. C. Lee, J. Fournier, K. Botzenhart, and C. Wolz. 2000. Adherence of Staphylococcus aureus to endothelial cells: influence of capsular polysaccharide, global regulator agr, and bacterial growth phase. Infect. Immun. 68:4865-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Projan, S., and R. Novick. 1997. The molecular basis of virulence, p. 55-81. In G. Archer and K. Crossley (ed.), Staphylococci in human disease. Churchill Livingstone, New York, N.Y.
- 18.Reichert, R., and G. Stern. 1984. Quantitative adherence of bacteria to human corneal epithelial cells. Arch. Ophthalmol. 102:1394-1395. [DOI] [PubMed] [Google Scholar]
- 19.Rhem, M. N., E. M. Lech, J. M. Patti, D. McDevitt, M. Hook, D. B. Jones, and K. R. Wilhelmus. 2000. The collagen-binding adhesin is a virulence factor in Staphylococcus aureus keratitis. Infect. Immun. 68:3776-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudner, X. L., K. A. Kernacki, R. P. Barrett, and L. D. Hazlett. 2000. Prolonged elevation of IL-1 in Pseudomonas aeruginosa ocular infection regulates macrophage-inflammatory protein-2 production, polymorphonuclear neutrophil persistence, and corneal perforation. J. Immunol. 164:6576-6582. [DOI] [PubMed] [Google Scholar]
- 21.Schaefer, F., O. Bruttin, L. Zografos, and Y. Guex-Crosier. 2001. Bacterial keratitis: a prospective clinical and microbiological study. Br. J. Ophthalmol. 85:842-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinha, B., P. Francois, Y. Que, M. Hussain, C. Heilmann, P. Moreillon, D. Lew, K. Krause, G. Peters, and M. Herrmann. 2000. Heterologously expressed Staphylococcus aureus fibronectin-binding proteins are sufficient for invasion of host cells. Infect. Immun. 68:6871-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinha, B., P. P. Francois, O. Nüβe, M. Foti, O. M. Hartford, P. Vaudaux, T. J. Foster, D. P. Lew, M. Herrmann, and K. H. Krause. 1999. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell. Microbiol. 1:101-117. [DOI] [PubMed] [Google Scholar]
- 24.Virtanen, I., K. Tervo, M. Korhonen, T. Paallysaho, and T. Tervo. 1992. Integrins as receptors for extracellular matrix proteins in human cornea. Acta Ophthalmol. 202(Suppl.):18-21. [DOI] [PubMed] [Google Scholar]
- 25.Wesson, C. A., L. E. Liou, K. M. Todd, G. A. Bohach, W. R. Trumble, and K. W. Bayles. 1998. Staphylococcus aureus Agr and Sar global regulators influence internalization and induction of apoptosis. Infect. Immun. 66:5238-5243. [DOI] [PMC free article] [PubMed] [Google Scholar]