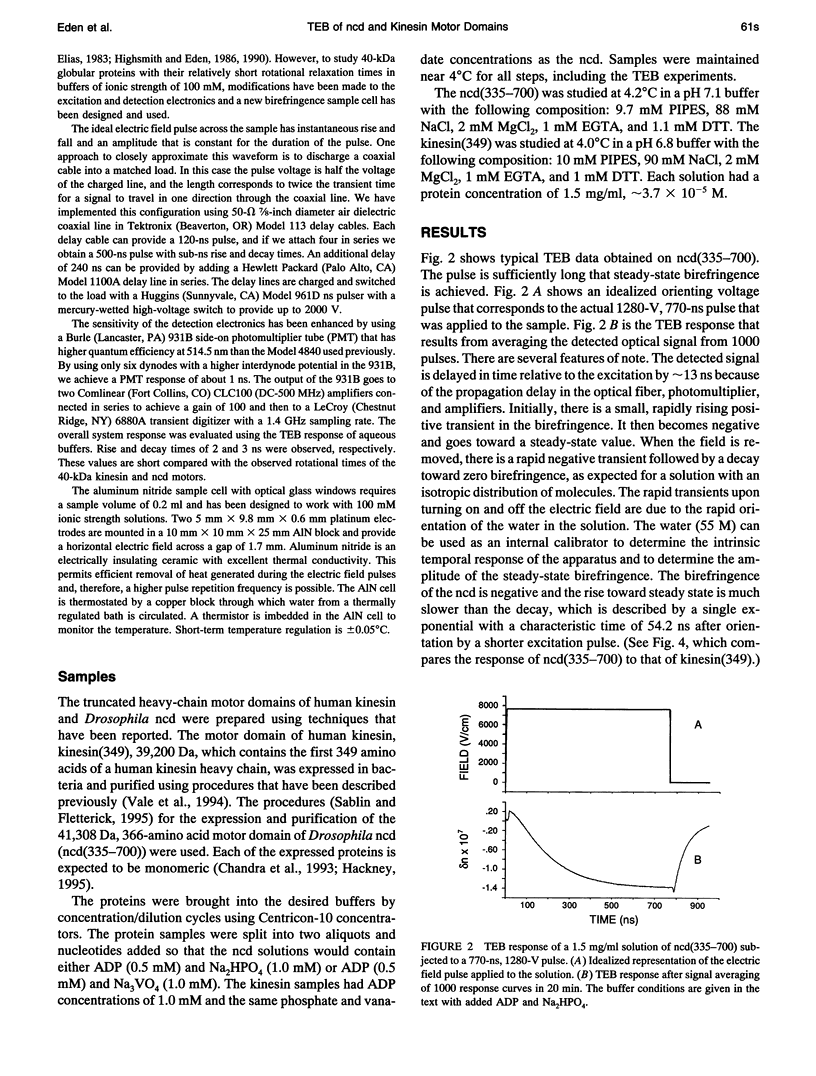
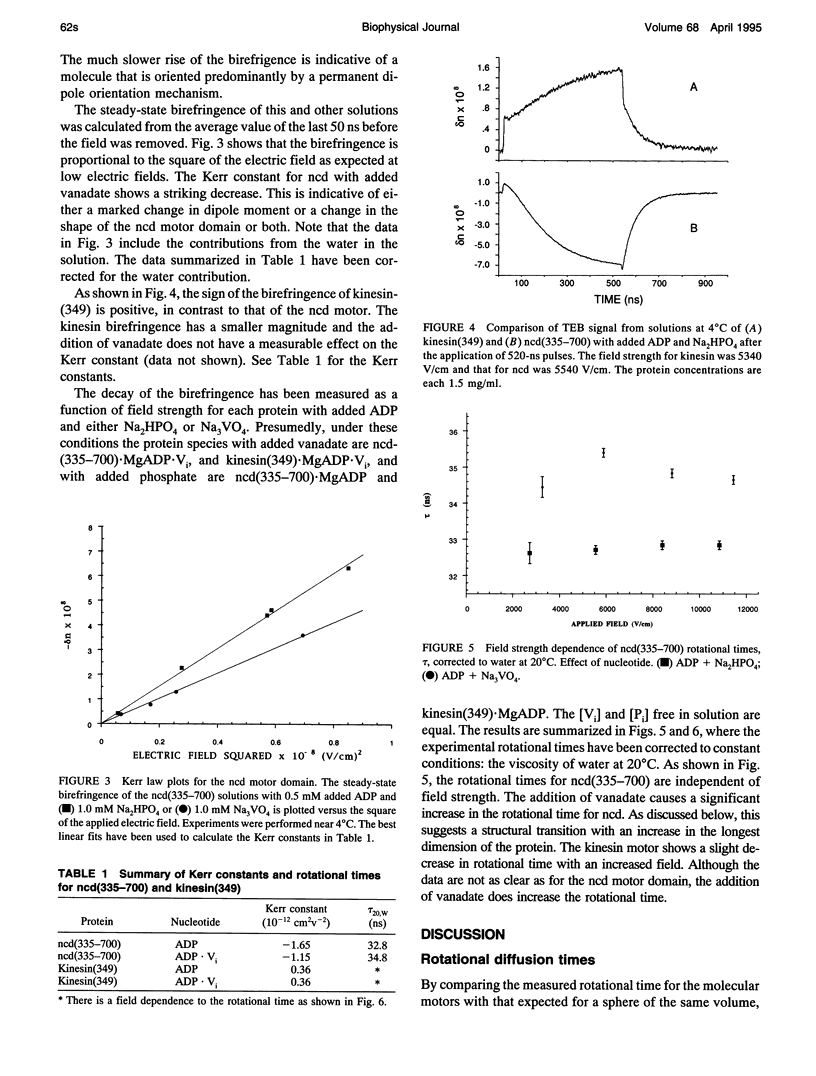
Abstract
The effects of selected ligands on the structure of the truncated heavy-chain chemomechanical motor domains of Drosophila ncd and human kinesin were compared using the technique of transient electric birefringence. The 366-amino acid C-terminal motor domain of Drosophila nonclaret disjunctional, ncd(335-700), and the 349-amino acid N-terminal motor domain of human kinesin, kinesin(349), were studied at 4 degrees C in neutral buffers with ionic strength of 100 mM to form complexes with either MgADP or MgADP.Vi. The rotational diffusion time adjusted to 20 degrees C and water, tau 20,W, for ncd(335-700).MgADP is 32.8 ns, and for ncd(335-700).MgADP.Vi is 34.8 ns, suggesting prolate ellipsoids with dimensions 9.40 x 3.77 nm and 9.73 x 3.70 nm, respectively. The specific Kerr constant, Ksp, of ncd is -1.65 x 10(-12) cm2V-2 for the MgADP complex and -1.15 x 10(-12) cm2V-2 for the MgADP.Vi complex. The large negative Ksp for a prolate protein suggests an unusual charge distribution with two long surfaces with opposite charge. The tau 20,W for kinesin(349).MgADP is longer than the corresponding ncd motor and shows a decrease with increased electric field. The kinesin(349).MgADP.Vi complex has a longer tau 20,W. The Ksp for kinesin(349) is 0.36 x 10(-12) cm2V-2 for each complex.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguirre R., Lin S. H., Gonsoulin F., Wang C. K., Cheung H. C. Characterization of the ethenoadenosine diphosphate binding site of myosin subfragment 1. Energetics of the equilibrium between two states of nucleotide.S1 and vanadate-induced global conformation changes detected by energy transfer. Biochemistry. 1989 Jan 24;28(2):799–807. doi: 10.1021/bi00428a058. [DOI] [PubMed] [Google Scholar]
- Chandra R., Salmon E. D., Erickson H. P., Lockhart A., Endow S. A. Structural and functional domains of the Drosophila ncd microtubule motor protein. J Biol Chem. 1993 Apr 25;268(12):9005–9013. [PubMed] [Google Scholar]
- Goodno C. C. Inhibition of myosin ATPase by vanadate ion. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2620–2624. doi: 10.1073/pnas.76.6.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney D. D. Implications of diffusion-controlled limit for processivity of dimeric kinesin head domains. Biophys J. 1995 Apr;68(4 Suppl):267S–270S. [PMC free article] [PubMed] [Google Scholar]
- Highsmith S., Eden D. Ligand-induced myosin subfragment 1 global conformational change. Biochemistry. 1990 May 1;29(17):4087–4093. doi: 10.1021/bi00469a010. [DOI] [PubMed] [Google Scholar]
- Highsmith S., Eden D. Myosin subfragment 1 has tertiary structural domains. Biochemistry. 1986 Apr 22;25(8):2237–2242. doi: 10.1021/bi00356a058. [DOI] [PubMed] [Google Scholar]
- Highsmith S., Eden D. Myosin-ATP chemomechanics. Biochemistry. 1993 Mar 16;32(10):2455–2458. doi: 10.1021/bi00061a001. [DOI] [PubMed] [Google Scholar]
- McDonald H. B., Goldstein L. S. Identification and characterization of a gene encoding a kinesin-like protein in Drosophila. Cell. 1990 Jun 15;61(6):991–1000. doi: 10.1016/0092-8674(90)90064-l. [DOI] [PubMed] [Google Scholar]
- Oldenbourg R., Ruiz T. Birefringence of macromolecules. Wiener's theory revisited, with applications to DNA and tobacco mosaic virus. Biophys J. 1989 Jul;56(1):195–205. doi: 10.1016/S0006-3495(89)82664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Coppin C. M., Malik F., Kull F. J., Milligan R. A. Tubulin GTP hydrolysis influences the structure, mechanical properties, and kinesin-driven transport of microtubules. J Biol Chem. 1994 Sep 23;269(38):23769–23775. [PubMed] [Google Scholar]
- Wakabayashi K., Tokunaga M., Kohno I., Sugimoto Y., Hamanaka T., Takezawa Y., Wakabayashi T., Amemiya Y. Small-angle synchrotron x-ray scattering reveals distinct shape changes of the myosin head during hydrolysis of ATP. Science. 1992 Oct 16;258(5081):443–447. doi: 10.1126/science.1411537. [DOI] [PubMed] [Google Scholar]


