Abstract
The ability of human neutrophils to aid in defense against pulmonary infection with Mycobacterium tuberculosis is controversial. In this study, we have shown that neutrophils respond to and phagocytose M. tuberculosis in human lesions. Neutrophils from healthy individuals were able to kill significant fractions of an inoculum of M. tuberculosis within 1 h of phagocytosis, and this ability was enhanced by tumor necrosis factor alpha but not by gamma interferon. The mycobactericidal mechanism was nonoxidative, as inhibitors of reactive oxygen or reactive nitrogen intermediates did not interfere with killing. However, the mycobactericidal mechanism was associated with increased exposure of intracellular M. tuberculosis to neutrophil defensins. In vitro, human neutrophil peptides 1 to 3 were not able to kill the bacilli even at much higher levels. These studies support the concept that human neutrophils are directly involved in defense against infection with M. tuberculosis.
The observation that humans are not uniformly susceptible to infection by Mycobacterium tuberculosis may imply the existence of resistance mechanisms capable of sterilizing inhaled inoculae which operate independently of acquired immunity. Cells and tissues of the airways and alveoli are the first points of contact with M. tuberculosis following inhalation (20). Interaction of tubercle bacilli with airway or alveolar cells results in a rapid influx of neutrophils (20). Experiments performed by numerous laboratories over the past 30 years indicate that human monocytes/macrophages (including alveolar macrophages) fail to kill M. tuberculosis when infected in culture, despite incubation with cytokines or activated T cells (4, 34). Although studies with rodent models indicate that alveolar macrophages are important for containment of experimental aerogenic infections, it may be necessary to look to other effector cells to understand innate immune mechanisms which protect against human infections.
There have been two reports that human neutrophils can kill virulent M. tuberculosis in vitro (5, 18). However, more-recent examinations of this issue have been unable to confirm these results (2, 10). Majeed et al. were able to demonstrate that cultured human neutrophils were able to kill an attenuated strain of M. tuberculosis, H37Ra, in a calcium-dependent manner (21). However, as H37Ra is not known to be virulent for humans, it remains important to resolve the issue of neutrophil capacity to kill virulent M. tuberculosis. Pedrosa et al. have suggested that murine neutrophils are protective against experimental infections of mice, although they found no evidence that the neutrophils phagocytosed the tubercle bacilli (27).
The goal of these studies was to understand whether the neutrophil response characteristic of the earliest responses to tuberculosis (TB) infection could be effective in reducing or eliminating the inoculum. To this end, we established a model of in vitro infection of human neutrophils and characterized some of the effector mechanisms which come into play during the response of neutrophils both in vitro and during pulmonary infection of humans. We show that neutrophils in human pulmonary lesions contain intracellular M. tuberculosis, confirming a phagocytic role for neutrophils in human TB. We also show that human neutrophils can kill virulent intracellular M. tuberculosis in vitro, but with variability between individuals. Mycobactericidal activity could be stimulated by exposure of infected neutrophils to tumor necrosis factor alpha (TNF-α), but not gamma interferon (IFN-γ). We also examine the role which neutrophil defensins play in the mycobactericidal mechanism.
MATERIALS AND METHODS
M. tuberculosis cultures.
M. tuberculosis Erdman was used as a target strain. Cultures were grown in 7H9 broth for 7 to 10 days and then diluted to the optical density of McFarland standard no. 1. This density of cells is approximately 108/ml. The bacterial suspension was then preserved in 1-ml aliquots at −70°C until the time of infection.
Human neutrophils.
Human neutrophils were isolated from healthy individuals according to a protocol reviewed and approved by the Institutional Review Board by using a Percoll (Pharmacia) density gradient (29). In brief, 4.4 ml of 3.8% (wt/vol) sodium citrate (Fisher Scientific, Pittsburgh, Pa.) was added to 40 ml of heparinized blood. The blood was then centrifuged at 400 × g for 20 min, after which the plasma layer was removed, 5 ml of 6% (wt/vol) dextran (Pharmacia) was added to the pelleted whole blood, and the total volume was brought up to 50 ml with saline and mixed gently. The cell suspension was then left for 30 min at room temperature to allow the red blood cells to settle. The upper white blood cell layer was removed and centrifuged at 400 × g for 10 min, the supernatant was discarded, and the pellet was resuspended in 2 ml of autologous plasma. The cell suspension was then underlaid with a 42% (wt/vol) Percoll gradient followed by a 51% (wt/vol) Percoll gradient and centrifuged at 350 × g for 10 min. The resulting neutrophil-rich layer was carefully removed. Neutrophils were then resuspended in phosphate-buffered saline (PBS) and centrifuged at 350 × g for 10 min, and the supernatant was discarded. The resulting neutrophil pellet was then resuspended in Hanks balanced saline solution.
Defensins.
Synthetic human neutrophil peptide 1 (HNP-1) was obtained from the Peptide Institute (Osaka, Japan), Alpha Diagnostics (San Antonio, Tex.), and Bachem Bioscience (Philadelpha, Pa.). HNP-1 to -3 were also isolated as a mixture from sputum produced by patients with cystic fibrosis as previously described (35). Antimicrobial activity of defensins was examined by incubation with Escherichia coli ML-35. Briefly, E. coli cells were grown to mid-log phase in Luria-Bertani (LB) broth. They were then washed in low-salt buffer consisting of 10 mM NaPO4, pH 7.4, containing 0.01× tryptic soy broth (Difco) (TSB-phosphate buffer). The bacteria were then diluted to 3.6 × 107/ml of TSB-phosphate buffer, and 100 μl was aliquoted into sterile 5-ml culture tubes. Defensin in 0.01% acetic acid or 0.01% acetic acid alone was added to the tubes, followed by incubation at 37°C for 24 h. The cultures were then diluted 1:100, 1:1,000, and 1:10,000, and 100 μl was spread on LB agar plates. The plates were incubated for a further 24 h at 37°C to allow colonies to develop. Colonies were counted visually, and minimal bactericidal activity was calculated as the concentration of defensin required to reduce the number of colonies by 90% relative to the number present in the initial inoculum.
Infection of neutrophils.
After resuspension in Hanks balanced saline solution the neutrophils were counted and diluted in macrophage serum-free medium (mSFM; Gibco, Gaithersburg, Md.) to 106/ml. Next, a bacterial suspension was diluted from the frozen aliquots in mSFM to 107/ml and placed with the neutrophil suspension in 35-mm-diameter dishes (Fisher). After 1 h, the adherent cells were washed twice with a 1:1 mixture of RPMI 1640 and saline, and then fresh mSFM was added depending on experimental conditions. For cytokine treatment, after the washing of the neutrophils, 100 U of TNF-α or IFN-γ (R&D Systems, Minneapolis, Minn.)/ml was added for 1 h. After that, the cells were washed twice and fresh medium was added depending on experimental conditions.
Enumeration of viable M. tuberculosis by colony counting.
Numbers of CFU were determined at various intervals after infection. Infected neutrophil monolayers were lysed at the end of the growth period with 1 ml of 0.25% sodium dodecyl sulfate (SDS) for 10 min. Wells were then scraped, and the lysate was transferred into plastic culture tubes. Lysate was diluted with 7H9 medium to neutralize the SDS and spread onto Middlebrook 7H11 plates for colonies to be counted after cultivation for 21 days at 37°C.
Terminal deoxynucleotidyltransferase-mediated dUTP-fluorescein nick end labeling (TUNEL) assay for apoptosis.
Neutrophil cultures were fixed with 4% paraformaldehyde for 1 h. The fixed cultures were then rinsed with 100 mM glycine in order to neutralize residual reactive aldehydes. Nicked DNA in the cells was modified with fluorescein-dUTP, and terminal deoxynucleotidyltransferase was modified according to the manufacturer's instructions (apoptosis detection system, fluorescein; Promega Inc., Madison, Wis.). The apoptotic cells were counted by using epifluorescence illumination. Total cells were counted under bright-field optics, and results were expressed as percentages of fluorescein-positive (apoptotic) cells. Results were determined from counts of 20 random 200× fields per culture.
Immunocytochemistry.
Samples of human neutrophils were cultured as described above with the exception that eight-well chamber slides (Nunc, Inc., Naperville, Ill.) were used in place of tissue culture dishes and M. tuberculosis was surface labeled with Oregon green succinimidyl ester (Molecular Probes, Eugene, Oreg.). Slides were fixed in formalin at 4°C overnight, followed by neutralization with 1 M glycine buffer, pH 7.0. Tissue from pneumonectomy or lobectomy for progressive TB was fixed in formalin for up to 6 weeks, followed by embedding in paraffin, sectioning to a thickness of 5 μm, and mounting on glass slides. The slides were deparaffinized with three changes of xylene, followed by rehydration with 100, 95, and 75% ethanol and rinsing with PBS.
Both tissue sections and fixed neutrophils were then incubated for 30 min with blocking/permeabilizing solution consisting of PBS containing 0.5% (wt/vol) bovine serum albumin (BSA), 0.1% (vol/vol) Tween 20, and 0.1% (wt/vol) saponin (Sigma). An antibody to HNP-1 to -3 was prepared from hybridoma D1-1 (gift from Tom Ganz, University of California, Los Angeles) by standard methods and diluted to 0.5 μg/ml in staining medium consisting of 3% BSA and 0.1% (vol/vol) Tween 20 in 1× PBS. Samples were incubated with the primary antibody or control immunoglobulin G (IgG) overnight at 4°C in a humidified tank.
Image analysis.
Images of the samples were viewed and captured with a Leica (Bannockburn, Ill.) DMRXA microscope, coupled with a digital camera (Sensicam; PCO Computer Optics, Kelheim, Germany). The microscope and camera were controlled by a Macintosh computer running Slidebook software (Intelligent Imaging Innovations, Denver, Colo.). Images were captured with a 40× (for Fig. 1A) or a 100× (all other micrographs) objective as a series of images in the z dimension (a stack). The image stacks were then subjected to constrained iterative deconvolution based on a point spread function captured and calculated on the same system by using the same configuration of optical components, including slides, coverslips, and mounting medium (31).
FIG. 1.
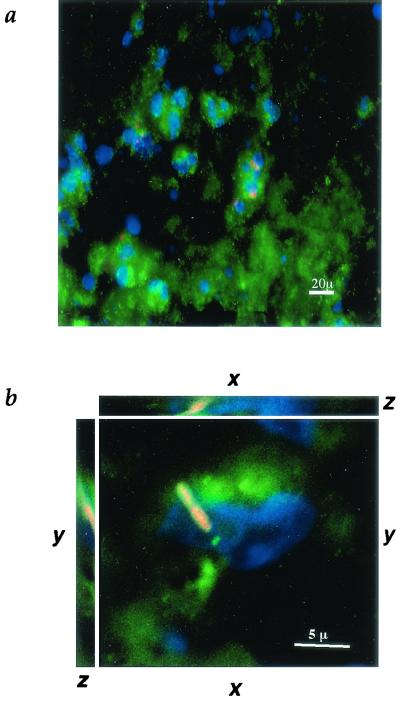
M. tuberculosis in human lung tissue after phagocytosis by neutrophils. (a) Fluorescence micrograph of human lung tissue from a patient with M. tuberculosis (magnification, ×400). Cell nuclei, stained with DAPI, are blue, including characteristic multilobed nuclei of neutrophils. Cell membranes, stained with wheat germ agglutinin-Oregon green, are green. Acid-fast M. tuberculosis bacilli, stained with auramine-rhodamine, are red. (b) Digitally deconvolved three-axis image of a neutrophil containing M. tuberculosis in human lung tissue (magnification, ×1,000). The nucleus is stained with DAPI (blue), the membranes are stained with mouse anti-human CD15 (green), and M. tuberculosis is stained with auramine-rhodamine (red). Out-of-plane fluorescence has been reassigned back to the plane of origin.
After deconvolution, the volumes representing intracellular mycobacteria were isolated by using a three-dimensional mask covering the mycobacterium-associated fluorophore (either Oregon green or auramine-rhodamine). The intensity of the anti-HNP-associated fluorophore (Cy-5) was then measured within the volumes occupied by the mycobacteria. Each measurement yielding the mean fluorescence of a mycobacterium was the average of 2,000 to 4,000 voxels covering the volume of the mycobacterium. Compensation was then performed to remove fluorescence due to the Oregon green or auramine-rhodamine used to identify the mycobacteria. This resulted in reduction of the raw Cy-5 fluorescence measurements by approximately 5%. Statistics were calculated based on 50 masks per sample, in which each mask was defined the volume of at least one mycobacterium. Nonspecific staining was examined by incubation of the mycobacteria with mouse IgG in place of the anti-HNP antibody, followed by incubation with the anti-mouse IgG-Cy-5 conjugate. Levels of fluorescence for unstained mycobacteria, mycobacteria incubated with mouse IgG and anti-mouse IgG-Cy-5, and mycobacteria incubated with specific anti-HNP-anti-mouse IgG-Cy-5 in the absence of HNP were comparable.
Statistics.
The Cy-5 anti-HNP intensity measurements were compared by using a t test for unpaired samples. A two-tailed P value of less than 0.05 was considered significant.
RESULTS
Neutrophils phagocytose M. tuberculosis in human tuberculous lesions.
Figure 1A and B show different magnifications of lung tissue from a patient diagnosed with progressive TB. Auramine-rhodamine staining of the tissue (red) reveals the presence of acid-fast bacilli within the lesion. DAPI (4′,6′-diamidino-2-phenylindole) staining of nuclei shows the characteristic multilobed nuclei characteristic of polymorphonuclear leukocytes (PMN; blue) containing acid-fast bacilli (Fig. 1A). Identification of the cells containing M. tuberculosis as neutrophils is based on staining with mouse anti-human CD15 (Fig. 1B, green). The location of the bacilli (red) within the PMN was confirmed via circumscription by the PMN cytoplasm and cell membranes (Fig. 1B, green). Fluorescence originating above or below the plane of this image was reassigned to the plane of origin by constrained-iterative deconvolution (31).
The lesions also contained numerous macrophages based on staining with mouse anti-human CD68. To determine the percentage of the visible M. tuberculosis cells which had been phagocytosed and by which cell type, archival specimens from three patients with progressive TB were examined after staining for acid-fast bacilli with auramine-rhodamine and counterstaining with either anti-CD15 to identify neutrophils or with anti-CD68 to identify macrophages. The results are shown in Table 1. Patient A had 42% of the tubercle bacilli associated with caseum, 27% associated with macrophages, 29% associated with neutrophils, and 2% associated with unidentified cells. Patient B had 0% associated with caseum, 4% associated with macrophages, and 96% associated with neutrophils. Patient C had 97% associated with caseum, 2% associated with macrophages, 0% associated with neutrophils, and 1% associated with unidentified cells. The heterogeneity of distribution among the patients may reflect differences in cellular responses among patients, differences in the area of the diseased tissue sampled, or both. Therefore, the finding of bacilli within the cytoplasm of neutrophils in the lesions supports a phagocytic role for neutrophils in human TB.
TABLE 1.
Association of M. tuberculosis in lung tissue of patients with progressive TB
| Cell type | % of tubercle bacilli in lungs of patient
|
||
|---|---|---|---|
| A | B | C | |
| Caseum | 42 | 0 | 97 |
| Macrophage | 27 | 4 | 2 |
| Neutrophil | 29 | 96 | 0 |
| Unidentified | 2 | 0 | 1 |
Neutrophils phagocytose M. tuberculosis in culture.
To characterize the ability of cultured neutrophils to phagocytose M. tuberculosis, we performed detailed analysis of the fate of M. tuberculosis within the first hour after exposure to neutrophils. In one example experiment, neutrophils were incubated with 1 ml of 7H9 containing 2.93 × 106 CFU of M. tuberculosis for 1 h. At the end of the phagocytosis period, the supernatant and rinses of the monolayers were removed and found to contain 2.21 × 106 CFU/ml, while the neutrophil monolayer contained 3.94 × 105 CFU. Therefore, the majority of the inoculum could be accounted for after incubation with the neutrophils. The infected cells were examined to determine the percentage of cells which phagocytosed M. tuberculosis and how many organisms were associated with each cell. In control cultures, approximately 28% ± 4% of the neutrophils contained one or more tubercle bacilli. In cultures treated with TNF-α, approximately 21% ± 3.2% of the cells contained tubercle bacilli. However, the difference was not significant based on a t test. Additionally, 6.7% of the cells contained from 1 to 5 bacilli, 9.4% contained 5 to 10 bacilli per cell, and 11.9% contained more than 10 bacilli per cell. The number of M. tuberculosis cells determined by light microscopy agrees roughly with the number of CFU recovered from the neutrophils after 1 h.
Killing of M. tuberculosis by human neutrophils in vitro is stimulated by TNF-α.
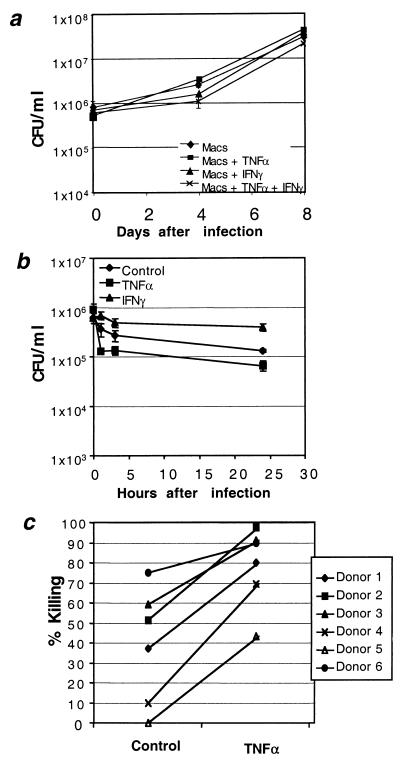
Phagocytosis of M. tuberculosis by neutrophils in human lesions led to the hypothesis that neutrophils may be capable of killing M. tuberculosis. We tested this hypothesis by incubating human neutrophils with virulent M. tuberculosis Erdman from frozen aliquots prepared and stored from the same culture. In parallel, the ability of human monocyte-derived macrophages to kill M. tuberculosis in culture was assessed. After allowing the neutrophils or macrophages to adhere to plastic and phagocytose the mycobacteria for 1 h, extracellular bacilli were removed by rinsing the monolayers. Some of the monolayers were then stimulated with TNF-α or IFN-γ to assess the effect of cytokine stimulation. The supernatants were harvested, and monolayers were lysed and plated at increasing intervals for assessment of viable bacilli. Macrophages were unable to kill M. tuberculosis in culture despite stimulation with cytokines, as shown in Fig. 2a. These data are in agreement with previous studies conducted by ourselves and others (11a, 18a). Neutrophils exhibited potent killing activity within 24 h after tubercle bacilli were added to the cultures (Fig. 2B). Figure 2B also shows that unstimulated neutrophils killed an average of 40.6% of the inoculum within 1 h. Treatment of the neutrophils with TNF-α for 1 h following infection resulted in killing 85.9% of the inoculum, while IFN-γ appeared to inhibit killing. The observed killing was not due to detachment of cells from the plastic or release of bacilli into the medium, as CFU counts in supernatants were included in the total. Of six donors' neutrophils assayed in this way, those of four were able to kill cell-associated M. tuberculosis with no stimulation, and all six donors' neutrophils were able to kill between 50 and 95% of the inoculum when treated for 1 h with TNF-α (Fig. 2C). Neutrophils from each donor killed significantly more tubercle bacilli after stimulation for 1 h with TNF-α (P < 0.01, t test for paired samples). The number of viable mycobacteria continued to decline somewhat for the remainder of the first 24 h.
FIG. 2.
Effect of human neutrophils on the viability of intracellular M. tuberculosis. (a) Time course of M. tuberculosis viability after infection of macrophages treated with medium only (♦) or 100 U of recombinant human TNF-α (rhTNF-α) (▪)/ml or 100 U of rhIFN-γ (▴)/ml or both (×). Results presented are from a single anonymous donor who was representative of over 30 examined. Data points represent three separate infections. CFU for each data point were calculated as the average count from four plates at two different dilutions. (b) Time course of M. tuberculosis viability after infection of neutrophils treated with medium only (♦), 100 U of rhTNF-α (▪)/ml, or 100 U of rhIFN-γ (▴)/ml. Results were obtained from a single anonymous donor. Data points represent three separate infections. CFU for each data point were calculated as the average count from four plates at two different dilutions. (c) Donor-to-donor neutrophil variation in spontaneous and TNF-α-stimulated killing of intracellular M. tuberculosis 24 h after infection. Percent killing relative to time zero (immediately after washout of uningested organisms) for each donor with and without 100 U of rhTNF-α/ml was calculated from three separate infections performed in parallel. All donors exhibited a significant increase in killing in response to TNF-α (P < 0.01; Student's t test).
Inhibitors or RNI or ROI production did not inhibit killing.
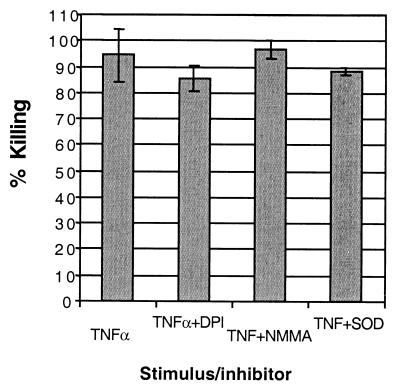
Growth inhibition of M. tuberculosis by human neutrophils has been reported to be independent of reactive oxygen intermediate (ROI) production (18). Therefore, we sought to confirm that this was the case in our system as well, and also to determine if reactive nitrogen intermediates (RNI) were involved. Figure 3 shows that killing was not inhibited by an inhibitor of inducible nitric oxide synthase, l-NG-monomethyl arginine (l-NMMA) (7). Neither infected neutrophils nor infected neutrophils treated with l-NMMA produced Griess-detectable NO2−. Killing was slightly inhibited by an inhibitor of NADPH oxidase, diphenylene iodonium (DPI) (8), and by the superoxide dismustase (SOD) mimic manganese(III) meso-tetrakis(N-methylpyridinium-2-yl)porphyrin (3). Both of these ROI inhibitors reduced H2O2 to undetectable levels (data not shown). However, the level of inhibition of mycobactericidal activity was not significant (P > 0.05). These results indicate that the main killing mechanism which neutrophils use for M. tuberculosis is nonoxidative, confirming the work of Jones et al. (18).
FIG. 3.
Lack of effect of ROI or RNI inhibitors on killing by neutrophils. M. tuberculosis-infected neutrophils were preincubated with medium, DPI, l-NMMA, or a SOD mimic prior to stimulation with TNF-α. The data represent triplicate wells and are percentages of reduction of CFU on four plates/sample relative to CFU at time zero (immediately after washout of uningested bacilli). Differences between treatments were not significant (P > 0.05; Student's t test).
Killing does not appear related to apoptosis of the cells.
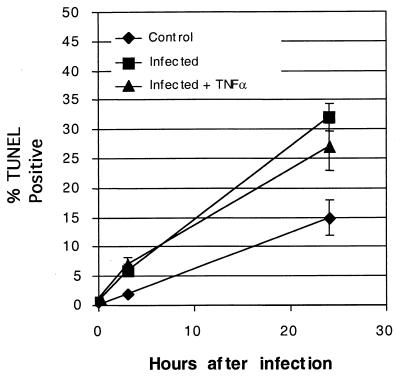
Fratazzi et al. demonstrated that M. tuberculosis bacilli within macrophages undergoing apoptosis become damaged (12). Since TNF-α stimulates apoptosis by human neutrophils (30), we sought to determine if apoptosis could account for the killing of the cell-associated M. tuberculosis. We measured what fraction of the cells underwent apoptosis during the period of the assay both with and without stimulation by TNF-α by labeling ends of fragmented DNA (TUNEL). Figure 4 shows that neutrophils exposed to M. tuberculosis underwent increased apoptosis relative to neutrophils alone, whether or not they were treated with TNF-α. The cultures which received M. tuberculosis had approximately 25 to 30% TUNEL-positive cells after 24 h. Some of the infected cells in the M. tuberculosis-treated cultures were TUNEL positive, but the frequency was not increased relative to that for uninfected cells. Therefore, it is unlikely that the killing of M. tuberculosis by human neutrophils was strictly an effect of apoptosis of the cells.
FIG. 4.
Enhanced killing is not associated with increased apoptosis. Shown are percentages of TUNEL-positive cells in neutrophil cultures exposed to medium alone (♦), M. tuberculosis (▪), or M. tuberculosis and 100 U of recombinant human TNF-α/ml (▴). Data shown are for neutrophils from a single donor; data points are the average counts and standard deviations of triplicate wells. Data are representative of other donors, which vary in the amount of apoptosis observed in 24 h but which show similar rates for M. tuberculosis-infected cells and infected cells treated with TNF-α.
Intracellular M. tuberculosis cells are exposed to HNP during killing.
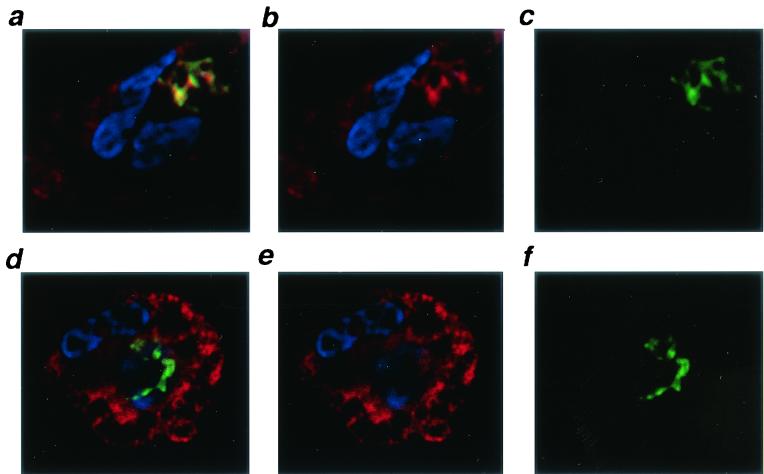
In addition to oxidative mechanisms, neutrophils contain a variety of substances which are directly antimicrobial, including cathelicidins (37), cathepsin G (26), and α-defensins (14). One of the α-defensins of neutrophils, HNP-1, has been shown to prevent growth of M. tuberculosis at 2 to 5 μg/ml (24). Defensins are contained in the primary granules of human neutrophils, which normally fuse with phagosomes during the killing of bacteria (14). N'diaye et al. have reported, however, that M. tuberculosis inhibits fusion of the primary granules with phagosomes and thus may avoid exposure to the antimicrobial substances contained within (25). We sought to determine whether TNF-α stimulated increased exposure of phagocytosed M. tuberculosis to defensins during killing. Figure 5b is a confocal image showing that M. tuberculosis-infected human neutrophils in culture contain substantial amounts of HNP (red). M. tuberculosis phagocytosed by neutrophils can be stained with an anti-HNP antibody, indicating that some of the defensin-containing granules were released into the phagosome containing the bacillus. Figure 5a to c shows a representative neutrophil after phagocytosis of M. tuberculosis and stimulation with TNF-α. The amount of HNP staining (red) coincident with the intracellular M. tuberculosis (green) is greater than that without TNF-α stimulation (Fig. 5d to f). The increased colocalization results in yellow staining of the bacilli.
FIG. 5.
Increased colocalization of HNP with intracellular M. tuberculosis after TNF-α treatment of neutrophils. (a to c) Digital confocal images of a neutrophil infected in vitro with M. tuberculosis and treated for 1 h with TNF-α (magnification, ×1,600). The nucleus is stained with DAPI (blue), and HNPs are stained with mouse anti-human HNP (primary) and goat anti-mouse Cy-5 (secondary) antibodies (b; red). M. tuberculosis was surface labeled with carboxyfluorescein succinimidyl ester prior to addition to the neutrophil culture (c; green). Note the yellow shift associated with the bacilli in panel a, the merged image. (d to f) Digital confocal images of neutrophils infected with M. tuberculosis. Colors represent stains as in panels a to c; however the intracellular bacilli remain green due to reduced colocalization with HNP.
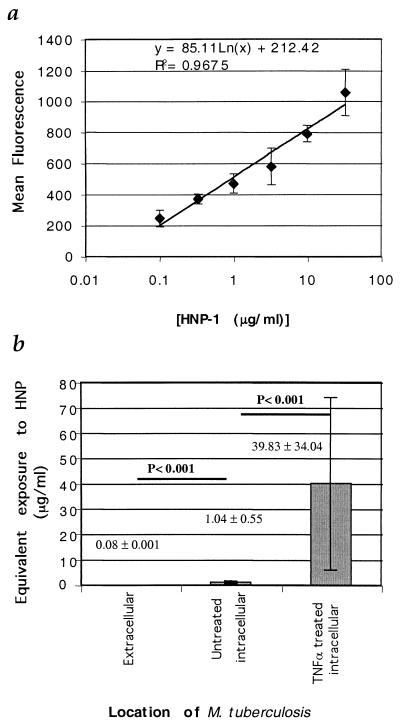
We next sought to determine whether the intracellular bacilli were exposed to a biologically relevant amount of defensin. Lehrer et.al. have investigated the relationship between the binding of defensins to candida and candidacidal activity (19). Such a detailed analysis of the relationship between the binding of HNP to M. tuberculosis and antimycobacterial activity has not yet been performed and is beyond the scope of this report. However, it is possible to detect HNP bound to M. tuberculosis by antibody staining, as seen in Fig. 5b. If the amount of HNP bound to M. tuberculosis varies with the amount of HNP in the medium, it should be possible to construct a standard curve relating the exposure to HNP to the amount of specific antibody staining. The MIC sufficient to prevent growth of M. tuberculosis has previously been reported to be 2.0 to 2.5 μg/ml (32, 33). Therefore, we incubated M. tuberculosis (Erdman strain) with HNP-1 at concentrations from 0.1 to 33 μg/ml for 24 h at 37°C, after which the tubercle bacilli were quickly washed and fixed in formalin. The bacilli were then dried onto coverslips and stained for the presence of HNP-1 to -3. Figure 6a shows that increasing exposure of M. tuberculosis to HNP-1 results in increasing staining for HNP-1, which can be measured by fluorescence microscopy and digital imaging. The relationship between exposure and fluorescence (y) staining was log-linear and fit a line with the equation y = 85.11 ln (x) + 212.42, with a correlation coefficient of 0.97. This equation was then rearranged to solve for x, or [HNP], yielding [HNP] = e(y − 212.42)/85.11, which was used to estimate defensin exposure of M. tuberculosis cells infecting neutrophils based on their fluorescence intensity. Figure 6b shows the results of estimating the amount of defensin exposure based on staining under several conditions. HNP exposure of extracellular M. tuberculosis is similar to background, while the HNP associated with mycobacteria within unstimulated neutrophils is significantly greater (P < 0.001; Student's t test). Stimulation of the neutrophils with TNF-α results in a further increase in HNP exposure of the bacilli, approximately 50-fold relative to that for the unstimulated cells (P < 0.001; Student's t test). These results demonstrate that the killing of intracellular M. tuberculosis by neutrophils is associated with mobilization of defensins onto the bacilli. To have achieved equivalent fluorescence intensity, the amount of defensin bound to the intracellular bacilli after TNF-α treatment of the neutrophils would have required an in vitro exposure of approximately 40 μg/ml, which is severalfold above the reported MIC of HNP-1 for M. tuberculosis of 2 to 2.5 μg/ml.
FIG. 6.
Quantitation of HNP binding to M. tuberculosis. (a) Fluorescence intensity of anti-HNP staining as a function of exposure to HNP-1. M. tuberculosis was exposed to increasing concentrations of HNP-1 and then stained with anti-HNP/Cy-5. Digital images were captured and deconvolved. The average intensity of Cy-5 fluorescence of individual organisms was calculated and plotted on the y axis versus the concentration of defensin on the x axis. Error bars, standard errors of 50 tubercle bacilli for each data point. (b) Exposure of M. tuberculosis to defensin during culture with human neutrophils. Average intensity and standard deviation of extracellular M. tuberculosis bacilli within 50 neutrophils infected with M. tuberculosis or 50 neutrophils infected with tuberculosis and treated with 100 U of TNF-α/ml were mapped to exposure via the equation for the standard curve shown in panel a.
Effects of HNP on growth and viability of M. tuberculosis in liquid culture.
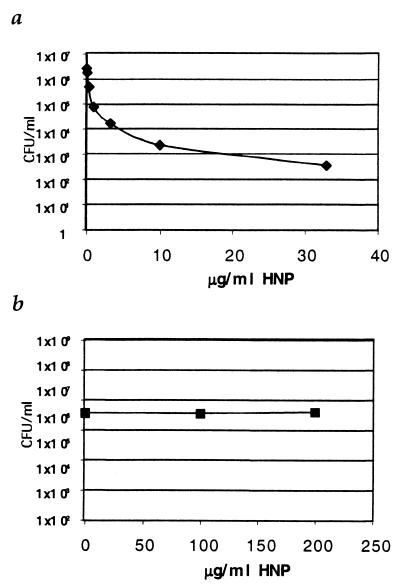
To determine if the level of defensin exposure in vivo could account for the mycobactericidal activity of the neutrophils, we performed in vitro antimicrobial assays using HNP obtained from commercial sources, in addition to HNP which we isolated from biological material. Figure 7a shows that, although HNPs were able to kill E. coli efficiently, we were unable to confirm that HNPs were able to kill M. tuberculosis (Erdman or H37Rv) at concentrations as high as 200 μg/ml, indicating that the minimal bactericidal concentration of HNP for M. tuberculosis exceeds 200 μg/ml for 24 h (Fig. 7b). Exposure to 200 μg/ml of HNP-1 in vitro results in high levels of HNP binding to the tubercle bacilli, exceeding the levels bound to M. tuberculosis in neutrophils during killing. Therefore, it is unlikely that neutrophil defensins can entirely account for the ability of neutrophils to kill M. tuberculosis.
FIG. 7.
Activity of HNP against M. tuberculosis and E. coli. (a) Numbers of E. coli CFU after 24 h of exposure to HNP at increasing concentration. (b) Numbers of M. tuberculosis (H37Rv) CFU after exposure to HNP at up to 200 μg/ml for 24 h.
DISCUSSION
Some individuals who are at high risk of infection by M. tuberculosis resist developing tuberculin reactivity much longer than others (1, 15, 17). The existence of innate mechanisms of immunity to M. tuberculosis infection was hypothesized in 1941, when Israel et al. noted in a study of nursing students working in a TB hospital that “In three persons the reaction to repeated tests was negative, becoming positive only on the tenth test at the end of three years. The persistence of a negative (tuberculin) reaction in these students after the majority of other students had long been infected is a striking phenomenon” (17). The report on the Prophit survey (9), which tracked purified protein derivative conversion rates among young nurses from TB hospitals, seems to bear out other anecdotal observations that there are people who resist infection with M. tuberculosis far longer than their peers, despite similar exposure levels.
Of the many cell types present in the naive human lung which may participate in killing inhaled M. tuberculosis, most efforts to demonstrate the ability to kill M. tuberculosis in culture have focused on alveolar macrophages or monocyte-derived macrophages. The ability of human macrophages to kill M. tuberculosis in culture has never been convincingly demonstrated, suggesting that other cell types present at the site or responding to the infection may also play important roles in innate resistance. Neutrophils are prominent in the earliest exudative TB lesions of human lungs (6). However, the ability of human neutrophils to kill virulent M. tuberculosis in vitro has not been widely reported, and some have strongly argued against it (10).
Pedrosa et al. have suggested a protective role for neutrophils in TB infection which does not involve phagocytosis of the bacilli (27). However, examination of lung tissue from patients with pulmonary TB indicates that neutrophils do indeed phagocytose M. tuberculosis in human lesions as shown in Fig. 1. Nevertheless, the ability of neutrophils to kill ingested bacilli cannot be inferred by this observation alone and was tested directly.
Figure 2 shows that human neutrophils from some donors did not kill M. tuberculosis unless they were stimulated with TNF-α. This result is in agreement with Brown et al., who used phorbol myristate acetate to stimulate mycobactericidal activity (5). We also noted spontaneous killing activity by neutrophils from several donors. This indicates that neutrophils from at least some individuals are able to spontaneously kill M. tuberculosis but that this capacity may not be universal. Our study utilized frozen aliquots of the same M. tuberculosis culture for infection, which provides highly reproducible infection in THP-1 cells. Therefore, these results reflect differences in the ability of the donor neutrophils to kill ingested M. tuberculosis. The variability observed among individuals may help to explain the conflicting observations of other workers in this regard. Additionally, in our studies it was necessary to ensure that the neutrophils were adherent and that all uningested organisms were removed to observe reliable killing. This may explain why Aston et al. (2) were unable to demonstrate killing of M. tuberculosis.
We have also explored the mycobactericidal mechanism of human neutrophils. Inducible production of NO by murine macrophages has been shown to be important for the ability of those cells to kill M. tuberculosis in culture (11). However, the mechanism of M. tuberculosis killing by neutrophils was not inhibited by l-arginine analog l-NMMA, which inhibits NO production (7). In fact, neither we nor others have observed production of NO by human neutrophils in response to M. tuberculosis (data not shown) (23), and so were not surprised by the lack of effect of l-NMMA. NADPH oxidase inhibitor DPI did not significantly diminish the killing capacity of neutrophils, nor did SOD mimic manganese(III) meso-tetrakis(N-methylpyridinium-2-yl)porphyrin, although at the concentrations used reactive oxygen production was inhibited by greater than 95% (data not shown). This indicates that the killing mechanism was independent of production of ROIs, which are known to be produced by neutrophils upon phagocytosis of M. tuberculosis (22). Our observations are in agreement with those of Jones et al., in that the majority of mycobactericidal activity appears to be nonoxidative (18).
Neutrophils are able to utilize several nonoxidative mechanisms to destroy ingested bacteria such as Pseudomonas aeruginosa, Listeria monocytogenes, and E. coli, including lipases (16), proteases (26), and several microbicidal peptides (13). In fact, approximately 30% of the proteins contained in the primary granules are α-defensins, HNP-1, -2, -3, and -4 (14). However, M. tuberculosis has been noted to inhibit phagosome fusion with primary granules in neutrophils (25). Using colocalization of HNP and phagocytosed M. tuberculosis as a marker of phagosome-primary granule fusion, we noted that TNF-α stimulated colocalization of ingested M. tuberculosis with HNP as shown in Fig. 6. However, even in the absence of TNF-α exposure M. tuberculosis bacilli within neutrophils were exposed to significantly more HNP than were extracellular bacilli. Lehrer et al. have previously found that the ability of rabbit defensins to kill Candida albicans is directly related to the binding of the peptides to the cell rather than just the concentration to which they were exposed (19), such that equivalent defensin binding resulted in equivalent candidacidal activity even in the presence of differing concentrations of defensins. Creation of a standard curve of defensin binding to M. tuberculosis after exposure at several different concentrations in vitro allowed us to estimate whether the amount of defensin which bound to the tubercle bacilli in neutrophils could have accounted for the mycobactericidal activity observed. This estimate bears some caveats. First, the binding of the anti-HNP antibodies to intracellular mycobacteria may not be as efficient as the binding to mycobacteria exposed to HNP in vitro. However, we have observed that the levels of staining of intracellular and extracellular M. tuberculosis with antimycobacterial antiserum are not significantly different (K. Kisich, unpublished observation). In addition, the conditions under which HNPs were bound to create the standard curve were probably quite different from the conditions under which binding took place in the neutrophil phagosome. However, since there are other potential mechanisms which may participate in mycobacterial killing by neutrophils, the “phagosomal MIC” may in fact be lower than the in vitro MIC. Despite these limitations, these data suggest that intracellular M. tuberculosis was exposed to HNP-1 at approximately 40 μg/ml after stimulation of the neutrophils with TNF-α.
Examination of the concentration of HNP required to kill M. tuberculosis in vitro revealed that concentrations up to 200 μg/ml for 24 h had no effect on the viability of the tubercle bacilli, despite potent antimicrobial activity against E. coli. These data are consistent with reports from Peschel et al., indicating that M. tuberculosis contains a gene or genes related to the MprF gene of Staphylococcus aureus, which mediates resistance to HNP in that species (28). Two groups have reported that HNP-1 can kill M. tuberculosis in vitro (24, 32). While we have used HNP from the same sources against the same strains of M. tuberculosis growing in the same medium as these groups, we have not observed mycobactericidal activity of HNP. It is possible that there may be subtle differences in our assay or between our mycobacterial strains which prevent us from observing mycobactericidal activity. However, it appears that, in our hands, M. tuberculosis is resistant to HNP relative to E. coli. M. tuberculosis is subject to growth inhibition by HNP, with exposure to 250 μg/ml inhibiting growth by greater than 90%. Growth rates of M. tuberculosis exposed to HNP remained depressed relative to those of untreated bacilli for at least 3 days, suggesting that while exposure to HNP does not result in destruction of M. tuberculosis, it may induce a dormant state which persists even after the HNPs are diluted or otherwise inactivated.
Neutrophils contain several antimicrobial substances in addition to defensins, including but not limited to cathelicidin (36), cathepsin G, myeloperoxidase, lactoferrin, elastase, bactericidal-permeability-increasing protein, and azurocidin (13). Through fractionation of neutrophil proteins, it may be possible to identify one or more of these substances which can account for the killing activity in isolation. However, it is also possible that the totality of the activities of these substances on the viability of M. tuberculosis are greater than their summed activities. This will be addressed in future studies.
Based on observations from ourselves and others, it is possible to consider a model of innate defense which is testable. An initial inoculum of M. tuberculosis is inhaled and phagocytosed by alveolar macrophages. The cytokines produced by the macrophages in response to M. tuberculosis stimulate extravasation and migration of neutrophils to the site. Phagocytosis of the tubercle bacilli by the neutrophils in the presence of TNF-α secreted by the macrophages stimulates neutrophil mycobactericidal mechanisms. If such mechanisms are operative to sterilize limited challenges, it is clear that they can be overwhelmed. Although several studies suggest that some individuals resist infection much longer than their peers (1, 15, 17), at least one study demonstrated that, in the face of persistent exposure to infectious aerosols, 100% infection was eventually observed (17).
In conclusion, we have demonstrated that human neutrophils respond to the presence of M. tuberculosis and actively phagocytose the bacilli in human lesions. Neutrophils adherent to tissue culture plastic are able to kill up to 95% of intracellular M. tuberculosis bacilli within 24 h of infection after stimulation of the infected neutrophils with TNF-α, but not IFN-γ, for 1 h after infection. These data suggest that neutrophils from healthy individuals have the capacity to rapidly kill inhaled M. tuberculosis. The killing mechanism cannot be blocked by addition of inhibitors of ROI or RNI production and coincides with the accumulation of significant levels of HNP on the intracellular bacilli. However, M. tuberculosis was resistant to comparable levels of HNP in vitro, suggesting that other mechanisms and/or substances may be involved in the intracellular destruction of M. tuberculosis by human neutrophils.
Acknowledgments
We are grateful to Tom Ganz for the gift of the anti-HNP-producing hybridoma cell line and helpful discussions. We are also indebted to Brian Day for the generous gift of manganese(III) meso-tetrakis(N-methylpyridinium-2-yl)porphyrin.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Amberson, J. B., and H. M. Riggins. 1936. Tuberculosis among student nurses: a five year study at Bellevue Hospital. Ann. Intern. Med. 10:156-165. [Google Scholar]
- 2.Aston, C., W. N. Rom, A. T. Talbot, and J. Reibman. 1998. Early inhibition of mycobacterial growth by human alveolar macrophages is not due to nitric oxide. Am. J. Respir. Crit. Care Med. 157:1943-1950. [DOI] [PubMed] [Google Scholar]
- 3.Batinic-Haberle, I., L. Benov, I. Spasojevic, and I. Fridovich. 1998. The ortho effect makes manganese(III) meso-tetrakis(N-methylpyridinium-2-yl)porphyrin a powerful and potentially useful superoxide dismutase mimic. J. Biol. Chem. 273:24521-24528. [DOI] [PubMed] [Google Scholar]
- 4.Bonecini-Almeida, M. G., S. Chitale, I. Boutsikakis, J. Geng, H. Doo, S. He, and J. L. Ho. 1998. Induction of in vitro human macrophage anti-Mycobacterium tuberculosis activity: requirement for IFN-gamma and primed lymphocytes. J. Immunol. 160:4490-4499. [PubMed] [Google Scholar]
- 5.Brown, A. E., T. J. Holzer, and B. R. Andersen. 1987. Capacity of human neutrophils to kill Mycobacterium tuberculosis. J. Infect. Dis. 156:985-989. [DOI] [PubMed] [Google Scholar]
- 6.Canetti, G. 1955. The tubercle bacillus in the pulmonary lesion of man; histobacteriology and its bearing on the therapy of pulmonary tuberculosis. Springer Verlag, New York, N.Y.
- 7.Cotter, G., E. Kaluski, A. Blatt, O. Milovanov, Y. Moshkovitz, R. Zaidenstein, A. Salah, D. Alon, Y. Michovitz, M. Metzger, Z. Vered, and A. Golik. 2000. L-NMMA (a nitric oxide synthase inhibitor) is effective in the treatment of cardiogenic shock. Circulation 101:1358-1361. [DOI] [PubMed] [Google Scholar]
- 8.Cross, A. R., and O. T. Jones. 1986. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem. J. 237:111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniels, M., F. Ridehalgh, V. H. Springett, and I. M. Hall. 1948. Tuberculosis in young adults: report on the Prophit tuberculosis survey 1935-1944. H. K. Lewis & Co. Ltd., London, United Kingdom.
- 10.Denis, M. 1991. Human neutrophils, activated with cytokines or not, do not kill virulent Mycobacterium tuberculosis. J. Infect. Dis. 163:919-920. [DOI] [PubMed] [Google Scholar]
- 11.Denis, M. 1991. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell. Immunol. 132:150-157. [DOI] [PubMed] [Google Scholar]
- 11a.Douvas, G. S., D. L. Looker, A. E. Vatter, and A. J. Crowle. 1985. Gamma interferon activates human macropages to become tumoricidal and leishmaniacidal but enhances replication of macrophage-associated mycobacteria. Infect. Immun. 50:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fratazzi, C., R. D. Arbeit, C. Carini, M. K. Balcewicz-Sablinska, J. Keane, H. Kornfeld, and H. G. Remold. 1999. Macrophage apoptosis in mycobacterial infections. J. Leukoc. Biol. 66:763-764. [DOI] [PubMed] [Google Scholar]
- 13.Gabay, J. E., R. W. Scott, D. Campanelli, J. Griffith, C. Wilde, M. N. Marra, M. Seeger, and C. F. Nathan. 1989. Antibiotic proteins of human polymorphonuclear leukocytes. Proc. Natl. Acad. Sci. USA 86:5610-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganz, T., M. E. Selsted, D. Szklarek, S. S. Harwig, K. Daher, D. F. Bainton, and R. I. Lehrer. 1985. Defensins. Natural peptide antibiotics of human neutrophils. J. Clin. Investig. 76:1427-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon, B., and W. M. Cashman. 1930. Tuberculosis in workers after residence in a tuberculosis hospital. JAMA 94:1643-1645. [Google Scholar]
- 16.Hagen, F. S., F. J. Grant, J. L. Kuijper, C. A. Slaughter, C. R. Moomaw, K. Orth, P. J. O'Hara, and R. S. Munford. 1991. Expression and characterization of recombinant human acyloxyacyl hydrolase, a leukocyte enzyme that deacylates bacterial lipopolysaccharides. Biochemistry 30:8415-8423. [DOI] [PubMed] [Google Scholar]
- 17.Israel, H. L., H. W. Hetherington, and J. G. Ord. 1941. A study of tuberculosis among students of nursing. JAMA 117:839-844. [Google Scholar]
- 18.Jones, G. S., H. J. Amirault, and B. R. Andersen. 1990. Killing of Mycobacterium tuberculosis by neutrophils: a nonoxidative process. J. Infect. Dis. 162:700-704. [DOI] [PubMed] [Google Scholar]
- 18a.Kisich, K. O., L. Heifets, M. Higgins, and G. Diamond. 2001. Antimycobacterial agent based on mRNA encoding human β-defensin 2 enables primary macrophages to restrict growth of Mycobacterium tuberculosis. Infect. Immun. 69:2692-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehrer, R. I., D. Szklarek, T. Ganz, and M. E. Selsted. 1985. Correlation of binding of rabbit granulocyte peptides to Candida albicans: correlation with candacidal activity. Infect. Immun. 49:207-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lurie, M. B. 1964. Resistance to tuberculosis: experimental studies in native and acquired defensive mechanisms. The Commonwealth Fund, Cambridge, United Kingdom.
- 21.Majeed, M., N. Perskvist, J. D. Ernst, K. Orselius, and O. Stendahl. 1998. Roles of calcium and annexins in phagocytosis and elimination of an attenuated strain of Mycobacterium tuberculosis in human neutrophils. Microb. Pathog. 24:309-320. [DOI] [PubMed] [Google Scholar]
- 22.May, M. E., and P. J. Spagnuolo. 1987. Evidence for activation of a respiratory burst in the interaction of human neutrophils with Mycobacterium tuberculosis. Infect. Immun. 55:2304-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miles, A. M., M. W. Owens, S. Milligan, G. G. Johnson, J. Z. Fields, T. S. Ing, V. Kottapalli, A. Keshavarzian, and M. B. Grisham. 1995. Nitric oxide synthase in circulating vs. extravasated polymorphonuclear leukocytes. J. Leukoc. Biol. 58:616-622. [DOI] [PubMed] [Google Scholar]
- 24.Miyakawa, Y., P. Ratnakar, A. G. Rao, M. L. Costello, O. Mathieu-Costello, R. I. Lehrer, and A. Catanzaro. 1996. In vitro activity of the antimicrobial peptides human and rabbit defensins and porcine leukocyte protegrin against Mycobacterium tuberculosis. Infect. Immun. 64:926-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.N'Diaye, E. N., X. Darzacq, C. Astarie-Dequeker, M. Daffe, J. Calafat, and I. Maridonneau-Parini. 1998. Fusion of azurophil granules with phagosomes and activation of the tyrosine kinase Hck are specifically inhibited during phagocytosis of mycobacteria by human neutrophils. J. Immunol. 161:4983-4991. [PubMed] [Google Scholar]
- 26.Odeberg, H., and I. Olsson. 1975. Antibacterial activity of cationic proteins from human granulocytes. J. Clin. Investig. 56:1118-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedrosa, J., B. M. Saunders, R. Appelberg, I. M. Orme, M. T. Silva, and A. M. Cooper. 2000. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect. Immun. 68:577-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peschel, A., R. W. Jack, M. Otto, L. V. Collins, P. Staubitz, G. Nicholson, H. Kalbacher, W. F. Nieuwenhuizen, G. Jung, A. Tarkowski, K. P. van Kessel, and J. A. van Strijp. 2001. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 193:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sala, A., S. Zarini, G. Folco, R. C. Murphy, and P. M. Henson. 1999. Differential metabolism of exogenous and endogenous arachidonic acid in human neutrophils. J. Biol. Chem. 274:28264-28269. [DOI] [PubMed] [Google Scholar]
- 30.Salamone, G., M. Giordano, A. S. Trevani, R. Gamberale, M. Vermeulen, J. Schettinni, and J. R. Geffner. 2001. Promotion of neutrophil apoptosis by TNF-alpha. J. Immunol. 166:3476-3483. [DOI] [PubMed] [Google Scholar]
- 31.Scalettar, B. A., J. R. Swedlow, J. W. Sedat, and D. A. Agard. 1996. Dispersion, aberration and deconvolution in multi-wavelength fluorescence images. J. Microsc. 182:50-60. [DOI] [PubMed] [Google Scholar]
- 32.Sharma, S., I. Verma, and G. K. Khuller. 2000. Antibacterial activity of human neutrophil peptide-1 against Mycobacterium tuberculosis H37Rv: in vitro and ex vivo study. Eur. Respir. J. 16:112-117. [DOI] [PubMed] [Google Scholar]
- 33.Sharma, S., I. Verma, and G. K. Khuller. 1999. Biochemical interaction of human neutrophil peptide-1 with Mycobacterium tuberculosis H37Ra. Arch. Microbiol. 171:338-342. [DOI] [PubMed] [Google Scholar]
- 34.Silver, R. F., Q. Li, W. H. Boom, and J. J. Ellner. 1998. Lymphocyte-dependent inhibition of growth of virulent Mycobacterium tuberculosis H37Rv within human monocytes: requirement for CD4+ T cells in purified protein derivative-positive, but not in purified protein derivative-negative subjects. J. Immunol. 160:2408-2417. [PubMed] [Google Scholar]
- 35.Soong, L. B., T. Ganz, A. Ellison, and G. H. Caughey. 1997. Purification and characterization of defensins from cystic fibrosis sputum. Inflamm. Res. 46:98-102. [DOI] [PubMed] [Google Scholar]
- 36.Sorensen, O., K. Arnljots, J. B. Cowland, D. F. Bainton, and N. Borregaard. 1997. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood 90:2796-2803. [PubMed] [Google Scholar]
- 37.Travis, S. M., N. N. Anderson, W. R. Forsyth, C. Espiritu, B. D. Conway, E. P. Greenberg, P. B. McCray, R. I. Lehrer, M. J. Welsh, and B. F. Tack. 2000. Bactericidal activity of mammalian cathelicidin-derived peptides. Infect. Immun. 68:2748-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]