Abstract
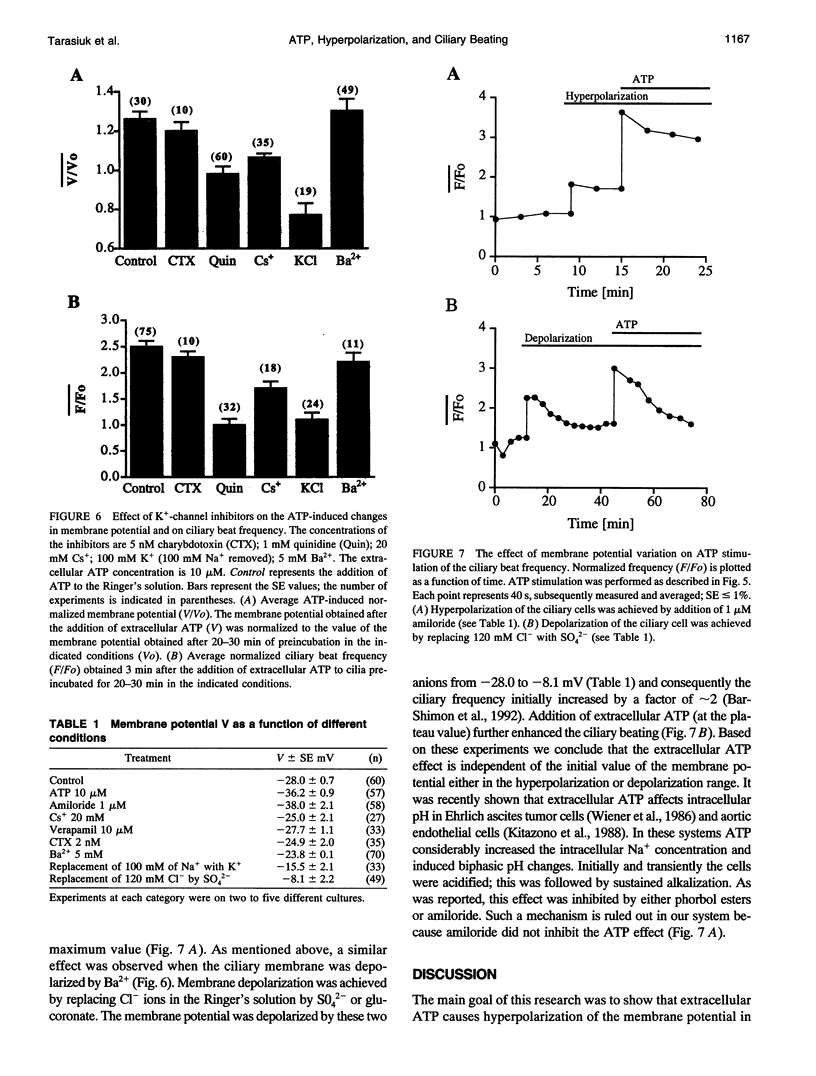
Cellular membrane potential and ciliary motility were examined in tissues cultures prepared from frog palate and esophagus epithelia. Addition of micromolar concentrations of extracellular ATP caused membrane hyperpolarization and enhanced the beat frequency. These two effects of ATP were 1) dose dependent, reaching a maximum at 10 microM ATP; 2) dependent on the presence of extracellular Ca2+ or Mg2+; 3) insensitive to inhibitors of voltage-gated calcium channels; 4) abolished after depleting the intracellular Ca2+ stores with thapsigargin; 5) attenuated by quinidine (1 mM), Cs+ (5-20 mM), and replacement of extracellular Na+ by K+; 6) insensitive to charybdotoxin (5-20 nM), TEA (1-20 microM), and apamin (0.1-1 microM); 7) independent of initial membrane potential; and 8) unaffected by amiloride. In addition, extracellular ATP induced an appreciable rise in intracellular Ca2+. Addition of thapsigargin caused an initial enhancement of the ciliary beat frequency and membrane hyperpolarization. These results strongly suggest the involvement of calcium-dependent potassium channels in the response to ATP. The results show that moderate hyperpolarization is closely associated with a sustained enhancement of ciliary beating by extracellular ATP.
Full text
PDF
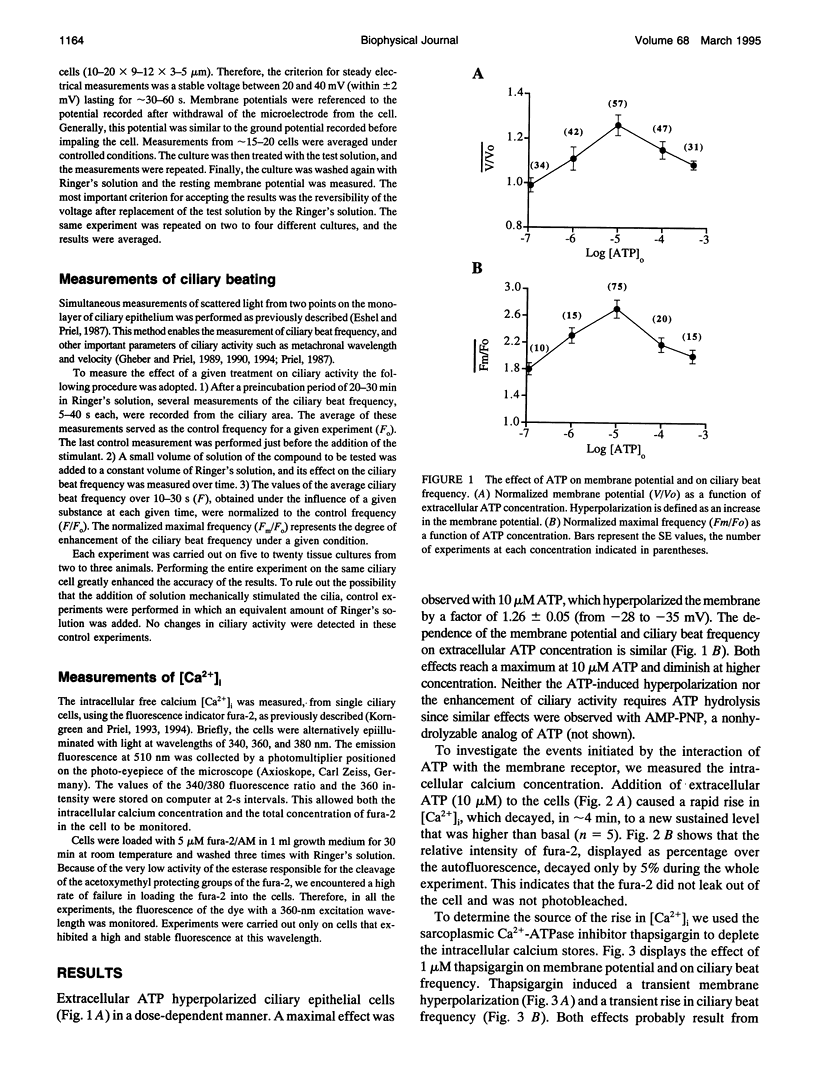


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonini N. M., Gustin M. C., Nelson D. L. Regulation of ciliary motility by membrane potential in Paramecium: a role for cyclic AMP. Cell Motil Cytoskeleton. 1986;6(3):256–272. doi: 10.1002/cm.970060303. [DOI] [PubMed] [Google Scholar]
- Bonini N. M., Nelson D. L. Differential regulation of Paramecium ciliary motility by cAMP and cGMP. J Cell Biol. 1988 May;106(5):1615–1623. doi: 10.1083/jcb.106.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini N. M., Nelson D. L. Phosphoproteins associated with cyclic nucleotide stimulation of ciliary motility in Paramecium. J Cell Sci. 1990 Feb;95(Pt 2):219–230. doi: 10.1242/jcs.95.2.219. [DOI] [PubMed] [Google Scholar]
- Brehm P., Eckert R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science. 1978 Dec 15;202(4373):1203–1206. doi: 10.1126/science.103199. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Cusack N. J., Hills J. M., MacKenzie I., Meghji P. Studies on the stereoselectivity of the P2-purinoceptor. Br J Pharmacol. 1983 Aug;79(4):907–913. doi: 10.1111/j.1476-5381.1983.tb10535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey T. E., Chandy K. G., Gupta S., Cahalan M. D. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature. 1984 Feb 2;307(5950):465–468. doi: 10.1038/307465a0. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993 Sep;265(3 Pt 1):C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Eshel D., Grossman Y., Priel Z. Spectral characterization of ciliary beating: variations of frequency with time. Am J Physiol. 1985 Jul;249(1 Pt 1):C160–C165. doi: 10.1152/ajpcell.1985.249.1.C160. [DOI] [PubMed] [Google Scholar]
- Eshel D., Priel Z. Characterization of metachronal wave of beating cilia on frog's palate epithelium in tissue culture. J Physiol. 1987 Jul;388:1–8. doi: 10.1113/jphysiol.1987.sp016597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher D. V. Are there purinergic receptors on parotid acinar cells? Nature. 1982 Mar 4;296(5852):83–86. doi: 10.1038/296083a0. [DOI] [PubMed] [Google Scholar]
- Gheber L., Priel Z. Ciliary activity under normal conditions and under viscous load. Biorheology. 1990;27(3-4):547–557. doi: 10.3233/bir-1990-273-433. [DOI] [PubMed] [Google Scholar]
- Gheber L., Priel Z. Metachronal activity of cultured mucociliary epithelium under normal and stimulated conditions. Cell Motil Cytoskeleton. 1994;28(4):333–345. doi: 10.1002/cm.970280407. [DOI] [PubMed] [Google Scholar]
- Gheber L., Priel Z. Synchronization between beating cilia. Biophys J. 1989 Jan;55(1):183–191. doi: 10.1016/S0006-3495(89)82790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygorczyk C., Grygorczyk R., Ferrier J. Osteoblastic cells have L-type calcium channels. Bone Miner. 1989 Sep;7(2):137–148. doi: 10.1016/0169-6009(89)90071-8. [DOI] [PubMed] [Google Scholar]
- Hansen M., Boitano S., Dirksen E. R., Sanderson M. J. Intercellular calcium signaling induced by extracellular adenosine 5'-triphosphate and mechanical stimulation in airway epithelial cells. J Cell Sci. 1993 Dec;106(Pt 4):995–1004. doi: 10.1242/jcs.106.4.995. [DOI] [PubMed] [Google Scholar]
- Inoue R., Brading A. F. The properties of the ATP-induced depolarization and current in single cells isolated from the guinea-pig urinary bladder. Br J Pharmacol. 1990 Jul;100(3):619–625. doi: 10.1111/j.1476-5381.1990.tb15856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean T., Klee C. B. Calcium modulation of inositol 1,4,5-trisphosphate-induced calcium release from neuroblastoma x glioma hybrid (NG108-15) microsomes. J Biol Chem. 1986 Dec 15;261(35):16414–16420. [PubMed] [Google Scholar]
- Keef K. D., Pasco J. S., Eckman D. M. Purinergic relaxation and hyperpolarization in guinea pig and rabbit coronary artery: role of the endothelium. J Pharmacol Exp Ther. 1992 Feb;260(2):592–600. [PubMed] [Google Scholar]
- Kennedy C., Burnstock G. Evidence for two types of P2-purinoceptor in longitudinal muscle of the rabbit portal vein. Eur J Pharmacol. 1985 Apr 23;111(1):49–56. doi: 10.1016/0014-2999(85)90112-8. [DOI] [PubMed] [Google Scholar]
- Kindmark H., Köhler M., Nilsson T., Arkhammar P., Wiechel K. L., Rorsman P., Efendić S., Berggren P. O. Measurements of cytoplasmic free Ca2+ concentration in human pancreatic islets and insulinoma cells. FEBS Lett. 1991 Oct 21;291(2):310–314. doi: 10.1016/0014-5793(91)81309-v. [DOI] [PubMed] [Google Scholar]
- Kitazono T., Takeshige K., Cragoe E. J., Jr, Minakami S. Intracellular pH changes of cultured bovine aortic endothelial cells in response to ATP addition. Biochem Biophys Res Commun. 1988 May 16;152(3):1304–1309. doi: 10.1016/s0006-291x(88)80427-3. [DOI] [PubMed] [Google Scholar]
- Korngreen A., Priel Z. Simultaneous measurement of ciliary beating and intracellular calcium. Biophys J. 1994 Jul;67(1):377–380. doi: 10.1016/S0006-3495(94)80492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R., Miller C. Conduction and selectivity in potassium channels. J Membr Biol. 1983;71(1-2):11–30. doi: 10.1007/BF01870671. [DOI] [PubMed] [Google Scholar]
- Nakaoka Y., Ooi H. Regulation of ciliary reversal in triton-extracted Paramecium by calcium and cyclic adenosine monophosphate. J Cell Sci. 1985 Aug;77:185–195. doi: 10.1242/jcs.77.1.185. [DOI] [PubMed] [Google Scholar]
- Nelson D. J., Wright E. M. The distribution, activity, and function of the cilia in the frog brain. J Physiol. 1974 Nov;243(1):63–78. doi: 10.1113/jphysiol.1974.sp010742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovadyahu D., Eshel D., Priel Z. Intensification of ciliary motility by extracellular ATP. Biorheology. 1988;25(3):489–501. doi: 10.3233/bir-1988-25309. [DOI] [PubMed] [Google Scholar]
- Paulmichl M., Pfeilschifter J., Wöll E., Lang F. Cellular mechanisms of ATP-induced hyperpolarization in renal epitheloid MDCK-cells. J Cell Physiol. 1991 Apr;147(1):68–75. doi: 10.1002/jcp.1041470110. [DOI] [PubMed] [Google Scholar]
- Priel Z. Direct measurement of the velocity of the metachronal wave in beating cilia. Biorheology. 1987;24(6):599–603. doi: 10.3233/bir-1987-24612. [DOI] [PubMed] [Google Scholar]
- Ruff R. L. Ionic channels: II. Voltage- and agonist-gated and agonist-modified channel properties and structure. Muscle Nerve. 1986 Nov-Dec;9(9):767–786. doi: 10.1002/mus.880090902. [DOI] [PubMed] [Google Scholar]
- Saimi Y., Hinrichsen R. D., Forte M., Kung C. Mutant analysis shows that the Ca2+-induced K+ current shuts off one type of excitation in Paramecium. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5112–5116. doi: 10.1073/pnas.80.16.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satow Y., Kung C. Ca-induced K+-outward current in Paramecium tetraurelia. J Exp Biol. 1980 Oct;88:293–303. doi: 10.1242/jeb.88.1.293. [DOI] [PubMed] [Google Scholar]
- Soltoff S. P., McMillian M. K., Talamo B. R. ATP activates a cation-permeable pathway in rat parotid acinar cells. Am J Physiol. 1992 Apr;262(4 Pt 1):C934–C940. doi: 10.1152/ajpcell.1992.262.4.C934. [DOI] [PubMed] [Google Scholar]
- VORHAUS E. F., DEYRUP I. J. The effect of adenosinetriphosphate on the cilia of the pharyngeal mucosa of the frog. Science. 1953 Nov 6;118(3071):553–554. doi: 10.1126/science.118.3071.553. [DOI] [PubMed] [Google Scholar]
- Van Coevorden A., Boeynaems J. M. Physiological concentrations of ADP stimulate the release of prostacyclin from bovine aortic endothelial cells. Prostaglandins. 1984 Apr;27(4):615–626. doi: 10.1016/0090-6980(84)90097-2. [DOI] [PubMed] [Google Scholar]
- Villalón M., Hinds T. R., Verdugo P. Stimulus-response coupling in mammalian ciliated cells. Demonstration of two mechanisms of control for cytosolic [Ca2+]. Biophys J. 1989 Dec;56(6):1255–1258. doi: 10.1016/S0006-3495(89)82772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss T., Gheber L., Shoshan-Barmatz V., Priel Z. Possible mechanism of ciliary stimulation by extracellular ATP: involvement of calcium-dependent potassium channels and exogenous Ca2+. J Membr Biol. 1992 May;127(3):185–193. doi: 10.1007/BF00231506. [DOI] [PubMed] [Google Scholar]
- Wiener E., Dubyak G., Scarpa A. Na+/H+ exchange in Ehrlich ascites tumor cells. Regulation by extracellular ATP and 12-O-tetradecanoylphorbol 13-acetate. J Biol Chem. 1986 Apr 5;261(10):4529–4534. [PubMed] [Google Scholar]
- el-Moatassim C., Dornand J., Mani J. C. Extracellular ATP and cell signalling. Biochim Biophys Acta. 1992 Feb 19;1134(1):31–45. doi: 10.1016/0167-4889(92)90025-7. [DOI] [PubMed] [Google Scholar]