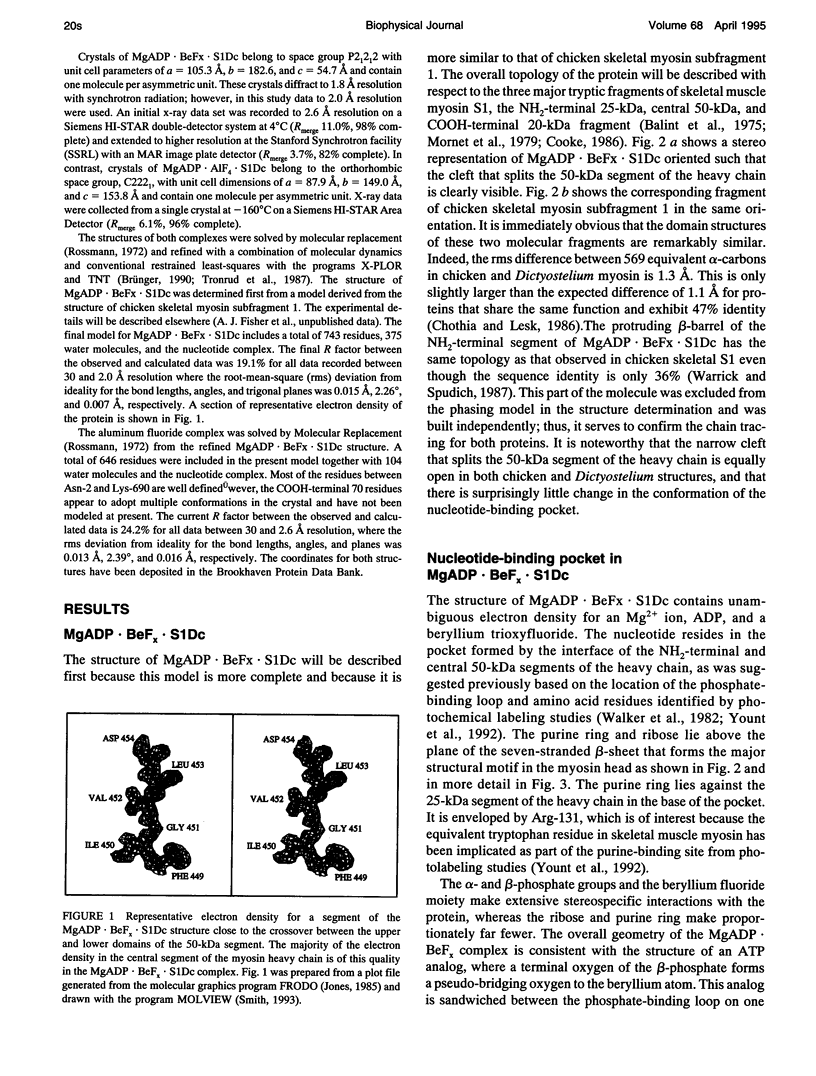
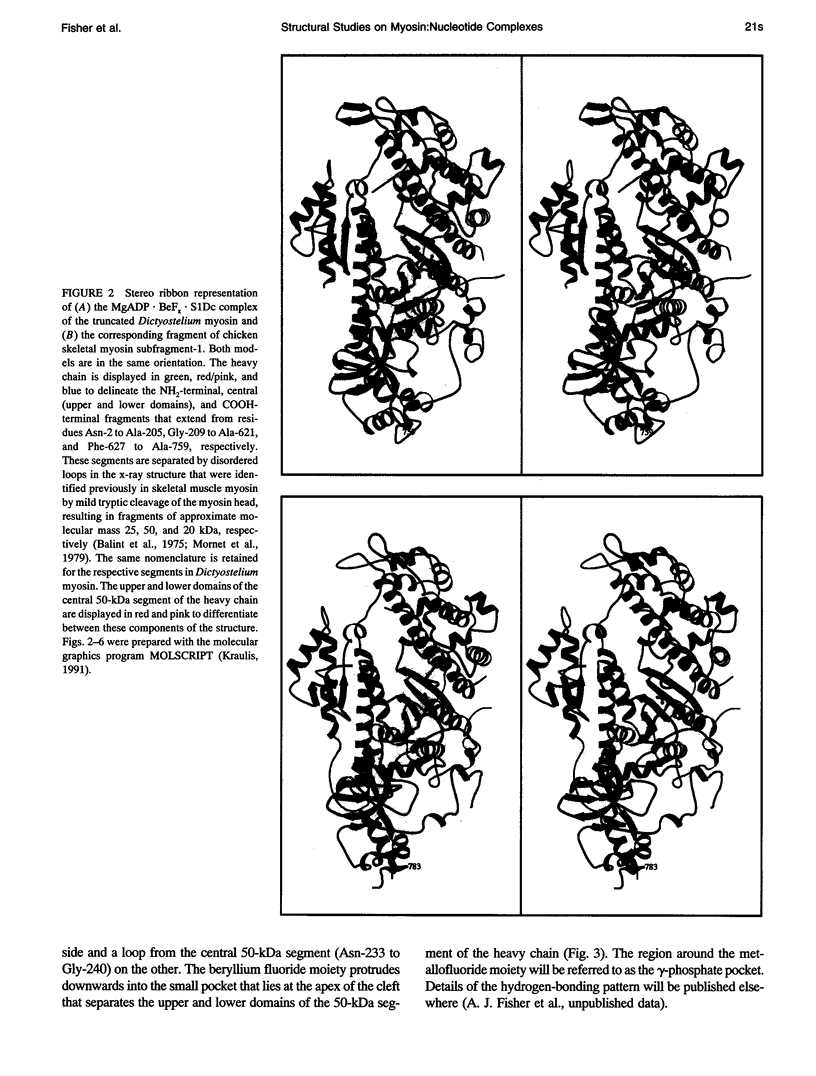
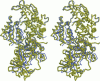Abstract
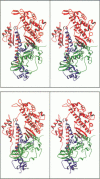
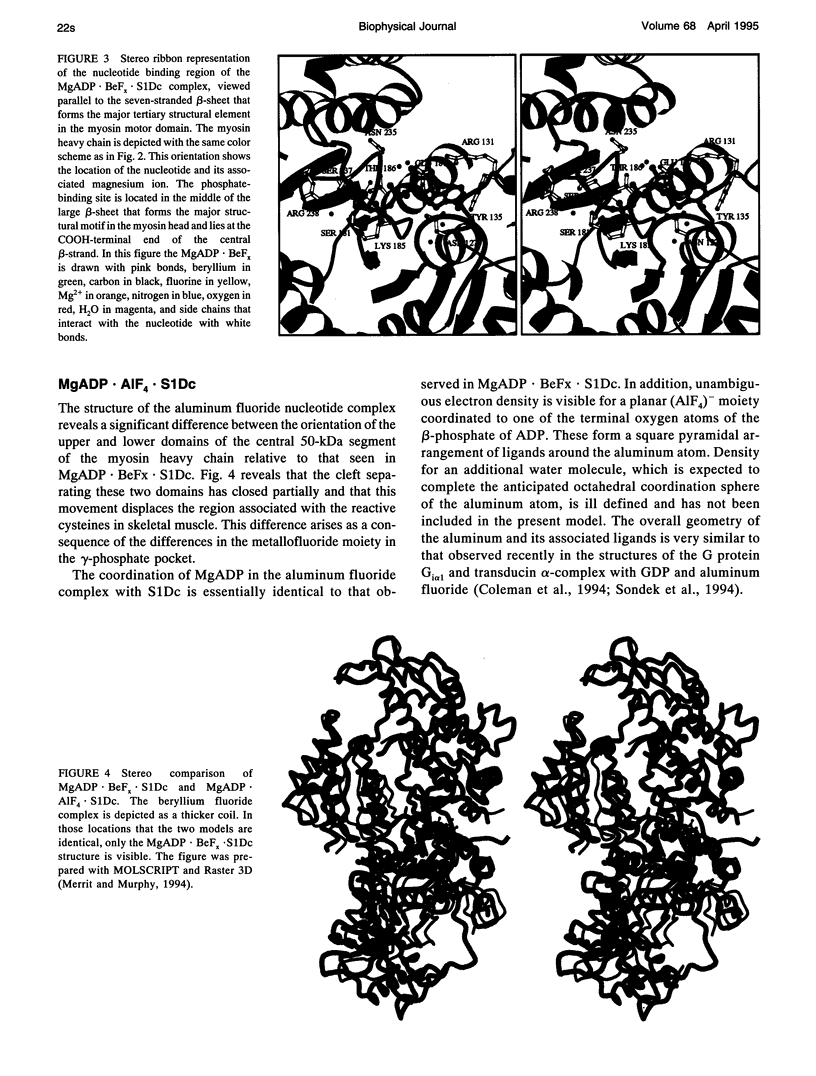
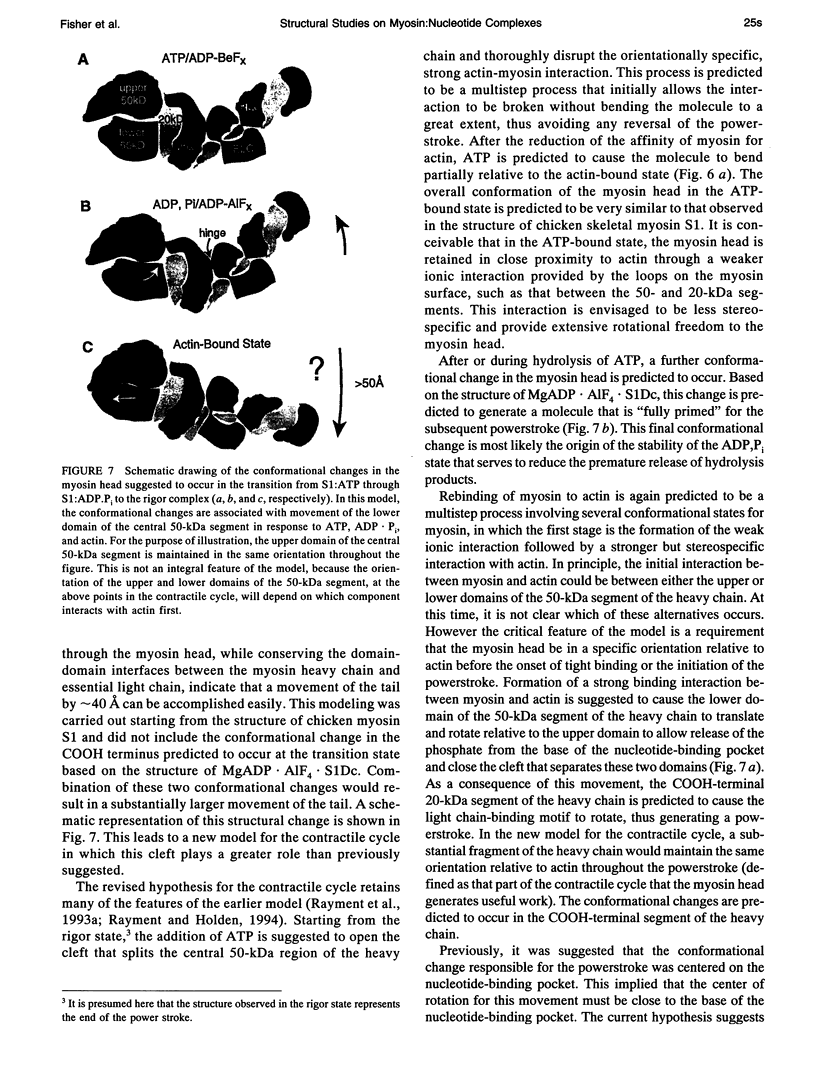
The structures of the MgADP-beryllium fluoride and MgADP-aluminum fluoride complexes of the truncated myosin head from Dictyostelium myosin II are reported. These reveal the location of the nucleotide complex and define the amino acid residues that form the active site. The tertiary structure of the beryllium fluoride complex is essentially identical to that seen previously in the three-dimensional structure of chicken skeletal muscle myosin. By contrast, significant domain movements are observed in the aluminum fluoride complex. These structural findings form the basis of a revised model for the structural basis of the contractile cycle. It is now suggested that the narrow cleft that splits the central 50-kDa segment of the heavy chain provides not only the communication route between the nucleotide-binding pocket and actin but also transmits the conformational change necessary for movement.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bálint M., Sréter F. A., Wolf I., Nagy B., Gergely J. The substructure of heavy meromyosin. The effect of Ca2+ and Mg2+ on the tryptic fragmentation of heavy meromyosin. J Biol Chem. 1975 Aug 10;250(15):6168–6177. [PubMed] [Google Scholar]
- Chothia C., Lesk A. M. The relation between the divergence of sequence and structure in proteins. EMBO J. 1986 Apr;5(4):823–826. doi: 10.1002/j.1460-2075.1986.tb04288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole D. G., Yount R. G. Photolabeling of the 6 and 10 S conformations of gizzard myosin with 3'(2')-O-(4-Benzoyl)benzoyl-ATP. Proline 324 is near the active site. J Biol Chem. 1990 Dec 25;265(36):22537–22546. [PubMed] [Google Scholar]
- Coleman D. E., Berghuis A. M., Lee E., Linder M. E., Gilman A. G., Sprang S. R. Structures of active conformations of Gi alpha 1 and the mechanism of GTP hydrolysis. Science. 1994 Sep 2;265(5177):1405–1412. doi: 10.1126/science.8073283. [DOI] [PubMed] [Google Scholar]
- Cooke R. The mechanism of muscle contraction. CRC Crit Rev Biochem. 1986;21(1):53–118. doi: 10.3109/10409238609113609. [DOI] [PubMed] [Google Scholar]
- Cremo C. R., Neuron J. M., Yount R. G. Interaction of myosin subfragment 1 with fluorescent ribose-modified nucleotides. A comparison of vanadate trapping and SH1-SH2 cross-linking. Biochemistry. 1990 Apr 3;29(13):3309–3319. doi: 10.1021/bi00465a023. [DOI] [PubMed] [Google Scholar]
- Crowder M. S., Cooke R. Orientation of spin-labeled nucleotides bound to myosin in glycerinated muscle fibers. Biophys J. 1987 Feb;51(2):323–333. doi: 10.1016/S0006-3495(87)83338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks-Skiba K., Cooke R. The conformation of the active site of myosin probed using mant-nucleotides. Biophys J. 1995 Apr;68(4 Suppl):142S–149S. [PMC free article] [PubMed] [Google Scholar]
- Franks-Skiba K., Hwang T., Cooke R. Quenching of fluorescent nucleotides bound to myosin: a probe of the active-site conformation. Biochemistry. 1994 Oct 25;33(42):12720–12728. doi: 10.1021/bi00208a025. [DOI] [PubMed] [Google Scholar]
- Gilson M. K., Straatsma T. P., McCammon J. A., Ripoll D. R., Faerman C. H., Axelsen P. H., Silman I., Sussman J. L. Open "back door" in a molecular dynamics simulation of acetylcholinesterase. Science. 1994 Mar 4;263(5151):1276–1278. doi: 10.1126/science.8122110. [DOI] [PubMed] [Google Scholar]
- Henry G. D., Maruta S., Ikebe M., Sykes B. D. Observation of multiple myosin subfragment 1-ADP-fluoroberyllate complexes by 19F NMR spectroscopy. Biochemistry. 1993 Oct 5;32(39):10451–10456. doi: 10.1021/bi00090a022. [DOI] [PubMed] [Google Scholar]
- Itakura S., Yamakawa H., Toyoshima Y. Y., Ishijima A., Kojima T., Harada Y., Yanagida T., Wakabayashi T., Sutoh K. Force-generating domain of myosin motor. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1504–1510. doi: 10.1006/bbrc.1993.2422. [DOI] [PubMed] [Google Scholar]
- Jones T. A. Diffraction methods for biological macromolecules. Interactive computer graphics: FRODO. Methods Enzymol. 1985;115:157–171. doi: 10.1016/0076-6879(85)15014-7. [DOI] [PubMed] [Google Scholar]
- Maruta S., Henry G. D., Sykes B. D., Ikebe M. Formation of the stable myosin-ADP-aluminum fluoride and myosin-ADP-beryllium fluoride complexes and their analysis using 19F NMR. J Biol Chem. 1993 Apr 5;268(10):7093–7100. [PubMed] [Google Scholar]
- Merritt E. A., Murphy M. E. Raster3D Version 2.0. A program for photorealistic molecular graphics. Acta Crystallogr D Biol Crystallogr. 1994 Nov 1;50(Pt 6):869–873. doi: 10.1107/S0907444994006396. [DOI] [PubMed] [Google Scholar]
- Mornet D., Pantel P., Audemard E., Kassab R. The limited tryptic cleavage of chymotryptic S-1: an approach to the characterization of the actin site in myosin heads. Biochem Biophys Res Commun. 1979 Aug 13;89(3):925–932. doi: 10.1016/0006-291x(79)91867-9. [DOI] [PubMed] [Google Scholar]
- Rayment I., Holden H. M. The three-dimensional structure of a molecular motor. Trends Biochem Sci. 1994 Mar;19(3):129–134. doi: 10.1016/0968-0004(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Rypniewski W. R., Holden H. M., Rayment I. Structural consequences of reductive methylation of lysine residues in hen egg white lysozyme: an X-ray analysis at 1.8-A resolution. Biochemistry. 1993 Sep 21;32(37):9851–9858. doi: 10.1021/bi00088a041. [DOI] [PubMed] [Google Scholar]
- Sondek J., Lambright D. G., Noel J. P., Hamm H. E., Sigler P. B. GTPase mechanism of Gproteins from the 1.7-A crystal structure of transducin alpha-GDP-AIF-4. Nature. 1994 Nov 17;372(6503):276–279. doi: 10.1038/372276a0. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrick H. M., Spudich J. A. Myosin structure and function in cell motility. Annu Rev Cell Biol. 1987;3:379–421. doi: 10.1146/annurev.cb.03.110187.002115. [DOI] [PubMed] [Google Scholar]
- White H. D., Rayment I. Kinetic characterization of reductively methylated myosin subfragment 1. Biochemistry. 1993 Sep 21;32(37):9859–9865. doi: 10.1021/bi00088a042. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Cremo C. R., Grammer J. C., Kerwin B. A. Photochemical mapping of the active site of myosin. Philos Trans R Soc Lond B Biol Sci. 1992 Apr 29;336(1276):55–61. doi: 10.1098/rstb.1992.0044. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Lawson D., Rayment I. Is myosin a "back door" enzyme? Biophys J. 1995 Apr;68(4 Suppl):44S–49S. [PMC free article] [PubMed] [Google Scholar]