Abstract
β-1,2-Oligomannosides (β-1,2-Man) derived from Candida albicans mannan have been shown to act as adhesins and to induce protective antibodies. We used monoclonal antibodies specific for β-1,2-Man in electron, confocal, and fluorescence microscopy to study the surface expression of β-1,2-Man epitopes. These monoclonal antibodies were also used for Western blotting of cell surface extracts to study the nature of the molecules expressing the β-Man epitopes. Evidence was obtained for the contribution of a glycolipid, phospholipomannan (PLM), to the complex expression of β-1,2-Man epitopes at the cell wall surfaces of yeasts grown on solid media. PLM was present in intercellular matrixes of colonies grown on agar and was detected as a contaminant in mannan batches prepared by conventional methods.
The opportunistic pathogen Candida albicans is the leading cause of mucosal and systemic fungal infections in developed countries. The increasing incidence of these medical problems has stimulated research on both the biology of this yeast and the pathophysiology of candidosis. Study of the C. albicans cell wall is at the interface of both areas of research, since regulation of cell wall biogenesis molds invasion and the nature of the molecules which act as adhesins and/or stimulators of the immune system. Among these, a growing body of evidence suggests that sequences of mannose residues linked through an unusual anomer type of linkage, β-1,2-oligomannosides, correlate positively with pathogenicity. These residues act as adhesins (9, 10, 20) for cells of the macrophage lineage, stimulate these cells to produce different mediators (18, 19), and elicit antibodies that are protective in rodent models of both systemic (11, 13, 14) and vaginal (7) candidosis. Most of these results have been obtained with β-1,2-oligomannosides released from C. albicans cell wall mannan, where they are linked to the rest of the molecule (essentially made of α-linked mannose residues) by phosphodiester bonds. However, by using antibodies specific for β-1,2-oligomannosides, it is possible to reveal the association of β-1,2-oligomannoside epitopes with several cell wall mannoproteins (31) and with a major glycolipid, phospholipomannan (PLM) (32). Structural analysis has confirmed the presence of β-1,2-oligomannosides within PLM (34), which is shed from the cell when C. albicans comes in contact with host cells (17). It is therefore very likely that the expression of β-1,2-oligomannosides at the C. albicans cell wall surface, as shown by fluorescence or electron microscopy using antibodies specific for these residues (3, 27), is the result of their association with different carrier molecules, either mannan, mannoproteins, or PLM. Among these molecules, PLM is the only one which expresses exclusively β-1,2-oligomannoside epitopes in the absence of α-linked mannose residues (33). On this basis, the contribution of PLM to the surface expression of C. albicans β-1,2-oligomannosides was examined by a combination of microscopy and Western blotting methods. Investigation of the growth conditions usually used to culture C. albicans on solid or liquid media, particularly for the preparation of mannan, suggested that PLM is an important component of colony matrixes and a very common contaminant of mannan preparations.
MATERIALS AND METHODS
Strains and growth conditions.
The reference strain C. albicans VW32, serotype A, was used for extraction procedures. This strain was grown on Sabouraud's dextrose agar (SDA) at 37°C after preincubation under the same conditions. Extraction procedures were performed on cells streaked in large quantities onto agar and grown for 24 h. Fluorescence and confocal microscopy was performed on colonies after 48 h of growth from single cells. Immunoelectron microscopy was performed on C. albicans strain ATCC 10231 grown on yeast extract agar for 48 h at 27°C (see below). For mannan extraction, C. albicans strains VW32 and NIH A207 were grown in a biofermentor as described previously (8).
Antibodies.
Monoclonal antibody (MAb) AF1 is a murine immunoglobulin M (IgM) raised against C. albicans glucomannoproteins (6). The specificity of this MAb for β-1,2-oligomannosides has been established previously (33). MAb EB-CA1 (Bio-Rad SA, Marnes la Coquette, France) reacts with α-linked oligomannosides from C. albicans (16) and was used as a control.
Microscopy. (i) Immunoelectron microscopy.
Plots (area, about 16 mm2; thickness, 2 mm) of solid medium with budding yeast cells of C. albicans strain ATCC 10231 were removed with a scalpel. They were placed on filter paper, helium cryofixed, and cryosubstituted in acetone with osmium tetroxide before being embedded in Epikote 812. Epon blocks were kindly furnished by H. Bobichon, Faculté de Pharmacie, Reims, France. This procedure allowed good preservation of the yeast cell wall and organelles, together with postembedding accessibility of saccharide units to ligands coupled to colloidal gold (2). Immunodetection was performed after fixation and embedding procedures on thin Epon sections (80 to 100 nm) collected on Formvar-coated grids. The grids were saturated on a drop of phosphate-buffered saline (PBS) with 1% bovine serum albumin (BSA) for 30 min at room temperature and then incubated with MAb AF1 diluted 1/50 in the same buffer for 1 h at 37°C in a moist chamber. After five washes in the same buffer, grids were incubated in an anti-mouse IgM colloidal gold conjugate (10 nm; Zymed Laboratories Inc., San Francisco, Calif.) diluted 1/50 in PBS-1% BSA for 1 h at 37°C. After one wash in the same buffer followed by four washes in PBS alone, grids were rinsed in distilled water and dried. The sections were then contrasted with uranyl acetate and lead nitrate. Because this step could interfere with the labeling, some grids were left unstained. As a control, the first antibody was also omitted. Slides were observed at 75 kV on a Hitachi H-600 electron microscope.
(ii) Confocal microscopy.
SDA was poured at 60°C onto microchamber slides (Labtek; Poly Labo, Strasbourg, France) and allowed to solidify. Each chamber was inoculated with 200 yeast cells of C. albicans strain VW32 determined by microscopic counting, which were grown for 48 h at 37°C. Cells were treated with MAb AF1 diluted 1/250 in PBS for 30 min at 37°C and then washed four times in PBS. Fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin (Zymed Laboratories) diluted 1/50 in PBS was then added to reveal AF1 binding. After extensive washing, slides were detached from medium chambers, mounted in Vectashield Mounting medium (AbCys, Paris, France), and observed with a TCS-NT Leica microscope at a 488-nm wavelength.
(iii) Fluorescence microscopy.
Microscopy slides were pressed firmly onto a 9-cm-diameter petri dish containing SDA on which C. albicans cells had been allowed to grow for 48 h at 37°C as separated colonies. The imprinted colonies were then washed extensively with tap water under high pressure to remove yeast cells. The slides were then treated as above with MAb AF1; controls consisted of slides incubated with concanavalin A (ConA) coupled to FITC for 1 h at room temperature. Slides were examined under a Zeiss Axiophot fluorescence microscope.
Extraction procedures. (i) AERC extraction.
Whole-cell extracts of C. albicans grown at 37°C on SDA were obtained by the alkaline extraction in reducing conditions procedure as described previously (33). Briefly, cells were treated for 15 min on ice with 0.5 ml of 1.85 M NaOH-5% mercaptoethanol. After addition of 0.5 ml of 50% trichloroacetic acid, cells were further incubated for 15 min on ice, harvested, washed with 1 M Tris (pH 11), and then extracted for 5 min at 100°C with 62.5 mM Tris-HCl buffer-2% SDS (pH 6.8).
(ii) β-Mercaptoethanol extraction.
The cell pellet was washed with 50 mM Tris-HCl buffer, pH 9, and then incubated in the same buffer containing 0.1 M β-mercaptoethanol for 2 h at 28°C. Cells were then centrifuged for 10 min at 800 × g, and the supernatant was filtered through a GF/F glass filter (Whatman International Ltd., Maidstone, England), lyophilized, dialyzed, and desiccated in a vacuum centrifuge.
(iii) SDS and water extraction.
SDS and water extracts were obtained by incubation of yeast cells for 30 min at room temperature in 4% SDS or deionized water, respectively, with gentle shaking. Water extracts were filtered through a GF/F glass filter and concentrated under a vacuum. SDS extracts were treated with 3 volumes of cold absolute ethanol, incubated for 24 h at 4°C, and harvested. The pellet of precipitated proteins was used for analysis.
(iv) Glycolipid extraction.
To confirm the presence of PLM in the cell surface extracts described above, chloroform-methanol extractions (10/10/3, vol/vol/vol) were performed for 10 min at room temperature with gentle shaking. After 10 min of centrifugation at 12,000 × g to pellet the insoluble proteins, the supernatants were concentrated in a vacuum centrifuge and then further treated like protein samples for electrophoresis.
(v) Mannan extraction.
Mannan was extracted and purified from C. albicans strains VW32 and NIH A207 grown in bioreactors in liquid medium according to our standard protocol (8), initially derived from the method of Kocourek and Ballou (22) with slight modifications. As a control, a mannan sample from strain NIH A207, kindly provided by S. Suzuki (Sendai Research Institute for Mycology, Sendai, Japan), was also used. This mannan was also derived from yeasts grown in liquid medium but was prepared by the standard method used by this group (21).
SDS-polyacrylamide gel electrophoresis and Western blotting.
Yeast extracts were resolved as described by Laemmli (23) on an SDS-5 to 15% polyacrylamide slab gel at a constant current of 4 mA. Gels were then electroblotted in a semidry apparatus onto a nitrocellulose sheet (Schleicher and Schuell, Dassel, Germany), stained with Ponceau S, blocked, and revealed with the appropriate MAb or ConA-peroxidase as described previously (5), except that nonfat milk was added at every step to eliminate nonspecific reactions.
RESULTS
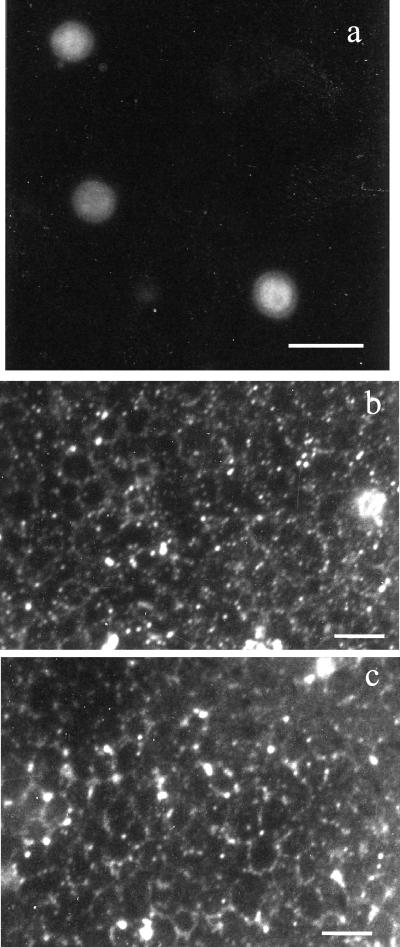
Analysis of the distribution of β-1,2-oligomannoside epitopes by immunoelectron, confocal, and fluorescence microscopy.
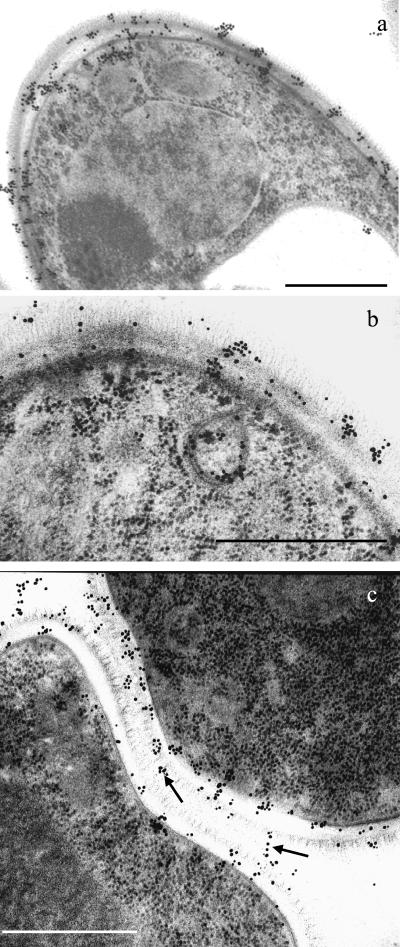
Cryosubstitution methods provided good preservation of C. albicans organelles, including the nucleolus, nuclear membranes, tonoplast, mitochondria, ribosomes, and endoplasmic reticulum (Fig. 1). Gold particles revealing the binding of the anti-β-1,2-oligomannoside MAb were distributed mainly at the periphery of the cytoplasm, close to the plasma membrane, and at the cell surface. Higher magnification showed the presence of epitopes both in the hyaloplasm and associated with vesicles (either inside or on their membranes). In the cell wall, crossing of the electron lucent layers resulted in a progressive coating of surface fimbriae with a patchy distribution. Examination of intact yeast cells by confocal microscopy (Fig. 2) confirmed that secretion was not homogeneously distributed at the cell periphery. Consideration of the presence of β-1,2-oligomannoside epitopes in the intercellular spaces, namely, at the point where fibrils from adjacent cells merged (Fig. 1c), poses the question of the nature of the molecule expressing β-1,2-oligomannosides which is present in colony matrixes.
FIG. 1.
Ultrathin sections of C. albicans cells grown on agar, fixed by cryosubstitution, and labeled with gold particles reveal the distribution of β-1,2-oligomannoside epitopes (MAb AF1 reactive). (a) Low-magnification micrograph showing C. albicans organelles and distribution of epitopes at the cell periphery, inside, and at the cell wall surface. (b) Details of the peripheral location of β-1,2-oligomannoside epitopes in the haloplasm or associated with a vesicle located close to the plasmalemma, where they are distributed either inside or on the membrane. (c) β-1,2-Oligomannoside epitopes cover the fibrils of adjacent cells up to the point where fibrils merge (arrows). Bars, 1 μm.
FIG. 2.
Confocal microscopy of C. albicans cells grown on agar. β-1,2-Oligomannoside epitopes are not homogeneously distributed at the cell surface, and some cells may be negative (see mother and daughter cells in panel a); others exhibit a patchy distribution (a and b) or double polarity in the secretion process (c). Bars, 5 μm
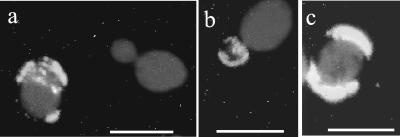
To answer this question, immunofluorescence experiments were carried out by staining ghosts released on microscope slides after imprinting of a C. albicans colony grown on agar and extensive washing to remove adhering cells. When such a slide was stained with ConA, specific for α-linked mannose residues (Fig. 3a), only the rare yeast cells remaining on the glass were stained, but no antigen-bearing α-linked mannose residues were detected on the slide between these cells. When the slide was stained with MAb AF1 (Fig. 3b and c), some of the remaining yeast cells were labeled. However, the staining also included an intense background and was distributed over a honeycomb structure which corresponded to intercellular yeast spaces. Since PLM is the only C. albicans molecule so far identified to express exclusively β-1,2-oligomannoside epitopes, these experiments strongly suggest that PLM is a component of the matrix of C. albicans colonies grown on agar.
FIG. 3.
Fluorescent staining of a colony print made on a microscopic slide after extensive washing. (a) Staining with ConA-FITC. A few cells which remain on the slide have their surfaces homogeneously stained by ConA, but this lectin does not bind to any amorphous material remaining on the slide. (b and c) Staining with MAb AF1, specific for β-1,2-oligomannoside epitopes. In contrast, this staining reveals material still adhering to the slide whose distribution corresponds to the spaces between cells that have been removed by washing. Bars, 5 μm
PLM is released by β-mercaptoethanol treatment of whole C. albicans cells.
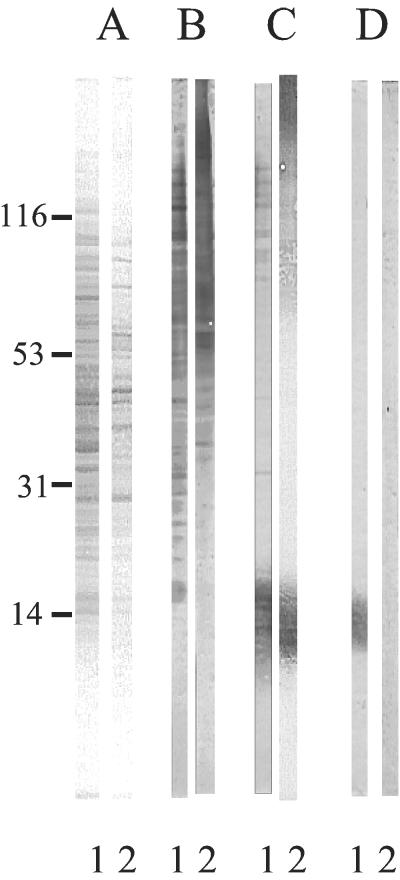
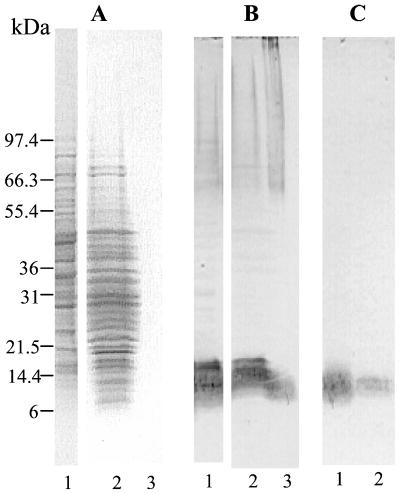
To confirm the expected presence of PLM in the cell walls of yeast cells grown on solid medium, C. albicans cells were treated with β-mercaptoethanol. This well-established, mild treatment does not affect either the membrane integrity (trypan blue staining) or the viability of the cells (CFU) (data not shown). Comparison of the profile of the extracted proteins with that of a whole-cell extract confirmed the selectivity of this extraction procedure (Fig. 4A). ConA staining labeled higher-molecular-weight mannoproteins in the β-mercaptoethanol extract than in the whole-cell extract and also led to quite different patterns (Fig. 4B). Staining patterns obtained with MAb AF1 differed strongly from the ConA staining pattern; the former involved only the upper fraction of the mannoproteins and a low-molecular-weight smear which was not stained by ConA (Fig. 4C). This selective expression of β-1,2-oligomannoside epitopes in a smear within this range of molecular weight is a characteristic feature of PLM. This was further confirmed by its specific extraction with chloroform-methanol-water (10/10/3, vol/vol/vol), a solvent mixture in which glycoproteins are insoluble (Fig. 4D).
FIG. 4.
Comparative Western blot analysis of protein patterns and glycoconjugates expressing α-linked oligomannoside and/or β-1,2-oligomannoside epitopes in AERC extracts (A through C, lanes 1) and β-mercaptoethanol extracts (A through C, lanes 2; D, lanes 1 and 2) prepared from C. albicans VW32 grown at 37°C on SDA. To control the efficiency of transfer onto the membranes, proteins were stained with Ponceau S prior to immunostaining (A). ConA-peroxidase (B, lanes 1 and 2; D, lane 2) and MAb AF1 (C, lanes 1 and 2; D, lane 1) were used to detect α-linked oligomannoside and β-1,2-oligomannoside epitopes, respectively. (D) A selective chloroform-methanol-water extraction performed on the β-mercaptoethanol extract confirmed the glycolipid nature of PLM, which expressed β-1,2-oligomannoside epitopes (lane 1) in the absence of α-linked oligomannoside epitopes (lane 2).
PLM appears noncovalently attached at the C. albicans surface and “contaminates” mannan preparations prepared under standard conditions.
To confirm the contribution of PLM to the cell surface expression of β-1,2-oligomannosides, cells grown on SDA were treated with water and 4% SDS, and the extracts were analyzed by Western blotting. Routine Ponceau red staining confirmed the specificity of the protein pattern of the SDS extract (Fig. 5A, lane 2) by comparison to a whole-cell extract of C. albicans (Fig. 5A, lane 1) and showed the lower efficiency of the water extraction procedure (Fig. 5A, lane 3). Staining of the extracts with MAb AF1 still revealed, even in the water extract, the presence of β-1,2-oligomannoside epitopes on a low-molecular-weight antigen (Fig. 5B). The solubility of this antigen in chloroform-methanol-water confirmed that this antigen corresponded to PLM (Fig. 5C).
FIG. 5.
(A and B) Western blot analysis of the distribution of protein patterns and β-1,2-oligomannoside epitopes on glycoconjugates in an AERC extract (lane 1), a 4% SDS extract (lane 2), and a water extract (lanes 3) prepared from C. albicans VW32 grown at 37°C. (C) Results of chloroform-methanol-water extraction performed on the 4% SDS (lane 1) and water extracts (lane 2). Staining was performed with Ponceau red (A) or MAb AF1 revealed with a phosphatase-labeled anti-mouse IgM antibody (B and C).
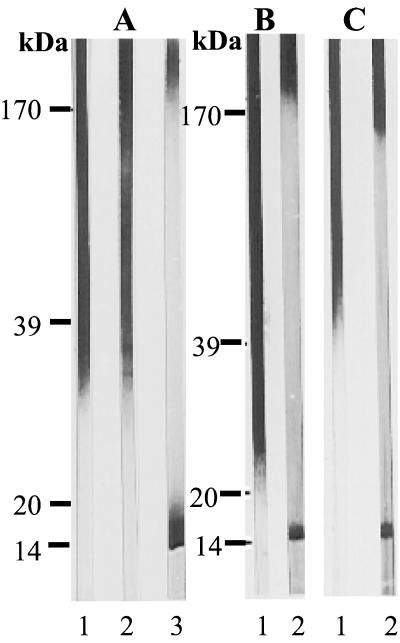
These observations, which suggest that PLM is bound noncovalently to the cell wall, led us to investigate the presence of PLM in a mannan extract, a very commonly used C. albicans antigen. Western blot analysis of our mannan preparation using the lectin ConA and MAb EB-CA1, both specific for α-linked mannose residues, revealed a wide smear of mannoproteins ranging from 30 to 300 kDa (Fig. 6A, lanes 1 and 2). On the same extract, MAb AF1 revealed mannan components above 170 kDa and the presence of PLM (Fig. 6A, lane 3). To check that this observation was not strain specific, mannan prepared from the reference strain NIH A207 was also tested, and the same results were observed (Fig. 6B, lane 2). Finally, to verify whether the presence of PLM was related to our extraction procedure, a mannan batch from strain NIH A207 which was kindly provided by S. Suzuki was also tested. As expected, PLM was present in this extract also (Fig. 6C, lane 2).
FIG. 6.
Western blots of different mannan batches extracted from strain VW32, serotype A (A), and strain NIH A207 (B and C). Mannans in panels A and B were prepared in our laboratory, and the mannan analyzed in panel C was prepared in S. Suzuki's laboratory in Sendai, Japan. Lanes 1 were revealed with MAb EB-CA1, specific for α-linked mannose residues. Lane A2 was stained with ConA-peroxidase, which is also specific for α-linked mannose residues. MAb AF1 was used to reveal β-1,2-linked oligomannosides (lanes A3, B2, and C2). PLM was always present in the mannan extracts, irrespective of the strain or preparation procedure, but never expressed α-linked oligomannoside epitopes.
DISCUSSION
The pathophysiology of the yeast C. albicans is the subject of much active research, due to the increasing medical importance of mucocutaneous and systemic candidosis. The complexity of C. albicans biology, the sequencing of its genome, and its similarities and differences to Saccharomyces cerevisiae have also led to an increased interest in this opportunistic pathogen. As far as the cell biology of C. albicans and S. cerevisiae is concerned, much remains to be discovered about the structural organization of the cell wall and about the assembly and variability of the macromolecular architecture according to growth conditions. Among the differences between the two species, several studies have highlighted the importance of β-1,2-linked mannose residues specifically expressed at the C. albicans cell surface as pathogenicity determinants. These sugars act as adhesins (25), bind to the macrophage membrane (10), stimulate these cells to produce mediators of the immune response (18, 19), and induce protective antibodies (7, 12, 13). The presence of β-1,2-oligomannosides in mannan was first demonstrated by Suzuki and coworkers (30). C. albicans mannan is homogeneously distributed at the cell surface and displays variable thickness and a fibrillar layer according to the growth conditions (15). Nevertheless, early studies with MAbs revealed that some mannan epitopes were not homogeneously distributed at the cell surface; the first immunofluorescence studies with anti-β-1,2-oligomannoside antibodies revealed a complex heterogeneity of surface expression and a patchy distribution of β-1,2-oligomannoside epitopes (3). Electron microscopy studies using immunogold labeling methods further confirmed the heterogeneous distribution and secretion of β-1,2-oligomannoside epitopes at the subcellular level (24, 27). The studies of Hazen and coworkers demonstrated a change in the degree of polymerization of β-1,2-oligomannosides that was related to the hydrophobic or hydrophilic status of C. albicans yeast cells (26) and which also appeared to correlate with the length of cell surface fibrils (15). All these studies and those concerning the role of β-1,2-oligomannosides in physiopathology have considered mannan to be the unique carrier molecule and source of β-1,2-oligomannosides. However, studies using anti-β-1,2-oligomannoside antibodies have suggested the presence of β-1,2-oligomannosides in the glycan moieties of several mannoproteins of C. albicans and in PLM (32). Chemical characterization of the PLM glycan moiety has recently confirmed that it contains phosphoinositol mannosides presenting long linear chains of β-1,2-oligomannosides (34). As β-1,2-oligomannosides have been shown to be involved in adherence, immunomodulation, and protection, the mode of their presentation to the host is an important question, since the way in which an immunoreactive sequence triggers the immune system is highly dependent on the nature of the carrier molecule. Because most microbial cell surface glycolipids are powerful immunomodulators (1, 29), the question of the contribution of PLM to β-1,2-oligomannoside surface expression in C. albicans has been addressed in this study. To answer this question by using microscopy and anti-β-1,2-oligomannoside antibodies is not easy, because β-1,2-oligomannosides may be expressed on mannan, mannoproteins, and glycolipids, and no mutant with a specific disruption in one of these biosynthetic pathways is currently available.
Most of the studies on β-1,2-oligomannoside expression have been performed with C. albicans grown in liquid medium to promote homogeneity of the cell population. However, such conditions, where yeasts are isolated from each other, are rarely encountered by the yeast in its “natural” life either as a saprophyte or a parasite. In the present study, therefore, cells grown on solid medium were used to promote contact interactions. There are at least three reasons for this choice: (i) C. albicans yeasts and/or mycelia in the host are constantly in contact with host cells or tissues or with each other; (ii) there is more and more evidence that C. albicans cell wall architecture is dependent on the response to contact, i.e., germ tube thigmotropism (28, 37) or sensor mechanisms conditioning yeast flocculation (4); and (iii) shedding of molecules has been demonstrated to be a natural physiological process during contact of C. albicans with solid surfaces (35) or host cells (17, 36). Under these growth conditions, fibrils, which are mainly composed of α-linked mannose residues, have been shown to be covered by β-1,2-oligomannosides, especially at the junction between yeasts growing as colonies on agar. This covering process is progressive, since yeasts in the deeper parts of colonies are entirely covered (data not shown), but not homogeneous, as revealed by confocal microscopy. Printing of colonies on microscope slides followed by extensive washing to remove the cells demonstrated that the intercellular spaces were filled with a material adhering to the slides which expressed β-1,2-oligomannoside epitopes in the absence of α-linked mannosides. As far as is known, according to data obtained on C. albicans mannoglycoconjugates by using chemical methods, lectins, and antibodies, the only molecule presenting such properties is C. albicans PLM. The possibility that this material could correspond to PLM was therefore explored. Like the well-established β-mercaptoethanol extraction procedure, simple water or SDS extraction of colonies at room temperature revealed PLM as a prominent molecular entity expressing β-1,2-oligomannoside epitopes. It is therefore reasonable to assume that this molecule is present in the cell wall and could be a component of the adherent material making up C. albicans colony matrixes. This observation poses the question of the potential role of PLM in cell cohesion and its physicochemical mechanics.
Previous experiments have also shown the presence of PLM in liquid media (32) as well as its shedding from the cell wall surface (17). This is coherent with the surprising observation from this study of the presence of PLM in mannan preparations, irrespective of the strain or the extraction procedure used. Mannan has been studied extensively to characterize its glycan moiety and its immunochemical and immunomodulatory properties, but this is the first evidence of the contamination of mannan preparations by PLM. Mannan preparation procedures now generally include a ConA-Sepharose step, which theorically eliminates PLM, as long as chromatography conditions avoid PLM-mannan molecular interactions. Nevertheless, our results clearly show the presence of PLM at the cell surface, which has been underestimated in previous studies due to the specific procedures necessary for its identification. Because of increasing evidence of the role of β-1,2-oligomannosides in the pathology of candidosis, until now mainly attributed to mannan, the results of this study pose the fundamental question of the contribution of PLM to the biology and pathogenicity of C. albicans.
Editor: T. R. Kozel
REFERENCES
- 1.Barnes, P. F., D. Chatterjee, J. S. Abrams, S. Lu, E. Wang, M. Yamamura, P. J. Brennan, and R. L. Modlin. 1992. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. Relationship to chemical structure. J. Immunol. 149:541-547. [PubMed] [Google Scholar]
- 2.Bobichon, H., D. Gache, and P. Bouchet. 1994. Ultrarapid cryofixation of Candida albicans: evidence for a fibrillar reticulated external layer and mannan channels within the cell wall. Cryo-Letters 15:161-172. [Google Scholar]
- 3.Brawner, D. L., J. E. Cutler, and W. L. Beatty. 1990. Caveats in the investigation of form-specific molecules of Candida albicans. Infect. Immun. 58:378-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calera, J. A., and R. Calderone. 1999. Flocculation of hyphae is associated with a deletion in the putative CaHK1 two-component histidine kinase gene from Candida albicans. Microbiology 145:1431-1442. [DOI] [PubMed] [Google Scholar]
- 5.Cantelli, C., P. A. Trinel, A. Bernigaud, T. Jouault, L. Polonelli, and D. Poulain. 1995. Mapping of β-1,2-linked oligomannosidic epitopes among glycoconjugates of Candida species. Microbiology 141:2693-2697. [DOI] [PubMed] [Google Scholar]
- 6.Cassone, A., A. Torosantucci, M. Boccanera, G. Pellegrini, C. Palma, and F. Malavasi. 1988. Production and characterisation of a monoclonal antibody to a cell-surface, glucomannoprotein constituent of Candida albicans and other pathogenic Candida species. J. Med. Microbiol. 27:233-238. [DOI] [PubMed] [Google Scholar]
- 7.De Bernardis, F., M. Boccanera, D. Adriani, E. Spreghini, G. Santoni, and A. Cassone. 1997. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect. Immun. 65:3399-3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faille, C., J. C. Michalski, G. Strecker, D. W. Mackenzie, D. Camus, and D. Poulain. 1990. Immunoreactivity of neoglycolipids constructed from oligomannosidic residues of the Candida albicans cell wall. Infect. Immun. 58:3537-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fradin, C., T. Jouault, A. Mallet, J. M. Mallet, D. Camus, P. Sinay, and D. Poulain. 1996. β-1,2-Linked oligomannosides inhibit Candida albicans binding to murine macrophage. J. Leukoc. Biol. 60:81-87. [DOI] [PubMed] [Google Scholar]
- 10.Fradin, C., D. Poulain, and T. Jouault. 2000. β-1,2-Linked oligomannosides from Candida albicans bind to a 32-kilodalton macrophage membrane protein homologous to the mammalian lectin galectin-3. Infect. Immun. 68:4391-4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han, Y., and J. E. Cutler. 1995. Antibody response that protects against disseminated candidiasis. Infect. Immun. 63:2714-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han, Y., T. Kanbe, R. Cherniak, and J. E. Cutler. 1997. Biochemical characterization of Candida albicans epitopes that can elicit protective and nonprotective antibodies. Infect. Immun. 65:4100-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han, Y., R. P. Morrison, and J. E. Cutler. 1998. A vaccine and monoclonal antibodies that enhance mouse resistance to Candida albicans vaginal infection. Infect. Immun. 66:5771-5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han, Y., M. H. Riesselman, and J. E. Cutler. 2000. Protection against candidiasis by an immunoglobulin G3 (IgG3) monoclonal antibody specific for the same mannotriose as an IgM protective antibody. Infect. Immun. 68:1649-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hazen, K. C., and B. W. Hazen. 1993. Surface hydrophobic and hydrophilic protein alterations in Candida albicans. FEMS Microbiol. Lett. 107:83-87. [DOI] [PubMed] [Google Scholar]
- 16.Jacquinot, P. M., Y. Plancke, B. Sendid, G. Strecker, and D. Poulain. 1998. Nature of Candida albicans-derived carbohydrate antigen recognized by a monoclonal antibody in patient sera and distribution over Candida species. FEMS Microbiol. Lett. 169:131-138. [DOI] [PubMed] [Google Scholar]
- 17.Jouault, T., C. Fradin, P. A. Trinel, A. Bernigaud, and D. Poulain. 1998. Early signal transduction induced by Candida albicans in macrophages through shedding of a glycolipid. J. Infect. Dis. 178:792-802. [DOI] [PubMed] [Google Scholar]
- 18.Jouault, T., C. Fradin, P. A. Trinel, and D. Poulain. 2000. Candida albicans-derived β-1,2-linked mannooligosaccharides induce desensitization of macrophages. Infect. Immun. 68:965-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jouault, T., G. Lepage, A. Bernigaud, P. A. Trinel, C. Fradin, J. M. Wieruszeski, G. Strecker, and D. Poulain. 1995. β-1,2-Linked oligomannosides from Candida albicans act as signals for tumor necrosis factor alpha production. Infect. Immun. 63:2378-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanbe, T., and J. E. Cutler. 1998. Minimum chemical requirements for adhesin activity of the acid-stable part of Candida albicans cell wall phosphomannoprotein complex. Infect. Immun. 66:5812-5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi, H., N. Shibata, H. Mitobe, Y. Ohkubo, and S. Suzuki. 1989. Structural study of phosphomannan of yeast-form cells of Candida albicans J-1012 strain with special reference to application of mild acetolysis. Arch. Biochem. Biophys. 272:364-375. [DOI] [PubMed] [Google Scholar]
- 22.Kocourek, J., and C. E. Ballou. 1969. Method for fingerprinting yeast cell wall mannans. J. Bacteriol. 100:1175-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 24.Li, R. K., and J. E. Cutler. 1991. A cell surface/plasma membrane antigen of Candida albicans. J. Gen. Microbiol. 137:455-464. [DOI] [PubMed] [Google Scholar]
- 25.Li, R. K., and J. E. Cutler. 1993. Chemical definition of an epitope/adhesin molecule on Candida albicans. J. Biol. Chem. 268:18293-18299. [PubMed] [Google Scholar]
- 26.Masuoka, J., G. Wu, P. M. Glee, and K. C. Hazen. 1999. Inhibition of Candida albicans attachment to extracellular matrix by antibodies which recognize hydrophobic cell wall proteins. FEMS Immunol. Med. Microbiol. 24:421-429. [DOI] [PubMed] [Google Scholar]
- 27.Molinari, A., M. J. Gomez, P. Crateri, A. Torosantucci, A. Cassone, and G. Arancia. 1993. Differential cell surface expression of mannoprotein epitopes in yeast and mycelial forms of Candida albicans. Eur. J. Cell Biol. 60:146-153. [PubMed] [Google Scholar]
- 28.Nikawa, H., H. Nishimura, T. Hamada, S. Makihira, and L. P. Samaranayake. 1998. Relationship between thigmotropism and Candida biofilm formation in vitro. Mycopathologia 144:125-129. [DOI] [PubMed] [Google Scholar]
- 29.Schofield, L., and S. D. Tachado. 1996. Regulation of host cell function by glycosylphosphatidylinositols of the parasitic protozoa. Immunol. Cell Biol. 74:555-563. [DOI] [PubMed] [Google Scholar]
- 30.Shibata, N., T. Ichikawa, M. Tojo, M. Takahashi, N. Ito, Y. Okubo, and S. Suzuki. 1985. Immunochemical study on the mannans of Candida albicans NIH A-207, NIH B-792, and J-1012 strains prepared by fractional precipitation with cetyltrimethylammonium bromide. Arch. Biochem. Biophys. 243:338-348. [DOI] [PubMed] [Google Scholar]
- 31.Torosantucci, A., M. Boccanera, I. Casalinuovo, G. Pellegrini, and A. Cassone. 1990. Differences in the antigenic expression of immunomodulatory mannoprotein constituents on yeast and mycelial forms of Candida albicans. J. Gen. Microbiol. 136:1421-1428. [DOI] [PubMed] [Google Scholar]
- 32.Trinel, P. A., M. Borg-von-Zepelin, G. Lepage, T. Jouault, D. Mackenzie, and D. Poulain. 1993. Isolation and preliminary characterization of the 14- to 18-kilodalton Candida albicans antigen as a phospholipomannan containing β-1,2-linked oligomannosides. Infect. Immun. 61:4398-4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trinel, P. A., C. Faille, P. M. Jacquinot, J. C. Cailliez, and D. Poulain. 1992. Mapping of Candida albicans oligomannosidic epitopes by using monoclonal antibodies. Infect. Immun. 60:3845-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trinel, P. A., Y. Plancke, P. Gerold, T. Jouault, F. Delplace, R. T. Schwarz, G. Strecker, and D. Poulain. 1999. The Candida albicans phospholipomannan is a family of glycolipids presenting phosphoinositolmannosides with long linear chains of β-1,2-linked mannose residues. J. Biol. Chem. 274:30520-30526. [DOI] [PubMed] [Google Scholar]
- 35.Tronchin, G., J. P. Bouchara, R. Robert, and J. M. Senet. 1988. Adherence of Candida albicans germ tubes to plastic: ultrastructural and molecular studies of fibrillar adhesins. Infect. Immun. 56:1987-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tronchin, G., D. Poulain, and A. Vernes. 1984. Cytochemical and ultrastructural studies of Candida albicans. III. Evidence for modifications of the cell wall coat during adherence to human buccal epithelial cells. Arch. Microbiol. 139:221-224. [DOI] [PubMed] [Google Scholar]
- 37.Watts, H. J., A. A. Very, T. H. Perera, J. M. Davies, and N. A. Gow. 1998. Thigmotropism and stretch-activated channels in the pathogenic fungus Candida albicans. Microbiology 144:689-695. [DOI] [PubMed] [Google Scholar]