Abstract
Serum antibodies to pertussis toxin (PT) have been shown to be protective against severe pertussis disease, although a specific level of anti-PT antibody that correlates with protection has not been demonstrated. Current animal models such as the intracerebral challenge model have significant limitations in correlating protection to a specific level of anti-PT antibody. This study examines the protective effects of priming with tetranitromethane-inactivated pertussis toxoid (PTx) vaccine in the aerosol challenge model and whether a measurable response to a priming dose of PTx is enough to initiate a protective secondary response when challenged with infection. The correlation of priming with markers of illness such as leukocytosis, weight loss, bacterial proliferation, and mortality after established infection with Bordetella pertussis was explored. BALB/c mice were immunized with PTx vaccine on day 6 of life and then challenged with B. pertussis using the aerosol challenge model. Data were analyzed according to the primary immunologic response, differentiating responders (anti-PT immunoglobulin G [IgG] ≥1 μg/ml) from nonresponders (anti-PT IgG <1 μg/ml). Mice that showed evidence of priming on the day of aerosol challenge were able to mount a secondary response to the challenge with a ≥2-fold rise in anti-PT IgG antibody by day 7 and a ≥10-fold rise by day 14 post-aerosol challenge. These primed mice were significantly better protected against leukocytosis, weight loss, and proliferation of B. pertussis in the lungs following aerosol challenge than the nonprimed group. This protection correlated with levels of anti-PT antibody in serum present on the day of aerosol challenge.
Immunization against pertussis was initiated in the 1950s with the introduction of pertussis whole-cell vaccine. With the changing epidemiology of pertussis and inadequate vaccine coverage rates in many populations, pertussis continues to cause significant morbidity and mortality (9-12, 27). A high rate of adverse reactions associated with whole-cell vaccines has led to the development of acellular pertussis vaccines. These vaccines all contain inactivated pertussis toxin (PT) in addition to various combinations of other protective antigens, including filamentous hemagglutinin, pertactin, and agglutinogens 1 to 3. Clinical trials have demonstrated that these acellular vaccines confer protection against pertussis (2, 16, 18, 44). These secondary antigens may offer added protection against severe pertussis, yet there is evidence that antibodies to PT alone are effective in protecting against severe pertussis as shown in single component acellular pertussis toxoid (PTx) vaccine trials in humans (1, 6; P. Olin and J. Storsaeter, Letter, JAMA 261:560, 1989). Although antibodies to PT in serum have been shown to be protective (47), to date none of the human pertussis vaccine trials or the studies of convalescing patients after natural infection have demonstrated a level of anti-PT antibody that correlates with protection (15, 16, 18, 22, 41, 44, 46). Without a surrogate marker of protection, all newly developed vaccines must undergo rigorous and costly human vaccine efficacy trials. Animal models that demonstrate a specific protective level of anti-PT antibody are needed. We sought to demonstrate that a primary response of anti-PT antibodies could be characterized following immunization with a priming dose of PTx in an aerosol challenge model in mice.
Several animal models have been developed to study the immune response to pertussis infection and immunization. Antibodies to PT have been shown to be protective in several of these animal models, including the histamine challenge model (28), the intracerebral inoculation model (26, 37, 38, 40), the intranasal challenge model (31), and the aerosol challenge model (17, 19, 28, 31, 36, 39). Although the current method of evaluating vaccine potency using the intracerebral inoculation model in mice highly correlates protection to anti-PT antibody response (14, 45), this protection is dependent on alterations of the blood-brain barrier by natural PT (25, 32; R. K. Gupta, S. N. Saxena, S. B. Sharma, and S. Ahuja, Letter, Vaccine 8:289, 1990). Some investigators have demonstrated that pertussis toxoid vaccines provide protection in the intracerebral challenge model (26, 37, 38, 40), but it has been postulated that this protection may be the result of alteration of the blood-brain barrier from small contaminating amounts of holotoxin. Other animal models are needed to study the primary immune response to PTx and its correlation with protection from Bordetella pertussis infection. This has not been thoroughly examined in the aerosol challenge model.
The aerosol challenge model as described by Sato et al. (36) has many features that resemble pertussis in infants. Protection does not depend on anti-PT antibodies crossing the blood-brain barrier (13, 28). Although mice do not whoop or cough, the infection is established by adherence of the organisms to the columnar respiratory epithelium of mice (36), followed by proliferation of organisms in the lungs, lymphocytosis, weight loss, and death. Illness in mice is produced by the elaboration of PT, which has been shown to cause lymphocytosis and weight loss (17, 19, 36). As in humans, infant mice manifest more-severe illness than adult mice (36).
Although the protective effects of anti-PT antibodies have been demonstrated in the aerosol challenge model, evidence of priming with PTx has not been described. In this study we sought to examine the effects of priming with PTx in mice utilizing the aerosol challenge model, specifically to determine if a measurable response to a priming dose of PTx is enough to initiate a protective secondary response when challenged with infection. Finally, we examined the correlation between a measurable primary and secondary antibody response to markers of illness such as leukocytosis, weight loss, and mortality after established infection with B. pertussis. These findings demonstrate the potential utility of this model for determining PTx vaccine efficacy and potency.
MATERIALS AND METHODS
Mice.
Female specific-pathogen-free BALB/c mice with natural litters from Charles River Laboratories were ordered to arrive 4 days after dropping their litters. Mice were housed one litter per cage prior to weaning and five mice per cage after weaning, in Low Profile Micro-Isolator (Lab Products Inc., Maywood, N.J.) filtered-top cages with standard bedding. Mice had free access to food and water. The cages of mice were placed in a portable HEPA-filtered ventilated animal rack in the biosafety level-2 containment suite. All procedures and handling of mice took place in a BiochemGARD hood. All mice were marked using standard ear-clipping methods. Because the mice were immunized on day 6 of life, not allowing for easy sorting based on sex of these natural litters, males and females were subjected to challenge and then later identified by sex at an older age.
Bacteria.
B. pertussis strain 18323 (ATCC 9797; American Type Culture Collection, Manassas, Va.) was recovered from a lyophilized stock from the Massachusetts Public Health Biologic Laboratories (MPHBL) and inoculated onto Bordet-Gengou agar plates (Difco Laboratories, Detroit, Mich.). Growth from 72-h cultures was transferred to fresh Bordet-Gengou plates and grown at 35°C for 21 h. Bacteria were then removed from the plate using a sterile loop and suspended in phosphate-buffered saline (PBS) (pH 7.4) for use in aerosolization.
Serologic methods.
Anti-PT immunoglobulin G (IgG) antibody concentrations were determined using methods modified to detect mouse specific antibodies from those previously described by Siber et al. (41). Briefly, 96-well plates (Immulon 2; Dynatech, Alexandria, Va.) were coated with purified PT (1.0 μg/ml) obtained from the MPHBL. Sensitized plates were incubated overnight at 4°C with serial twofold dilutions of a known mouse anti-PT serum from the MPHBL (10 dilutions) or serial fourfold dilutions of the unknown test sera (5 dilutions). Bound antibodies were detected by using goat anti-mouse IgG human-absorbed alkaline phosphatase conjugated antibodies (Caltag Laboratories, San Francisco, Calif.).
The anti-PT IgG antibody concentration for the mouse anti-PT serum standard was determined by the Zollinger method as previously described (43, 48). Using the Center for Biologics Evaluation and Review (CBER) pertussis reference antiserum (lot 3), 1 μg/ml was equivalent to 10 CBER units/ml for PT IgG.
WBC concentrations.
Total leukocyte (WBC) concentrations were measured by obtaining 20 μl of blood by retro-orbital bleed using the Unopette capillary tube system (Becton Dickinson, Rutherford, N.J.). Anesthetized mice were bled at specified times post-aerosol challenge. WBC counts were calculated in thousand cells per cubic millimeter by using a hemocytometer and a 40×-objective microscope.
Vaccines and toxin.
Siber and colleagues at the MPHBL prepared the PTx as previously described (G. R. Siber, L. Herzog, C. D. Marchant, N. Cohen, L. W. Winberry, and C. W. Todd, Abstr. Sixth Int. Symp. Pertussis, abstr. 82, 1990). Briefly this vaccine is prepared by chemically modifying purified PT from culture supernatants with tetranitromethane (TNM). The lot was adsorbed to aluminum hydroxide (Alhydogel; Superfos, Vedbaek, Denmark) at a concentration of 50 μg of protein adsorbed to 4 mg per 1.0 ml. Animal doses (2.5, 0.5, and 0.1 μg) were prepared by making fivefold serial dilutions in aluminum hydroxide (4 mg/ml) diluent and given in a volume of 50 μl, subcutaneously.
Purified PT (pool F; MPHBL, Boston, Mass.) was obtained in a concentration of 1.52 mg protein per ml, was diluted 1:304 in PBS to a final concentration of 5.0 μg/ml, and was given to animals in a dose of 0.1 ml (0.5 μg) intraperitoneally.
Aerosol challenge.
Mice were removed from their cages, weighed, and placed on a stainless steel rack that fits inside of the Plexiglas aerosol chamber (40 by 40 by 40 cm). The 21-h culture of B. pertussis was suspended in sterile PBS to a concentration of approximately 2 × 109 CFU/ml of inoculum. This inoculum was delivered to the mice using a standard nebulizer (model 647; Devilbis, Somerset, Pa.) with a set pressure of 1.5 kg/cm2. The chamber and the nebulizer were enclosed in a biosafety level-2 hood and certified prior to use to document that airflow barriers were maintained. Uniformity of aerosol in the chamber was maintained with the use of two PABST 900 series AC fans (Newark Supply, Newark, N.J.). The even dispersion of the aerosol was confirmed with a light laser. Mice were exposed to nebulization for 30 min and removed 30 min after termination of aerosol. The completion of the aerosol represented time 0. Mice were removed from the box and replaced into their cages. Cages were checked daily for mortality. Serum was obtained from anesthetized mice by retro-orbital bleeding as previously described (5). Evidence of infection included weight loss, elevated WBC, and positive quantitative cultures of B. pertussis from lung tissue.
Statistical methods.
Data organization and analysis was performed on the PROPHET system, a national computer system sponsored by the National Center for Research Resources of the National Institutes of Health. For all antibody measurements, geometric means were calculated. Values below the lower limit of sensitivity of the assay were assigned a value of half the lower limit for purposes of taking logarithms. Comparisons of means or geometric means were performed by the two-sided t test for normally distributed values and by the Mann-Whitney test for nonnormally distributed values. A two-sided Fisher exact test was performed for comparisons of proportions.
RESULTS
Preliminary work for the establishment of the model are presented first, including the determination of the youngest age of mice that show evidence of priming, the determination of the optimal priming dose, and evidence of a priming dose effect. The final Results section will provide further evidence of priming and characterization of the primary response.
Establishment of priming age.
In order to determine the youngest age at which BALB/c mice can be primed with PTx, we first investigated effects of varying the age of the primary immunization. Mice were immunized subcutaneously with either the TNM-inactivated PTx vaccine described above or with AlPO4 placebo. In the first experiment mice were immunized with a dose of 2.5 μg PTx in a volume of 50 μl on day 5, 7, or 9 of life. The PTx immunized mice and AlPO4 controls were subsequently challenged on day 18 of life with a nonimmunogenic intraperitoneal dose (0.5 μg) of purified PT or saline. Anti-PT IgG antibodies and WBC counts were measured on days 18 and 25 of life (Table 1).
TABLE 1.
Effect of age on anti-PT IgG antibody response following primary immunization
| Age (days) of mice at immunization | Vaccine | Type of challenged | Levele of anti-PT IgG in serum (μg/ml) on day of life:
|
|
|---|---|---|---|---|
| 18a | 25b | |||
| 5 | PTxc | 0.5 μg of PT | 0.3 | 3.3 |
| PTx | Saline | 0.7 | 8.1 | |
| AlPO4 | 0.5 μg of PT | 0.3 | 0.5 | |
| 7 | PTx | 0.5 μg of PT | 0.6 | 5.4 |
| PTx | Saline | 0.4 | 6.4 | |
| AlPO4 | 0.5 μg of PT | 0.4 | 0.7 | |
| 9 | PTx | 0.5 μg of PT | 0.3 | 4.7 |
| PTx | Saline | 0.4 | 5.3 | |
| AlPO4 | 0.5 μg of PT | 0.2 | 0.4 | |
No statistical difference between any of the groups (P > 0.05).
Approximately 10-fold rise in antibody for all PTx-immunized groups (P < 0.05).
TNM-inactivated PTx vaccine.
On day 18 of life.
Geometric mean.
On the day of challenge (day 18 of life), the geometric mean anti-PT IgG antibody concentrations for PTx-immunized mice showed no statistical difference between any of the groups (P > 0.05) (Table 1). These values were also similar to those measured in AlPO4-immunized controls (P > 0.05) on the day of challenge. By day 25 of life, the geometric mean anti-PT IgG antibody concentrations in PTx recipients that were PT challenged on day 18 of life had risen nearly 10-fold, to 3.3, 5.4, and 4.7 μg/ml, respectively. This was a significant rise from values on day 18 of life in all groups (P < 0.05), and there was no statistical difference between any of the groups on day 25 of life (P > 0.05) (Table 1). AlPO4 immunized mice that were challenged with PT demonstrated no rise in anti-PT IgG antibody, thus confirming that the 0.5-μg PT dose was nonimmunogenic.
Establishment of priming dose.
After we determined that mice could be primed as early as 5 days of life, we investigated the dose response effects of PTx vaccine in order to determine if any level of priming as measured by anti-PT IgG antibody production correlated with protection. Mice were immunized subcutaneously with 2.5, 0.5, or 0.1 μg of PTx in a volume of 50 μl or AlPO4 in a volume of 50 μl on day 6 of life. All mice were then challenged on either day 18 or day 36 of life using the aerosol challenge model as described above. A nonchallenged control cohort was used to measure the anti-PT IgG antibody response over time. Anesthetized cohorts of mice were bled at various times for anti-PT IgG antibodies and WBC counts as previously described. Other cohorts of anesthetized mice were sacrificed and lungs were harvested for quantitative cultures and pathology as previously described.
The geometric mean anti-PT IgG antibody levels on day 18 of life in the immunized-unchallenged controls were 0.5, 0.6, and 0.3 μg/ml for recipients of the PTx doses of 2.5, 0.5, and 0.1 μg, respectively, and did not differ significantly from AlPO4 recipient levels of 0.4 μg/ml (P > 0.05). By day 39 of life, these levels had risen to 73.1, 3.1, and 0.5 μg/ml, respectively. Based on these results mice challenged with aerosolized B. pertussis on day 18 of life would not be expected to show much survival since none of the groups had detectable anti-PT antibodies and did not yet show evidence of a primary response. In fact we confirmed this by challenging a cohort of mice on day 18 of life. Survival at 14 days post-aerosol challenge was 0 of 16 for the 2.5-μg dose recipients, 0 of 13 for the 0.5-μg dose recipients, 0 of 15 for the 0.1-μg dose recipients, and 0 of 6 for AlPO4 recipients. Subsequently all challenges with aerosolized B. pertussis were conducted on day 36 of life. Because the aerosol challenge model is not as lethal in older mice, leukocytosis, weight loss, and quantitative lung cultures were used as outcome measures in these older mice.
Dose response of priming.
Mice were immunized on day 6 of life with the same doses of PTx and AlPO4 and were then challenged on day 36 of life. The characteristics of the different groups on the day of aerosol challenge (day 0) are shown in Table 2. Using a Fisher exact test to compare the ratios of females to males, there were no statistical differences among the groups (P > 0.05). Female recipients of the PTx dose of 0.1 μg were slightly larger than female recipients of the 2.5-μg dose (P = 0.04); otherwise, there were no statistical differences (P > 0.05) among the groups (Table 2). The primary response to PTx as shown by geometric mean anti-PT IgG antibody levels on day 0 (day of aerosol challenge) exhibits dose-dependent characteristics that are consistent with the values presented above for unchallenged mice. The difference between values was significant for all doses (P < 0.05) except between the 0.1-μg PTx dose and AlPO4, which was not statistically different (P > 0.05) (Table 3). The geometric mean anti-PT IgG antibody concentrations for recipients of PTx at doses of 2.5, 0.5, and 0.1 μg and AlPO4 had risen to 85.0, 7.8, 1.1, and 0.3 μg/ml, respectively by day 7 post-aerosol challenge and to 598.0, 139.3, 0.7, and 0.3 μg/ml, respectively, by day 14 (Table 3).
TABLE 2.
Characteristics of mice on the day of aerosol challenge
| Characteristicb | Value for group challenged with:
|
|||
|---|---|---|---|---|
| PTx
|
AlPO4 (n = 9) | |||
| 2.5 μg (n = 13) | 0.5 μg (n = 12) | 0.1 μg (n = 12) | ||
| Sex Ratio (female/male) | 8/5 | 5/7 | 8/4 | 7/2 |
| Mean wt ± SD (g) | ||||
| Females | 16.1 ± 0.3 | 16.8 ± 0.4 | 16.9 ± 0.2 | 15.4 ± 0.2 |
| Males | 19.4 ± 0.2 | 19.0 ± 0.3 | 18.8 ± 0.6 | 17.9 ± 0.4 |
| Mean WBC count ± SD (103 cells/mm3) | 5.9 ± 0.7 | 5.5 ± 0.5 | 5.2 ± 0.5 | 5.9 ± 0.4 |
| Anti-PT IgG titer (μg/ml) | 26.5 | 3.3 | 0.8 | 0.5 |
Female recipients of the PTx dose of 0.1 μg were slightly larger than female recipients of the PTx dose of 2.5 μg (P = 0.04); otherwise, there were no differences between any of the groups (P > 0.05).
Weights and WBC counts are presented as means ± standard deviations and antibody titers are presented as geometric means.
TABLE 3.
Anti-PT IgG antibody and total leukocyte response of mice after aerosol challenge with B. pertussis
| Challenge | Anti-PT IgG titera (μg/ml) on day:
|
Mean WBC count ± SD (103 cells/mm3) on day:
|
||||
|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 0b | 7c | 14c | |
| PTx dose (μg) | ||||||
| 2.5 (n = 13) | 26.5 | 85.0 | 598.0 | 5.9 ± 0.7 | 11.1 ± 2.1 | 17.5 ± 4.3 |
| 0.5 (n = 12) | 3.3 | 7.8 | 139.3 | 5.5 ± 0.5 | 24.5 ± 3.9 | 30.7 ± 7.5 |
| 0.1 (n = 12) | 0.8 | 1.1 | 0.7 | 5.2 ± 0.5 | 32.2 ± 3.8 | 171.1 ± 30.6 |
| AlPO4 (n = 9) | 0.5 | 0.3 | 0.3 | 5.9 ± 0.4 | 31.5 ± 5.2 | 135.7 ± 64.6 |
Anti-PT IgG titers (presented as geometric means) exhibited dose-dependent characteristics, and differences are significant for all groups and doses (P < 0.05) except between the recipients of the PTx dose of 0.1 μg and the AlPO4 recipients on days 0 and 14 (P > 0.05).
No statistical difference between any of the groups (P > 0.05)
Total WBC counts on days 7 and 14 for the recipients of the PTx dose of 2.5 μg were statistically different from those of lower-dose PTx recipients and AlPO4 recipients (P < 0.01).
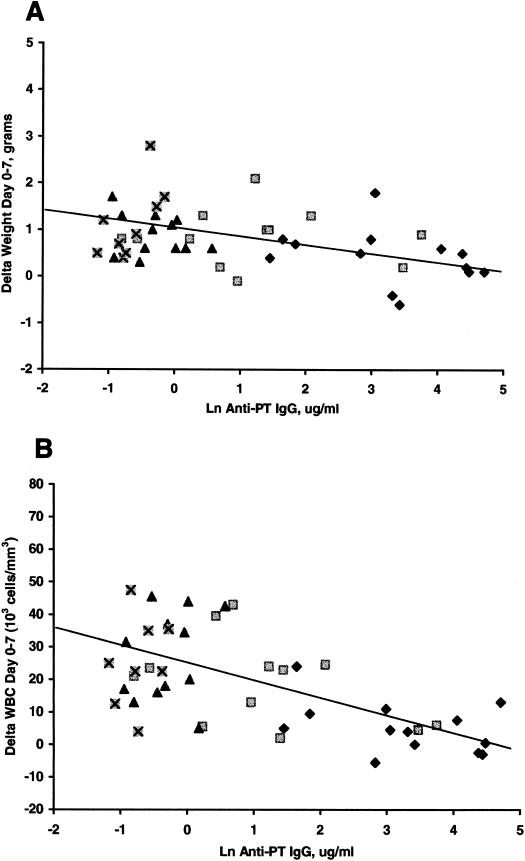
WBC counts rose following the aerosol challenge and the degree of rise was dependent on the PTx priming dose (Table 3). The WBC concentration on days 7 and 14 for recipients of the PTx dose of 2.5 μg were statistically lower than those of recipients of all other doses of PTx and AlPO4 (P < 0.05). The WBC for recipients of these lower doses of PTx and for AlPO4 were not significantly different. At 14 days postchallenge all groups continued to have rising WBCs; however, the rise for the recipients of the PTx dose of 2.5 μg was only modest. The values for recipients of the 2.5- and 0.5-μg PTx doses did not differ significantly (P > 0.05), and values for recipients of the 0.1-μg dose of PTx and AlPO4 also did not differ significantly (P > 0.05); however, the two higher-dose-recipient groups differed significantly from the two lower-dose-recipient groups (P < 0.05). The changes in WBCs and weights from day 0 to day 7 correlated highly for all PTx groups (P < 0.05) with the amount of anti-PT IgG antibody present on day 0 (Fig. 1).
FIG. 1.
Correlation of natural log of the anti-PT IgG antibodies on day 0 with change in weight from day 0 to 7 (A) (R = 0.37; P < 0.05) and change in WBC count from day 0 to 7 (B) (R = 0.6.; P < 0.05). ⊠, AlPO4; ▴, 0.1 μg of PTx; ▪, 0.5 μg of PTx; ⧫, 2.5 μg of PTx.
Quantitative cultures of B. pertussis from the lungs of mice were taken on days 7 and 14 post-aerosol challenge. These were inversely related to the level of anti-PT IgG antibody. On day 7 postchallenge, the mean numbers of CFU of B. pertussis per ml for all dose groups were similar (P > 0.05). By day 14 postchallenge, the higher-dose recipients of PTx (2.5 μg, 0.5 μg) showed a decrease in mean CFU of B. pertussis per ml, while the recipients of PTx at a dose of 0.1 μg or AlPO4 showed a proliferation of B. pertussis.
The mean weights for 7 days postchallenge for recipients of PTx doses of 2.5, 0.5, and 0.1 μg and AlPO4 were 16.5 ± 0.3, 17.8 ± 0.4, 17.7 ± 0.3, and 16.4 ± 0.2 g, respectively, for females and were 19.9 ± 0.5, 19.8 ± 0.3, 19.8 ± 0.7, and 19.5 ± 0.8 g, respectively, for males. The male and female recipients of the PTx dose of 2.5 μg and the male recipients of the PTx dose of 0.5 μg continued to gain weight throughout the 14 days postchallenge; however, all other groups began to manifest weight loss by day 14 post-aerosol challenge. By 7 days post-aerosol challenge the change in weight (day 0 to day 7) was highly correlated with the level of anti-PT IgG antibodies (R = 0.44; P < 0.05) (Fig. 1).
Evidence of priming.
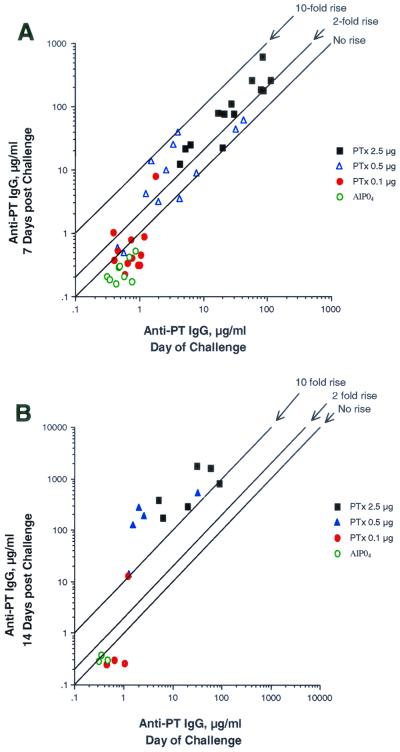
When levels of anti-PT antibodies at the time of aerosol challenge are examined more closely, there is a clear breakpoint between those animals that demonstrated a primary response (anti-PT antibody ≥1 μg/ml) and those animals that showed no evidence of a primary response (anti-PT antibody <1 μg/ml) (Table 4). The responders, who had ≥1 μg of anti-PT IgG antibody/ml on the day of aerosol challenge, were then able to mount a secondary response to the challenge, as evidenced by a ≥2-fold rise in anti-PT IgG antibody by day 7 (P < 0.05), and a ≥10-fold rise in anti-PT IgG antibody by day 14 (P < 0.05) post-aerosol challenge (Fig. 2).
TABLE 4.
Evidence of priming with PTx based on anti-PT antibody response (≥2-fold rise on day 7) following aerosol challenge (day 0)a
| Response group | WBC count (103 cells/mm3) on day:
|
Δ Wt (g) on days:
|
Lung bac- terial counts (106 CFU/ml) on day:
|
|||||
|---|---|---|---|---|---|---|---|---|
| 0b | 7 | 14 | 0-7 | 7-14 | 0-14 | 7b | 14 | |
| Nonrespondersd | 5.1 | 29.9 | 175.4 | 0.5 | −3.7 | −3.3 | 59.7 | 469.5 |
| Responderse | ||||||||
| All on day 7 | 5.2 | 14.3c | 23.0c | 0.6 | 0.9c | 1.5c | 36.1 | 0.8c |
| <2-fold rise on day 7 | 4.8 | 20.8 | 30.7 | 0.8 | −0.7 | 0.0 | 50.2 | 3.2 |
| ≥2-fold rise on day 7 | 5.5 | 11.8c | 20.1c | 0.6c | 2.0c | 2.5c | 36.5 | 0.3c |
| ≥10-fold rise on day 14 | 5.0 | 14.9c | 20.6c | 0.5 | 1.9c | 2.4c | NAf | 0.3c |
All values presented in geometric means.
No statistical difference between any of the groups (P >0.05).
Statistically different (P < 0.05) from nonprimed (anti-PT IgG <1 μg/ml) groups.
Day 0 anti-PT IgG titer, <1 μg/ml.
Day 0 anti-PT IgG titer, ≥1 μg/ml
NA, not available.
FIG. 2.
Increase in anti-PT IgG antibodies 7 days (A) and 14 days (B) post-aerosol challenge.
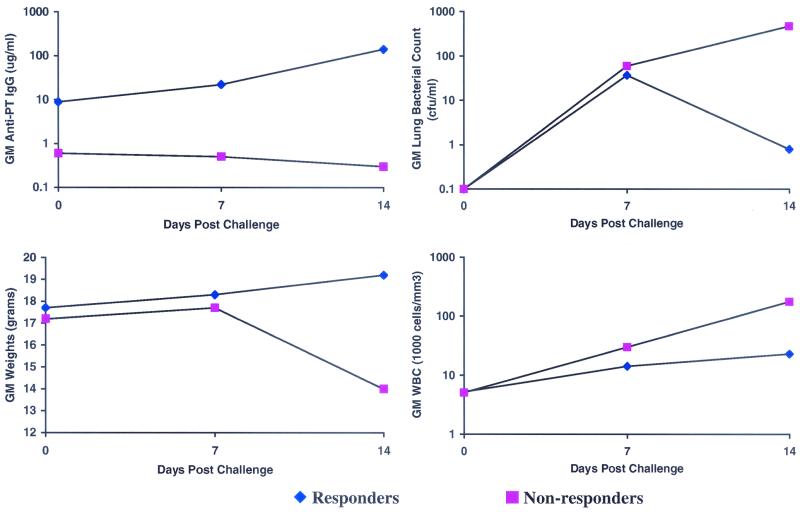
The primary responders (anti-PT IgG ≥ 1 μg/ml) were significantly better protected against leukocytosis, weight loss, and proliferation of B. pertussis in the lungs following aerosol challenge than the nonresponders (anti-PT IgG < 1 μg/ml) (Fig. 3). These responders also demonstrated a rise in anti-PT IgG antibodies from day 0 to day 14 postchallenge, whereas those characterized as nonresponders actually had a decline in anti-PT IgG antibodies (Fig. 3). Responders also showed significantly less weight loss, lower bacteria counts on lung culture, and near normal WBCs compared to nonresponders (Fig. 3).
FIG. 3.
Benefits of primary response after aerosol challenge with B. pertussis in mice (responders, anti-PT IgG ≥1 μg/ml; nonresponders, anti-PT IgG ≤ 1 μg/ml). All values are geometric means (GM).
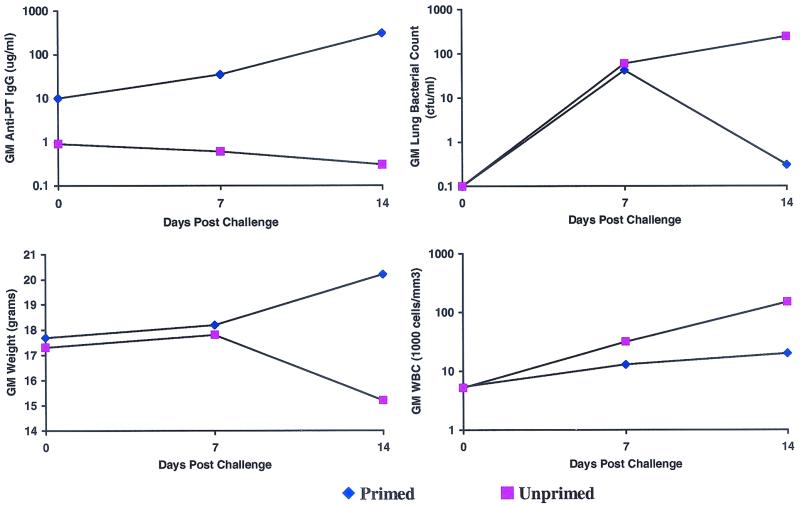
There was also a clear breakpoint between unprimed animals and those animals that demonstrated evidence of priming, defined as a ≥2-fold rise in anti-PT IgG by day 7 postchallenge and a ≥10-fold rise in anti-PT IgG antibody by day 14 postchallenge (Fig. 2). The primed animals showed a significantly greater increase in anti-PT IgG antibodies from day 0 to day 14 postchallenge, whereas those characterized as unprimed did not mount an anti-PT IgG antibody response after aerosol challenge (Fig. 4). Primed animals were significantly better protected against leukocytosis, weight loss, and proliferation of B. pertussis in the lungs following aerosol challenge than the nonprimed animals (Fig. 4). Primed mice showed significantly less weight loss, lower bacteria counts on lung culture, and normal WBCs compared to unprimed mice (Fig. 4).
FIG. 4.
Benefits of anamnestic response after aerosol challenge with B. pertussis in mice (primed, ≥2-fold rise in anti-PT IgG; unprimed, <2-fold rise in anti-PT IgG). All values are geometric means (GM).
DISCUSSION
We have demonstrated that mice immunized with TNM-inactivated PTx vaccine as early as 5 days of life were able to mount a primary immunologic response. We demonstrated that leukocytosis, weight loss, and proliferation of B. pertussis in the lung were blunted with immunogenic doses of PTx prior to aerosol challenge with virulent B. pertussis. This protection correlated with serum levels of anti-PT antibody that were present on the day of aerosol challenge. The higher the anti-PT IgG level was on the day of aerosol challenge, the less likely the mice were to develop leukocytosis or lose weight. In fact the animals with high antibody levels actually continue to gain weight.
These findings were consistent with those of other investigators who have studied the active anti-PT antibody response in the aerosol challenge model (17, 19, 29, 31, 36). Oda et al. showed that mice immunized with glutaraldehyde-inactivated PTx at 5 days of life and then given boosters at 12 days of life were protected, as measured by weight loss, leukocytosis, and mortality, from aerosol challenge with B. pertussis at 20 days of life (26). Although they mentioned that there was very little antibody 1 week after the first dose, the mice did mount an anti-PT antibody response in a dose-dependent fashion. The correlation between anti-PT antibody and protection was not thoroughly characterized. Shahin et al. alluded to a priming effect in mice immunized at 5 days of life with glutaraldehyde-inactivated B-oligomer of PT and given boosters at 12 days of life, followed by aerosol challenge, by showing that immunized mice mounted a >30-fold rise in anti-PT IgG 7 days following aerosol challenge (42). This was not further characterized or compared to results for uninfected, unimmunized mice. Our report is the first to describe the effects of immunologic priming in mice of this age using the aerosol challenge model. Similar to the findings of Shahin et al., there appears to be a delay in the priming response (42). We have demonstrated that priming takes between 18 and 36 days to develop but, once established, exhibits dose-dependent characteristics.
Pittman has hypothesized that the systemic manifestations of pertussis are mediated by PT (29); however, the mechanism by which PT might cause paroxysmal coughing or the correlates of PT-induced immunity have not been elucidated. Although it has been widely believed that immunity to pertussis is primarily dependant on a humoral response, there has been work suggesting cell-mediated immunity plays an important role in protection against pertussis both in humans and animals (3, 7, 8, 20, 21, 23, 30, 33). This cell-mediated response has also been shown to be important in conferring immunity after active immunization with both whole-cell and acellular pertussis vaccines (23, 30, 34, 35). This cell-mediated immunity in mice infected with B. pertussis is characterized by the induction of a T-cell-mediated response. There is some evidence that pertussis infection and whole-cell vaccines both induce a CD4+ Th1 response, whereas acellular pertussis vaccines induce a response more characteristic of CD4+ Th2 (4, 30, 34, 35).
In a recent study by Mills et al. (24), it was demonstrated that cell-mediated immunity and PT-IgG antibody response play a complementary role in conferring immunity in the aerosol challenge model. They compared three whole-cell and five acellular pertussis vaccines and demonstrated a high correlation between clinical vaccine efficacy in children and B. pertussis clearance from the lungs of immunized mice using the aerosol challenge model. Despite this correlation, the precise mechanisms for immunity need to be further investigated. In this study we have provided further evidence for the important role of anti-PT IgG antibodies in the immune response but do not exclude the role of cell-mediated immunity.
We have demonstrated that priming correlates to a measurable amount of anti-PT IgG antibody (≥1 μg/ml) present at the time of aerosol challenge. Mice that had evidence of priming were able to mount an anamnestic response, as measured by a ≥2-fold rise in anti-PT antibodies 7 days following aerosol challenge and a ≥10-fold rise by 14 days postchallenge. Animals that showed evidence of priming were significantly better protected, as measured by leukocytosis, weight loss, and quantitative lung culture, than were animals that exhibited no evidence of priming. In both human vaccine trials and several different animal models, no one to date has been able to show a protective level of anti-PT antibody. Although we have not shown that a specific level of anti-PT antibody is protective in the aerosol challenge model, we have shown that there is a measurable priming level (≥1 μg/ml) that seems to confer protection from the adverse effects of respiratory infection with B. pertussis. This priming effect in the aerosol challenge model may have great value for evaluating potency and efficacy of vaccines that contain PTx, while not having some of the limitations of the intracerebral challenge model. This model might also be valuable in testing the priming effects of other pertussis vaccine preparations, including diphtheria and tetanus toxoids with acellular pertussis vaccines.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health.
We are grateful to Claudette Thompson for her endless assistance as laboratory manager. We are grateful to the Massachusetts Public Health Biologic Laboratories for providing vaccine and toxins.
Editor: R. N. Moore
REFERENCES
- 1.Ad Hoc Group for the Study of Pertussis Vaccines. 1988. Placebo-controlled trial of two acellular pertussis vaccines in Sweden—protective efficacy and adverse events. Lancet i:955-960. [PubMed] [Google Scholar]
- 2.Ad Hoc Group for the Study of Pertussis Vaccines. 1997. A randomized controlled trial of a two-component, a three component and a five-component acellular pertussis vaccine and a British whole-cell pertussis vaccine. Lancet 350:1569-1577. [DOI] [PubMed] [Google Scholar]
- 3.Ausiello, C. M., F. Urbani, A. La Sala, R. Lande, and A. Cassone. 1997. Vaccine- and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines. Infect. Immun. 65:2168-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnard, A., B. P. Mahon, J. Watkins, K. Redhead, and K. H. G. Mills. 1996. Th1/Th2 cell dichotomy in acquired immunity to Bordetella pertussis: variables in the in vivo priming and in vitro cytokine detection techniques affect the classification of T cell subsets as Th1, Th2 or Th0. Immunology 87:372-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bivin, W. S., and G. D. Smith. 1984. Techniques of experimentation, p. 565-566. In J. G. Fox, B. J. Cohen, and F. M. Loew (ed.), Laboratory animal medicine. Academic Press, Inc., New York, N.Y.
- 6.Blackwelder, W. C. 1990. Vaccine efficacy for a variety of clinical case definitions. Food and Drug Administration publication 260. U.S. Food and Drug Administration, Bethesda, Md.
- 7.Bromberg, K., G. Tannis, and P. Steiner. 1991. Detection of Bordetella pertussis associated with the alveolar macrophages of children with human immunodeficiency virus infection. Infect. Immun. 59:4715-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassone, A., C. M. Ausiello, F. Urbani, R. Lande, and S. Giuliano for the Progetto Pertosse-CMI Working Group. 1997. Cell-mediated and antibody responses to Bordetella pertussis antigens in children vaccinated with acellular or whole-cell pertussis vaccines. Arch. Pediatr. Adolesc. Med. 151:283-289. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 1989. Summary of notifiable diseases, United States. Morb. Mortal. Wkly. Rep. 38:53-59. [Google Scholar]
- 10.Centers for Disease Control and Prevention. 1990. Pertussis surveillance—United States, 1986-1988. Morb. Mortal. Wkly. Rep. 39:57-66. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 1992. Pertussis surveillance—United States, 1989-1991. Morb. Mortal. Wkly. Rep. 41:11-19. [Google Scholar]
- 12.Centers for Disease Control and Prevention. 1993. Resurgence of pertussis—United States, 1993. Morb. Mortal. Wkly. Rep. 42:952-960. [PubMed] [Google Scholar]
- 13.Cherry, J. D., P. A. Brunnell, G. S. Golden, and D. T. Karzon. 1988. Report of the task force on pertussis and pertussis immunization 1988. Pediatrics 81(Suppl.):939-984. [Google Scholar]
- 14.Dellepiane, N. I., M. A. Manghi, P. V. Eriksson, G. Di Paola, and A. Cangelosi. 1992. Pertussis whole cell vaccines: relation between intracerebral protection in mice and antibody response to pertussis, filamentous hemagglutinin and adenylate cyclase. Zbl. Bakteriol 277:65-73. [DOI] [PubMed] [Google Scholar]
- 15.Edwards, K. M., B. D. Meade, M. D. Decker, G. F. Reed, M. B. Rennels, M. C. Steinhoff, E. L. Anderson, J. A. Enguland, M. E. Pichichero, and M. A. Deloria. 1995. Comparison of thirteen acellular pertussis vaccines: overview and serological response. Pediatrics 96:548-557. [PubMed] [Google Scholar]
- 16.Greco, D., S. Salmaso, P. Mastrantonio, M. Guiliano, A. G. Tozzi, A. Anemona, M. L. Giori Delgi Atti, A. Giammanco, P. Panel, W. C. Blackwelder, D. L. Klein, S. G. F. Wassilak, and the Progetto Pertosase Working Group. 1996. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. N. Engl. J. Med. 334:341-348. [DOI] [PubMed] [Google Scholar]
- 17.Gupta, R. K., S. N. Saxena, B. S. Suniti, and S. Ahuja. 1988. The effects of purified pertussis components and lipopolysaccharide on the results of the mouse weight gain test. J. Biol. Stand. 16:321-331. [DOI] [PubMed] [Google Scholar]
- 18.Gustafsson, L., H. O. Hallander, P. Olin, E. Reizenstein, and J. Storsaeter. 1996. A controlled trial of a two-component acellular, a five component acellular, and a whole cell pertussis vaccine. N. Engl. J. Med. 334:349-355. [DOI] [PubMed] [Google Scholar]
- 19.Halperin, S. A., S. A. Heifetz, and A. Kasina. 1988. Experimental respiratory infection with Bordetella pertussis in mice: comparison of two methods. Clin. Investig. Med. 11:297-303. [PubMed] [Google Scholar]
- 20.Mahon, B. P., M. Ryan, F. Griffin, and K. H. G. Mills. 1996. Interleukin-12 is produced by macrophages in response to live or killed Bordetella pertussis and enhances the efficacy of an acellular pertussis vaccine by promoting induction of Th1 cells. Infect. Immun. 64:5295-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahon, B. P., F. Griffin, B. Sheahan, and K. H. G. Mills. 1997. Atypical disease following Bordetella pertussis respiratory infection of mice with targeted disruptions in IFN-receptor or immunoglobulin μ chain genes. J. Exp. Med. 186:1843-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller, E., L. A. E. Ashworth, K. Redhead, C. Thornton, P. A. Waight, and T. Coleman. 1997. Effect of schedule on reactogenicity and antibody persistence of acellular and whole-cell pertussis vaccines: value of laboratory tests as predictors of clinical performance. Vaccine 15:51-60. [DOI] [PubMed] [Google Scholar]
- 23.Mills, K. H. G., A. Barnard, J. Watkins, and K. Redhead. 1993. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect. Immun. 61:399-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mills, K. H. G., A. Barnard, J. Watkins, and K. Redhead. 1998. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infect. Immun. 66:594-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munoz, J. J., and M. G. Peacock. 1989. Role of pertussigen (pertussis toxin) on the mouse protective activity of vaccines made from Bordetella species. Microbiol. Immun. 33:341-355. [DOI] [PubMed] [Google Scholar]
- 26.Oda, M., J. L. Cowell, and D. G. Burstyn. 1984. Protective activities of the filamentous hemagglutinin and the lymphocytosis-promoting factor of Bordetella pertussis in mice. J. Infect. Dis. 150:6823-6833. [DOI] [PubMed] [Google Scholar]
- 27.Olson, L. C. 1975. Pertussis. Medicine 54:427-469. [DOI] [PubMed] [Google Scholar]
- 28.Pittman, M., B. L. Furman, and A. C. Wardlaw. 1980. Bordetella pertussis respiratory tract infection in the mouse: pathophysiological responses. J. Infect. Dis. 142:56-66. [DOI] [PubMed] [Google Scholar]
- 29.Pittman, M. 1984. The concept of pertussis as a toxin-mediated disease. Pediatr. Infect. Dis. J. 3:467-486. [DOI] [PubMed] [Google Scholar]
- 30.Redhead, K., A. Barnard, J. Watkins, and K. H. G. Mills. 1993. Effective immunization against Bordetella pertussis respiratory infection in mice is dependent on the induction of cell mediated immunity. Infect. Immun. 61:3190-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson, A., A. Gorringe, and M. Fernandez. 1989. Serospecific protection of mice against intranasal infection with Bordetella pertussis. Vaccine 7:321-324. [DOI] [PubMed] [Google Scholar]
- 32.Robinson, A., and L. I. Irons. 1983. Synergistic effect of Bordetella pertussis lymphocytosis factor on protective activities of isolated Bordetella antigens in mice. Infect. Immun. 40:523-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan, M., G. Murphy, L. Gothefors, L. Nilsson, J. Storsaeter, and K. H. G. Mills. 1997. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 Th cells. J. Infect. Dis. 175:1246-1250. [DOI] [PubMed] [Google Scholar]
- 34.Ryan, M., L. Gothefors, J. Storsaeter, and K. H. G. Mills. 1997. B. pertussis-specific Th1/Th2 cells generated following respiratory infection or immunization with an acellular vaccine: comparison of the T cell cytokine profiles in infants and mice. Dev. Biol. Stand. 89:251-259. [PubMed] [Google Scholar]
- 35.Ryan, M., G. Murphy, L. Nilsson, F. Shackley, L. Gothefors, K. Ømar, E. Miller, J. Storsaeter, and K. H. G. Mills. 1998. Distinct Th cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology 93:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato, Y., K. Izumiya, H. Sato, J. L. Cowell, and C. R. Manclark. 1980. Aerosol infection of mice with Bordetella pertussis. Infect. Immun. 29:261-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato, Y., I. Kazumi, H. Sato, J. L. Cowell, and C. R. Manclark. 1985. Role of antibody to filamentous hemagglutinin and to leukocytosis promoting factor-hemagglutinin in immunity to pertussis. Semin. Infect. Dis. 4:380-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato, H., and Y. Sato. 1984. Bordetella pertussis infection in mice: correlation of specific antibodies against two antigens, pertussis toxin, and filamentous hemagglutinin with mouse protectivity in an intracerebral or aerosol challenge system. Infect. Immun. 46:415-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato, Y. 1981. Role of antibody to leukocytosis-promoting factor, hemagglutinin and to filamentous hemagglutinin in immunity to pertussis. Infect. Immun. 1223-1231. [DOI] [PMC free article] [PubMed]
- 40.Sato, H., and Y. Sato. 1988. Animal models of pertussis, p. 309-325. In Alastair C. Wardlaw and Roger Parton (ed.), Pathogenesis and immunity in pertussis. John Wiley & Sons Ltd., New York, N.Y.
- 41.Shahin, R. D., M. J. Brennan, Z. M. Li, B. D. Meade, and C. R. Manclark. 1990. Characterization of the protective capacity and immunogenicity of the 69-kD outer membrane protein of Bordetella pertussis. J. Exp. Med. 171:63-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shahin, R. D., M. H. Witvliet, and C. R. Manclark. 1990. Mechanism of pertussis toxin B oligomer-mediated protection against Bordetella pertussis respiratory infection. Infect. Immun. 58:4063-4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siber, G. R., N. Thakrar, and B. A. Yancey. 1991. Safety and immunogenicity of hydrogen peroxide-inactivated pertussis toxoid in 18-month-old children. Vaccine 9:735-740. [DOI] [PubMed] [Google Scholar]
- 44.Simondon, F., M.-P. Preziosi, A. Yam, C. T. Kane, L. Chabirand, I. Iteman, G. Sanden, S. Mboup, A. Hoffenbach, K. Knudsen, N. Guiso, S. Wassilak, and M. Cadoz. 1997. A randomized double blind trial comparing a two-component acellular to a whole-cell pertussis vaccine in Senegal. Vaccine 15:1606-1612. [DOI] [PubMed] [Google Scholar]
- 45.Standfast, A. F. B. 1958. The comparison between field trials and mouse protection tests against intranasal and intracerebral challenges with Bordetella pertussis. Immunology 2:135. [PMC free article] [PubMed] [Google Scholar]
- 46.Trollofors, B., J. Taranger, T. Lagergard, L. Lind, V. Sundh, G. Zackrisson, C. U. Lowe, W. Blackwelder, and J. B. Robbins. 1995. A placebo-controlled trial of a pertussis-toxoid vaccine. N. Engl. J. Med. 333:1045-1050. [DOI] [PubMed] [Google Scholar]
- 47.Zackrisson, G., J. Taranger, and B. Trollfors. 1990. History of whooping cough in nonvaccinated Swedish children, related to serum antibodies to pertussis toxin and filamentous hemagglutinin. J. Pediatr. 116:190-194. [DOI] [PubMed] [Google Scholar]
- 48.Zollinger, W. D., and J. W. Boslego. 1981. A general approach to standardization of the solid-phase radioimmunoassay for quantitation of class-specific antibodies. J. Immunol. Methods 46:129-140. [DOI] [PubMed] [Google Scholar]