Abstract
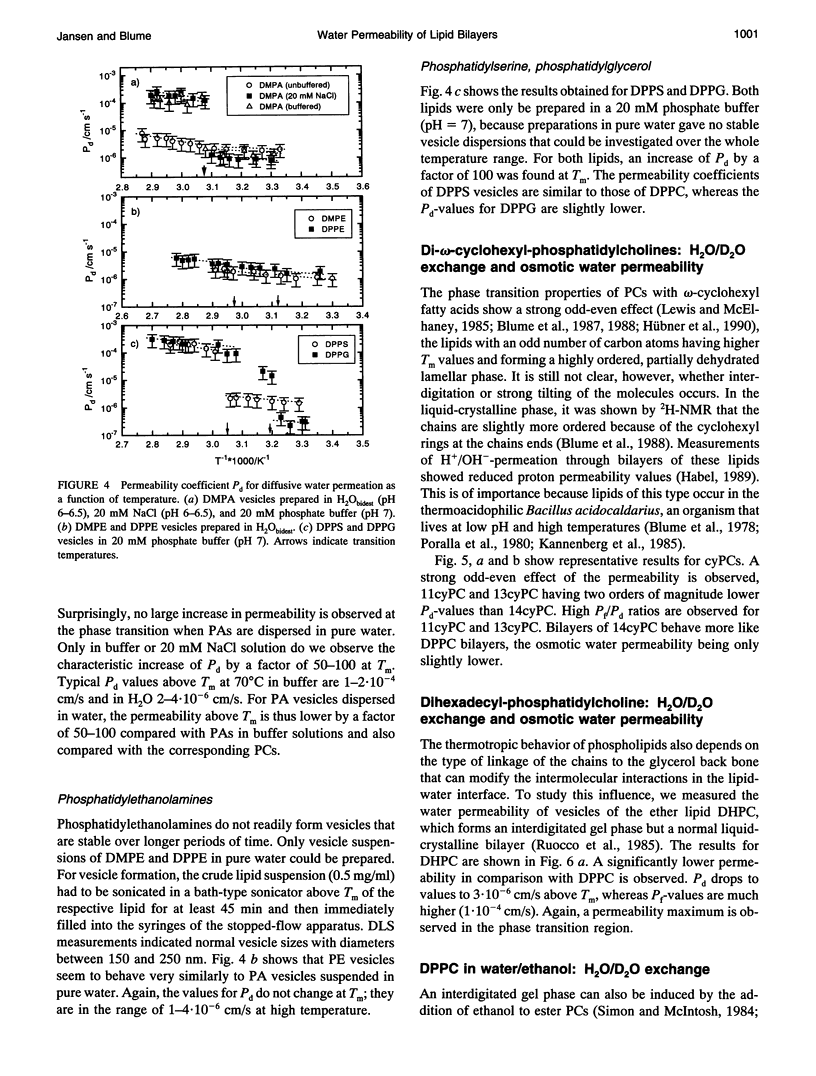
Osmotic and diffusive water permeability coefficients Pf and Pd were measured for lipid vesicles of 100-250 nm diameter composed of a variety of phospholipids with different head groups and fatty acyl chains. Two different methods were applied: the H2O/D2O exchange technique for diffusive water flow, and the osmotic technique for water flux driven by an osmotic gradient. For phosphatidylcholines in the liquid-crystalline state at 70 degrees C, permeability constants Pd between 3.0 and 5.2.10(-4) cm/s and ratios Pf/Pd 7 and 23 were observed. The observation of a permeability maximum in the phase transition region and the fact that osmotically driven water flux is higher than diffusive water exchange suggest that water is diffusing through small transient pores arising from density fluctuations in the bilayers. The Pd values depend on the nature of the head group, on the chemical structure of the chains, and on the type of chain linkage. In the case of charged lipids, the ionic strength of the solution has a strong influence. For phosphatidylethanolamines, phosphatidic acids, and ether phosphatidylcholines, permeability constants Pd were considerably lower (2-4.10(-6) cm/s at 70 degrees C). For liquid-crystalline phosphatidylcholines, a strong reduction of Pd after addition of ethanol was observed (2-4.10(-6) cm/s at 70 degrees C). The experimental values are discussed in connection with different permeation models.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Beckers F., Zimmermann U. Reversible electrical breakdown of lipid bilayer membranes: a charge-pulse relaxation study. J Membr Biol. 1979 Jul 16;48(2):181–204. doi: 10.1007/BF01872858. [DOI] [PubMed] [Google Scholar]
- Benz R., Zimmermann U. The resealing process of lipid bilayers after reversible electrical breakdown. Biochim Biophys Acta. 1981 Jan 8;640(1):169–178. doi: 10.1016/0005-2736(81)90542-3. [DOI] [PubMed] [Google Scholar]
- Bittman R., Blau L. The phospholipid-cholesterol interaction. Kinetics of water permeability in liposomes. Biochemistry. 1972 Dec 5;11(25):4831–4839. doi: 10.1021/bi00775a029. [DOI] [PubMed] [Google Scholar]
- Blume A., Dreher R., Poralla K. The influence of branched-chain and omega-alicyclic fatty acids on the transition temperature of bacillus subtilis lipids. Biochim Biophys Acta. 1978 Oct 4;512(3):489–494. doi: 10.1016/0005-2736(78)90159-1. [DOI] [PubMed] [Google Scholar]
- Blume A., Eibl H. The influence of charge on bilayer membranes. Calorimetric investigations of phosphatidic acid bilayers. Biochim Biophys Acta. 1979 Nov 16;558(1):13–21. doi: 10.1016/0005-2736(79)90311-0. [DOI] [PubMed] [Google Scholar]
- Blume A., Hübner W., Messner G. Fourier transform infrared spectroscopy of 13C = O-labeled phospholipids hydrogen bonding to carbonyl groups. Biochemistry. 1988 Oct 18;27(21):8239–8249. doi: 10.1021/bi00421a038. [DOI] [PubMed] [Google Scholar]
- Boggs J. M. Lipid intermolecular hydrogen bonding: influence on structural organization and membrane function. Biochim Biophys Acta. 1987 Oct 5;906(3):353–404. doi: 10.1016/0304-4157(87)90017-7. [DOI] [PubMed] [Google Scholar]
- Cevc G. Membrane electrostatics. Biochim Biophys Acta. 1990 Oct 8;1031(3):311–382. doi: 10.1016/0304-4157(90)90015-5. [DOI] [PubMed] [Google Scholar]
- Chiou J. S., Krishna P. R., Kamaya H., Ueda I. Alcohols dehydrate lipid membranes: an infrared study on hydrogen bonding. Biochim Biophys Acta. 1992 Oct 5;1110(2):225–233. doi: 10.1016/0005-2736(92)90363-q. [DOI] [PubMed] [Google Scholar]
- Cruzeiro-Hansson L., Mouritsen O. G. Passive ion permeability of lipid membranes modelled via lipid-domain interfacial area. Biochim Biophys Acta. 1988 Sep 15;944(1):63–72. doi: 10.1016/0005-2736(88)90316-1. [DOI] [PubMed] [Google Scholar]
- Damodaran K. V., Merz K. M., Jr A comparison of DMPC- and DLPE-based lipid bilayers. Biophys J. 1994 Apr;66(4):1076–1087. doi: 10.1016/S0006-3495(94)80889-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta S. K., Rice D. M., Griffin R. G. Synthesis of isotopically labeled saturated fatty acids. J Lipid Res. 1982 Jan;23(1):197–200. [PubMed] [Google Scholar]
- Deamer D. W., Bramhall J. Permeability of lipid bilayers to water and ionic solutes. Chem Phys Lipids. 1986 Jun-Jul;40(2-4):167–188. doi: 10.1016/0009-3084(86)90069-1. [DOI] [PubMed] [Google Scholar]
- Eibl H., Blume A. The influence of charge on phosphatidic acid bilayer membranes. Biochim Biophys Acta. 1979 Jun 2;553(3):476–488. doi: 10.1016/0005-2736(79)90303-1. [DOI] [PubMed] [Google Scholar]
- Eibl H., Woolley P. Electrostatic interactions at charged lipid membranes. Hydrogen bonds in lipid membrane surfaces. Biophys Chem. 1979 Nov;10(3-4):261–271. doi: 10.1016/0301-4622(79)85015-2. [DOI] [PubMed] [Google Scholar]
- Engelbert H. P., Lawaczeck R. Isotopic light scattering of lipid vesicles. Water permeation and effect of alpha-tocopherol. Chem Phys Lipids. 1985 Nov-Dec;38(4):365–379. doi: 10.1016/0009-3084(85)90030-1. [DOI] [PubMed] [Google Scholar]
- Ertel A., Marangoni A. G., Marsh J., Hallett F. R., Wood J. M. Mechanical properties of vesicles. I. Coordinated analysis of osmotic swelling and lysis. Biophys J. 1993 Feb;64(2):426–434. doi: 10.1016/S0006-3495(93)81383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein A. Water and nonelectrolyte permeability of lipid bilayer membranes. J Gen Physiol. 1976 Aug;68(2):127–135. doi: 10.1085/jgp.68.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S. A., Wang M. A., Weaver J. C. Theory of electroporation of planar bilayer membranes: predictions of the aqueous area, change in capacitance, and pore-pore separation. Biophys J. 1994 Jul;67(1):42–56. doi: 10.1016/S0006-3495(94)80453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R. W., Leikin S. L., Chernomordik L. V., Pastushenko V. F., Sokirko A. I. Reversible electrical breakdown of lipid bilayers: formation and evolution of pores. Biochim Biophys Acta. 1988 May 24;940(2):275–287. doi: 10.1016/0005-2736(88)90202-7. [DOI] [PubMed] [Google Scholar]
- Hallett F. R., Marsh J., Nickel B. G., Wood J. M. Mechanical properties of vesicles. II. A model for osmotic swelling and lysis. Biophys J. 1993 Feb;64(2):435–442. doi: 10.1016/S0006-3495(93)81384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanai T., Haydon D. A. The permeability to water of bimolecular lipid membranes. J Theor Biol. 1966 Aug;11(3):370–382. doi: 10.1016/0022-5193(66)90099-3. [DOI] [PubMed] [Google Scholar]
- Hirsch-Ayalon P. Precipitation membranes: III. Reversible changes of membrane properties induced by alterations in ionic concentrations. J Membr Biol. 1979 Dec 12;51(1):1–6. doi: 10.1007/BF01869339. [DOI] [PubMed] [Google Scholar]
- Huang C., Thompson T. E. Properties of lipid bilayer membranes separating two aqueous phases: water permeability. J Mol Biol. 1966 Feb;15(2):539–554. doi: 10.1016/s0022-2836(66)80126-2. [DOI] [PubMed] [Google Scholar]
- Hübner W., Wong P. T., Mantsch H. H. The effect of hydrostatic pressure on the bilayer structure of phosphatidylcholines containing omega-cyclohexyl fatty acyl chains. Biochim Biophys Acta. 1990 Sep 7;1027(3):229–237. doi: 10.1016/0005-2736(90)90312-c. [DOI] [PubMed] [Google Scholar]
- Jendrasiak G. L., Hasty J. H. The hydration of phospholipids. Biochim Biophys Acta. 1974 Jan 23;337(1):79–91. doi: 10.1016/0005-2760(74)90042-3. [DOI] [PubMed] [Google Scholar]
- Jähnig F. Electrostatic free energy and shift of the phase transition for charged lipid membranes. Biophys Chem. 1976 Jul;4(4):309–318. doi: 10.1016/0301-4622(76)80012-9. [DOI] [PubMed] [Google Scholar]
- Knott R. B., Schoenborn B. P. Quantitation of water in membranes by neutron diffraction and X-ray techniques. Methods Enzymol. 1986;127:217–229. doi: 10.1016/0076-6879(86)27018-4. [DOI] [PubMed] [Google Scholar]
- Laggner P., Lohner K., Degovics G., Müller K., Schuster A. Structure and thermodynamics of the dihexadecylphosphatidylcholine-water system. Chem Phys Lipids. 1987 Jun;44(1):31–60. doi: 10.1016/0009-3084(87)90004-1. [DOI] [PubMed] [Google Scholar]
- Lawaczeck R. Water permeability through biological membranes by isotopic effects of fluorescence and light scattering. Biophys J. 1984 Mar;45(3):491–494. doi: 10.1016/S0006-3495(84)84184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D. G. A new theory of transport for cell membrane pores. I. General theory and application to red cell. Biochim Biophys Acta. 1974 Nov 27;373(1):115–131. doi: 10.1016/0005-2736(74)90111-4. [DOI] [PubMed] [Google Scholar]
- Levitt D. G. Kinetics of diffusion and convection in 3.2-A pores. Exact solution by computer simulation. Biophys J. 1973 Feb;13(2):186–206. doi: 10.1016/S0006-3495(73)85979-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D. G., Subramanian G. A new theory of transport for cell membrane pores. II. Exact results and computer simulation (molecular dynamics). Biochim Biophys Acta. 1974 Nov 27;373(1):132–140. doi: 10.1016/0005-2736(74)90112-6. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing omega-cyclohexyl fatty acids. Differential scanning calorimetric and 31P NMR spectroscopic studies. Biochemistry. 1985 Aug 27;24(18):4903–4911. doi: 10.1021/bi00339a027. [DOI] [PubMed] [Google Scholar]
- Lieb W. R., Stein W. D. Biological membranes behave as non-porous polymeric sheets with respect to the diffusion of non-electrolytes. Nature. 1969 Oct 18;224(5216):240–243. doi: 10.1038/224240a0. [DOI] [PubMed] [Google Scholar]
- Mui B. L., Cullis P. R., Evans E. A., Madden T. D. Osmotic properties of large unilamellar vesicles prepared by extrusion. Biophys J. 1993 Feb;64(2):443–453. doi: 10.1016/S0006-3495(93)81385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel N. E., Cevc G., Kirchner S. The mechanism of the solute-induced chain interdigitation in phosphatidylcholine vesicles and characterization of the isothermal phase transitions by means of dynamic light scattering. Biochim Biophys Acta. 1992 Nov 9;1111(2):263–269. doi: 10.1016/0005-2736(92)90319-h. [DOI] [PubMed] [Google Scholar]
- Needham D., Evans E. Structure and mechanical properties of giant lipid (DMPC) vesicle bilayers from 20 degrees C below to 10 degrees C above the liquid crystal-crystalline phase transition at 24 degrees C. Biochemistry. 1988 Oct 18;27(21):8261–8269. doi: 10.1021/bi00421a041. [DOI] [PubMed] [Google Scholar]
- PAGANELLI C. V., SOLOMON A. K. The rate of exchange of tritiated water across the human red cell membrane. J Gen Physiol. 1957 Nov 20;41(2):259–277. doi: 10.1085/jgp.41.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos D., Jacobson K., Nir S., Isac T. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim Biophys Acta. 1973 Jul 6;311(3):330–348. doi: 10.1016/0005-2736(73)90314-3. [DOI] [PubMed] [Google Scholar]
- Poralla K., Kannenberg E., Blume A. A glycolipid containing hopane isolated from the acidophilic, thermophilic Bacillus acidocaldarius, has a cholesterol-like function in membranes. FEBS Lett. 1980 Apr 21;113(1):107–110. doi: 10.1016/0014-5793(80)80506-0. [DOI] [PubMed] [Google Scholar]
- Ruocco M. J., Siminovitch D. J., Griffin R. G. Comparative study of the gel phases of ether- and ester-linked phosphatidylcholines. Biochemistry. 1985 May 7;24(10):2406–2411. doi: 10.1021/bi00331a003. [DOI] [PubMed] [Google Scholar]
- SIDEL V. W., SOLOMON A. K. Entrance of water into human red cells under an osmotic pressure gradient. J Gen Physiol. 1957 Nov 20;41(2):243–257. doi: 10.1085/jgp.41.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J. Depth of water penetration into lipid bilayers. Methods Enzymol. 1986;127:511–521. doi: 10.1016/0076-6879(86)27041-x. [DOI] [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J. Interdigitated hydrocarbon chain packing causes the biphasic transition behavior in lipid/alcohol suspensions. Biochim Biophys Acta. 1984 Jun 13;773(1):169–172. doi: 10.1016/0005-2736(84)90562-5. [DOI] [PubMed] [Google Scholar]
- Sugar I. P., Förster W., Neumann E. Model of cell electrofusion. Membrane electroporation, pore coalescence and percolation. Biophys Chem. 1987 May 9;26(2-3):321–335. doi: 10.1016/0301-4622(87)80033-9. [DOI] [PubMed] [Google Scholar]
- Tonomura B., Nakatani H., Ohnishi M., Yamaguchi-Ito J., Hiromi K. Test reactions for a stopped-flow apparatus. Reduction of 2,6-dichlorophenolindophenol and potassium ferricyanide by L-ascorbic acid. Anal Biochem. 1978 Feb;84(2):370–383. doi: 10.1016/0003-2697(78)90054-4. [DOI] [PubMed] [Google Scholar]
- Träuble H., Eibl H. Electrostatic effects on lipid phase transitions: membrane structure and ionic environment. Proc Natl Acad Sci U S A. 1974 Jan;71(1):214–219. doi: 10.1073/pnas.71.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsong T. Y. Electroporation of cell membranes. Biophys J. 1991 Aug;60(2):297–306. doi: 10.1016/S0006-3495(91)82054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsong T. Y., Greenberg M., Kanehisa M. I. Anesthetic action of membrane lipids. Biochemistry. 1977 Jul 12;16(14):3115–3121. doi: 10.1021/bi00633a012. [DOI] [PubMed] [Google Scholar]
- Tyäuble H., Teubner M., Woolley P., Eibl H. Electrostatic interactions at charged lipid membranes. I. Effects of pH and univalent cations on membrane structure. Biophys Chem. 1976 Jul;4(4):319–342. doi: 10.1016/0301-4622(76)80013-0. [DOI] [PubMed] [Google Scholar]


