Abstract
Fusobacterium necrophorum, a gram-negative, rod-shaped, anaerobic bacterium, is a primary or secondary etiological agent in a variety of necrotic, purulent infections in humans and animals. Its major virulence factor is leukotoxin, a high-molecular-weight secreted protein, primarily toxic to ruminant leukocytes. In this study, bovine peripheral blood leukocytes were exposed to various concentrations of immunoaffinity-purified leukotoxin and the cytotoxicity was analyzed by flow cytometry and scanning and transmission electron microscopy. At very low toxin concentrations, polymorphonuclear leukocytes (PMNs) showed activation, as indicated by translocation of primary and secondary granules to the periphery of the cytoplasm. Furthermore, these cells showed changes characteristic of apoptosis, including decreased cell size, organelle condensation, cytoplasmic membrane blebbing (zeiosis), and chromatin condensation and margination, and decrease in cellular DNA content. At moderately high concentrations of leukotoxin, bovine mononuclear cells were also induced to undergo programmed cell death. At very high concentrations, leukotoxin caused necrotic cell death of bovine peripheral leukocytes. The ability of F. necrophorum leukotoxin to modulate the host immune system by its toxicity, including cellular activation of PMNs and apoptosis-mediated killing of phagocytes and immune effector cells, represents a potentially important mechanism of its pathogenesis.
Fusobacterium necrophorum is a gram-negative, obligately anaerobic, and pleomorphically rod-shaped bacterium. The organism is implicated as an etiological agent in a variety of necrotic diseases, such as Lemierre's syndrome in humans (11, 26) and hepatic abscesses, foot rot, and necrotic laryngitis (calf diphtheria) in cattle (22, 27, 28, 43). F. necrophorum is classified into two subspecies: F. necrophorum subsp. necrophorum and F. necrophorum subsp. funduliforme, formerly biotypes A and B, respectively (22, 38). F. necrophorum subsp. necrophorum is more virulent and is isolated more frequently from infections than F. necrophorum subsp. funduliforme (27, 28).
The bacterium produces many virulence factors (8, 28) including a potent, high-molecular-weight (336,000) leukotoxin specifically toxic to ruminant polymorphonuclear leukocytes (PMNs) (45). The importance of leukotoxin as a virulence factor is evidenced by the correlation between toxin production and the ability of F. necrophorum to induce abscesses in laboratory animals (7) and an inability of non-leukotoxin-producing strains to induce foot abscesses in cattle following intradermal inoculation (10). Furthermore, experimental challenge studies to induce liver abscesses in cattle vaccinated with leukotoxoid have established a relationship between neutralizing antileukotoxin antibody titers and protection against infection (32-34).
Biological effects of leukotoxins secreted by Mannheimia (Pasteurella) haemolytica and Actinobacillus actinomycetemcomitans (both repeats-in-toxins [RTX]-containing glycine-rich repeats) and Staphylococcus aureus have been characterized (3, 5, 6, 12, 23, 24). Apoptosis has been reported in target cells exposed to leukotoxins from P. haemolytica and A. actinomycetemcomitans (20, 21, 40, 42, 47). The nucleotide sequence encoding F. necrophorum leukotoxin and the deduced amino acid sequence suggested that the leukotoxin is a novel protein unrelated to any known leukotoxins or other bacterial exotoxins (29). Therefore, the mode of action for F. necrophorum leukotoxin is of interest. Our earlier studies utilizing F. necrophorum leukotoxin and bovine PMNs indicated that leukotoxin causes a dose-dependent decrease in the tetrazolium-reducing capacity of these cells (44). This functional impairment of the target cell cytochrome oxidase system detected in the MTT (3-[4,5-dimethylthiazoyl-2-yl]2,5-diphenylterazolium bromide) dye reduction assay was associated with a decrease in the number of cells excluding trypan blue (16, 35) and an increase in 51Cr released from target cells (9). Studies on target cell specificity showed that F. necrophorum leukotoxin is highly toxic to bovine and ovine PMNs, moderately toxic to horse PMNs, and nontoxic to swine and rabbit PMNs (45). However, the mechanism by which leukotoxin exerts its lethal effects on target cells and the sequence of events in the overall toxicity are not known.
The focus of the present study was to characterize the biological effects of F. necrophorum leukotoxin on bovine peripheral leukocytes. We utilized flow-cytometric and electron microscopy techniques to evaluate changes induced in the target cells exposed to immunoaffinity-purified leukotoxin of F. necrophorum.
MATERIALS AND METHODS
Preparation of leukocytes.
Bovine peripheral leukocytes were prepared from blood collected from the jugular vein (44). The leukocytes were suspended in RPMI medium (pH 7.2), and concentrations of viable cells were determined by the trypan blue exclusion method (44). The percentages of viable cells ranged from 95 to 98% during different sampling periods. Fresh cells were prepared for use each day, as the percentage of dead cells increased when cells were stored for over 8 h.
Preparation of F. necrophorum leukotoxin.
F. necrophorum subsp. necrophorum strain A25 was grown to log phase (7 h or optical density at 600 nm [OD600] of 0.6) in prereduced, anaerobically sterilized brain heart infusion broth (44). Cells were removed by centrifugation and filtration through a 0.2-μm-pore-size filter (Millipore Corp., Bedford, Mass.). The supernatant was concentrated 60-fold with Ultrafree-Biomax 100 filters (Millipore Corp.) to concentrate molecules over 100 kDa. Affinity purification of leukotoxin was carried out with monoclonal antibody F7B10 (46) in an Affigel Hz column (Bio-Rad Corp. Carlsbad, Calif). Purified leukotoxin was standardized for its activity by an MTT dye reduction assay with bovine PMNs as the target cells (44). The leukotoxin unit was defined as the reciprocal of the sample dilution causing a 10% decrease in MTT dye reduction activity. The affinity-purified leukotoxin had a final concentration of 2 × 105 U/ml.
Leukotoxin treatment of target cells.
Peripheral bovine leukocytes in complete RPMI medium were exposed to various concentrations of affinity-purified leukotoxin (0.0005 to 200,000 U/ml) for 45 min at 37°C in a humidified environment containing 5% CO2. Cells were removed from the medium by centrifugation at 500 × g for 10 min and resuspended in complete RPMI medium or washed with buffered salt solutions (phosphate-buffered saline [PBS] or Hanks' balanced salt solution [HBSS]). Toxin-treated cells that had aggregated were treated with DNase I (Sigma Chemical Corp., St. Louis, Mo.; final concentration in PBS, 100 μg/ml) for 30 min at 37°C in a water bath in an attempt to disperse the cells. Treated cells were washed twice and resuspended in sterile PBS.
Flow cytometry. (i) Immunophenotyping.
Bovine peripheral leukocytes were phenotyped by the procedure of Sun et al. (42). Monoclonal antibodies (2.5 μg/ml) against various leukocyte surface receptors (CD3, CD4, CD8, GM1, and immunoglobulin M [IgM]; VMRD Inc., Pullman, Wash.) were utilized. The secondary antibody was fluorescein isothiocyanate-conjugated goat anti-mouse IgG F(ab′)2. Samples were processed on a FACScan flow cytometer using an argon laser (Becton Dickinson, San Jose, Calif.). Data were analyzed by using Cell Quest analysis software (Becton Dickinson).
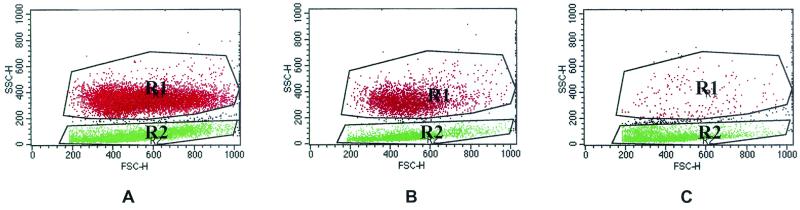
Unlabeled cells consisted of two distinct populations based on light scatter properties (Fig. 1). The populations were gated according to size based on forward scatter (FSC) and according to granularity based on 90o side scatter (SSC). The upper region (R1) consisted of granulocytes, and the lower region (R2) contained mononuclear cells (Fig. 1A). For each gated population, the percentages of cells labeled with the leukocyte differentiation monoclonal antibodies were determined by detection of green fluorescence signals with a 530 ± 15-nm band-pass filter.
FIG. 1.
Flow cytometry scatter plot analysis of untreated peripheral blood leukocytes (A) and leukocytes treated with 20 (B) or 200 U of leukotoxin/ml (C). The upper gated region (R1) represents granulocytes, whereas the lower gated region (R2) includes PBMC. FSC-H (x axis), fluorescence channels for FSC of cells (proportional to size); SSC-H (y axis), fluorescence channels for orthogonal light scatter of cells (proportional to granularity).
Alterations in size and granularity of leukocytes induced by exposure to leukotoxin were determined by recording the maximum values of the fluorescence channels imparted by cells in FSC and SSC parameters.
(ii) Assessment of cell viability.
One milliliter of bovine peripheral blood PMNs (9 × 106 cells/ml) was incubated with various concentrations of affinity-purified leukotoxin (0.02 to 2,000 U/ml) for 45 min at 37°C in a chamber containing 5% CO2. The cells were then washed twice in 2 ml of HBSS (pH 7.2) and resuspended in 200 μl of HBSS. These cells were treated for 10 min in the dark at room temperature with 10 μl of 5-μg/ml propidium iodide (PI; Sigma Chemical Co.). The proportion of cells stained with PI was determined by detection of red fluorescence signals with a 585 ± 21-nm band-pass filter (41). Percentages of positive cells were determined by quadrant statistics.
(iii) Simultaneous phagocytosis and oxidative burst activity assays.
Heat-killed S. aureus (Pansorbin; Calbiochem-Novabiochem Corp., La Jolla, Calif.) was labeled with PI as described previously (39). Briefly, 50 μl of Pansorbin was mixed with 1 ml of PI solution (100 μg/ml in a 100 mM carbonate buffer, pH 9.6) and incubated at 4°C in the dark for 24 h. The PI-stained S. aureus was pelleted by centrifugation at 13,000 × g for 30 s and washed twice by centrifugation and resuspension in HBSS. The PI-stained S. aureus was opsonized by incubation with 40% heat-inactivated pooled cattle serum for 30 min in the dark at 37°C. The opsonized PI-stained S. aureus was centrifuged and washed twice and resuspended in cold HBSS. One milliliter of toxin-treated or untreated peripheral blood leukocytes in HBSS (9 × 106 cells/ml) was incubated for 15 min with 50 μl of PI-stained S. aureus in the dark at 37°C (final ratio of bacterial cells to PMNs was 25:1). Cells were then incubated with 100 μl of dihydrorhodamine 123 (DHR; final concentration of 72.5 μM; Molecular Probes Inc., Eugene, Oreg.) for 15 min at 37°C. The nonfluorescent dye DHR is oxidized to a green fluorescent product by hydrogen peroxide produced when phagocytized bacteria stimulate NADPH oxidase (1, 2). Simultaneous phagocytosis and oxidative burst activities were determined by two-color (bivariate) flow-cytometric analysis. The number of cells phagocytosing PI-stained S. aureus was proportional to the FL-2 (585 ± 42-nm) shift, whereas the shift in FL-1 (530 ± 30 nm) was proportional to hydrogen peroxide production. Trypan blue was added to each tube before flow-cytometric analysis to quench the fluorescence of extracellular or adherent bacteria. Controls included untreated cells, cells treated with PI-stained S. aureus alone, and those treated with DHR alone.
(iv) Evaluation of cellular DNA content.
Routine assays for determining apoptotic cells such as annexin V assay and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling did not provide reliable results due to the inadequacy of the commercially available reagents for use with bovine cells, For example, we observed that 90% of untreated bovine peripheral leukocytes bound the annexin V reagent. Therefore, we opted to use flow cytometry to compare the DNA contents of untreated and toxin-treated leukocytes (13, 36). One milliliter aliquots of peripheral bovine neutrophils (6,000,000 cells/ml) in complete RPMI medium were incubated with various concentrations of affinity-purified native leukotoxin at 37°C for 45 min in a chamber containing 5% CO2. Cells were washed twice in PBS and resuspended in 1 ml of medium containing RPMI medium and fetal calf serum (1:1 [vol/vol]). Three milliliters of ice-cold ethanol was added drop-wise to achieve a final concentration of approximately 50% ethanol, and the tubes were incubated at 4°C for 30 min. Ethanol-fixed cells were centrifuged at 400 × g for 5 min and washed in PBS twice before resuspension in 200 μl of PBS. Twenty microliters of RNase solution (5 mg/ml; Sigma) and 200 μl of PI solution (10 μg/ml) were added, and the cells were incubated in the dark at 4°C for 30 min. The cells were analyzed for DNA content and the presence of hypodiploid nucleated cells in a FACScan flow cytometer (Becton Dickinson) after gating for R1 and R2 regions as described above.
All flow cytometry experiments were performed on two different days with duplicates on each day (n = 4), and results were expressed as means ± standard deviations.
Scanning electron microscopy (SEM).
One milliliter of bovine peripheral leukocytes (initial concentration of 6 × 106/ml), untreated or treated with various concentrations of F. necrophorum leukotoxin, was fixed overnight at 4°C in 20 volumes of modified Karnowsky's fixative (2% paraformaldehyde, 2.5% glutaraldehyde, 1.7 mM CaCl2 in 0.1 M cacodylate buffer [pH 7.4]). After being fixed, the cells were washed twice in 0.1 M cacodylate buffer (pH 7.4) and postfixed in an osmium solution (1% osmium tetroxide in 0.1 M cacodylate buffer) overnight at 4°C. After being washed twice in double-distilled water, the cells were resuspended in double-distilled water and mounted on poly-l-lysine coverslips and dehydrated in ascending grades of alcohol (30, 50, 70, 95, and 100%). Dehydrated samples were treated for 5 min with hexamethyldisilazane (Electron Microscopy Supplies, Ft. Washington, Pa), dried, mounted, and sputtered with gold (S150A sputter coater). Samples were viewed in a Hitachi H-300 electron microscope with a 3010 scanning image accessory.
Transmission electron microscopy (TEM).
Toxin-treated and control cells were pelleted and fixed in modified Karnowsky's fixative, washed, and postfixed in 1% osmium tetroxide as described above. After three 5-min washes in cold double-distilled water, the cell pellets were immobilized in 2% Trypticase soy agar and dehydrated in a series of solutions with increasing strength of ethanol (50, 70, 70, 95, and 100%). Dehydrated specimens were stained for 60 min en bloc with 5% uranyl acetate in 70% ethanol, and the dehydration was completed by two 20-min rinses in 100% acetone, which was used as the transitional solvent. The cell blocks were then infiltrated with three increasing concentrations of resin in acetone ending in 100% resin for periods of 2 to 12 h, 2 to 12 h, and 8 to 12 h, respectively. All tissues were then embedded in Epon LX112 embedding medium at 42°C for 18 to 20 h followed by 60°C for 24 h. Embedded tissues were trimmed and sectioned on an Ultracut E-Reichert-Jung ultramicrotome (C. Reichert Optische Werke AG, Vienna, Austria). Silver and gold thin sections (approximately 80 μm thick) were retrieved with copper grids and stained with uranyl acetate and lead citrate. Grids were examined and viewed with a Hitachi H-300 electron microscope.
LDH assay.
A suspension of 4.5 × 106 peripheral leukocytes/ml in 200 μl of complete RPMI medium was prepared as described above. The suspension was exposed to dilutions of F. necrophorum leukotoxin in 100 μl of PBS for 45 min at 37°C in a chamber containing 5% CO2. After incubation with or without toxin, the cells were centrifuged at 5,000 × g for 10 min and the supernatant was assayed for lactate dehydrogenase (LDH) with an LDH kit (Sigma). The LDH in the extracellular fluid that leaked from the cytoplasm of the toxin-treated cells was compared to that of untreated cells and cells treated with 0.1% Triton X-100, used as negative and positive controls, respectively, and results were expressed as percent activities. Percent LDH activity at a particular toxin concentration was calculated as [1 − (OD525 for supernatant from toxin-treated cells − OD525 for supernatant from positive control cells)/(OD525 for supernatant from untreated cells − OD525 for supernatant from positive control cells)] × 100. Assays were done on two different days with duplicates on each day (n = 4).
RESULTS
Immunophenotyping.
Bovine peripheral blood leukocyte preparations consisted of 31% granulocytes and 69% mononuclear cells (PBMC). Regions were placed around the granulocytes (upper region) and PBMC (lower region, containing lymphocytes [84%] and monocytes [12%]). Among the lymphocyte population, approximately 87% of the cells were CD3+, 46% were CD4+, 27% were CD8+, and 12% were IgM+.
Evaluation of FSC and SSC.
FSC (axial) and SSC (orthogonal) properties of granulocytes and mononuclear cells were monitored following treatment with various concentrations of affinity-purified leukotoxin (Fig. 1). Granulocytes showed a decrease in cell size even at very low concentrations of toxin (0.02 U/ml), although there was no significant increase in granularity (Tables 1 and 2). Toxin-treated granulocytes decreased from 1,000 to 640 FSC channels. Mononuclear cells showed a dose-dependent decrease in cell size and increase in granularity at higher concentrations of toxin (0.2 to 200 U/ml). For example, at 200 U of toxin/ml, 90% of the mononuclear cells decreased from 800 to 600 FSC channels (Fig. 1C) and the mean number of SSC channels increased from 80 to 160 (Tables 1 and 2). At 625 U of toxin/ml, very few granulocytic cells were present. These cells reacted with the anti-CD3 antibody and not with the anti-GM1 antibody, suggesting that the cells were lymphocytes and not granulocytes or macrophages. Apoptosis was suggested by the reduction in cell size (of granulocytes at low toxin concentrations and mononuclear cells at high toxin concentrations) coupled with an with an increase in granularity observed in toxin-treated lymphocytes. Although PMNs and lymphocytes appeared as two distinct cell populations due to their differences in size and granularity, even after fixing them with ethanol, there is a possibility that massive degranulation of PMNs may cause a decrease in their SSC and/or FSC leading to a shift to the lymphocyte gate. At very high concentrations of toxin (>1,250 U/ml) the numbers of both granulocytes and mononuclear cells decreased significantly, suggesting complete cell lysis.
TABLE 1.
Flow-cytometric analysis of F. necrophorum leukotoxin-treated bovine peripheral PMNsa
| Toxin concn (U/ml) | No. of channels (maximum linear scale of >90% cells)
|
PI-excluding cells (%) | Cells showing phagocytosis + oxidative burst (fluorescence)b | DNA content (fluorescence)b | |
|---|---|---|---|---|---|
| FSC | SSC | ||||
| 0 | 1,000 ± 0 | 460 ± 23 | 98.2 ± 1.1 | 215.4 ± 15.4 | 95.8 ± 4.1 |
| 0.02 | 680 ± 23 | 420 ± 23 | 98.3 ± 1.4 | 237.7 ± 19.6 | 78.6 ± 1.4 |
| 0.2 | 680 ± 23 | 420 ± 23 | 98.0 ± 1.4 | 284.9 ± 23.0 | 74.5 ± 1.1 |
| 0.4 | 640 ± 23 | 440 ± 46 | 97.3 ± 1.0 | 292.5 ± 19.8 | 65.0 ± 2.7 |
| 2 | 640 ± 23 | 480 ± 23 | 93.0 ± 2.4 | 312.4 ± 28.3 | 54.8 ± 7.0 |
| 4 | 640 ± 23 | 480 ± 23 | 90.0 ± 1.8 | 357.0 ± 26.0 | 295.4 ± 6.8 |
| 20 | 640 ± 23 | 480 ± 23 | 89.4 ± 1.8 | 475.3 ± 38.9 | 300.3 ± 4.2 |
| 40 | 640 ± 23 | 480 ± 23 | 65.6 ± 11.1 | 442.4 ± 26.2 | 303.5 ± 3.7 |
| 200 | Scattered | Scattered | 10.3 ± 4.6 | 197.3 ± 14.7 | Clumps |
| 625 | No cells | No cells | 8.2 ± 3.1 | 201.8 ± 13.8 | Clumps |
| 1,250 | No cells | No cells | 4.6 ± 1.0 | 202.4 ± 7.3 | Clumps |
| 2,000 | No cells | No cells | 3.5 ± 1.8 | 214.0 ± 7.6 | Clumps |
Each value is the mean ± standard deviation (n = 4).
The phagocytosis and respiratory burst activity and DNA content are expressed as the geometric means of the fluorescence.
TABLE 2.
Flow-cytometric analysis of F. necrophorum leukotoxin-treated bovine peripheral mononuclear leukocytesa
| Toxin concn (U/ml) | No. of channels (maximum linear scale of >90% cells)
|
PI-excluding cells (%) | Cells showing phagocytosis + oxidative burst (fluorescence)b | DNA content (fluorescence)b | |
|---|---|---|---|---|---|
| FSC | SSC | ||||
| 0 | 850 ± 11.6 | 80 ± 23.1 | 90.8 ± 2.8 | 65.2 ± 2.4 | 342.4 ± 2.8 |
| 0.02 | 850 ± 11.6 | 80 ± 23.1 | 90.9 ± 2.3 | 65.9 ± 4.6 | 320.6 ± 4.4 |
| 0.2 | 850 ± 11.6 | 80 ± 23.1 | 90.9 ± 2.9 | 59.1 ± 4.6 | 310.9 ± 1.7 |
| 0.4 | 850 ± 11.6 | 80 ± 23.1 | 89.9 ± 2.5 | 60.2 ± 3.7 | 191.1 ± 8.7 |
| 2 | 850 ± 11.6 | 80 ± 23.1 | 88.0 ± 2.5 | 62.0 ± 3.0 | 143.7 ± 3.8 |
| 4 | 780 ± 46.2 | 80 ± 23.1 | 87.7 ± 2.7 | 61.6 ± 2.5 | 125.6 ± 13.1 |
| 20 | 780 ± 46.2 | 80 ± 23.1 | 86.5 ± 5.4 | 60.1 ± 3.8 | 678.4 ± 8.5 |
| 40 | 700 ± 23.1 | 135 ± 41.2 | 85.2 ± 2.9 | 69.9 ± 1.6 | 782.4 ± 11.4 |
| 200 | 600 ± 46.2 | 135 ± 41.2 | 73.6 ± 4.3 | 53.2 ± 2.4 | Clumps |
| 625 | 590 ± 57.7 | 135 ± 41.2 | 51.2 ± 6.0 | 55.4 ± 2.3 | Clumps |
| 1,250 | 540 ± 0 | 80 ± 0 | 16.7 ± 4.6 | 63.4 ± 1.2 | Clumps |
| 2,000 | 520 ± 0 | 80 ± 0 | 5.1 ± 1.0 | 67.1 ± 2.0 | Clumps |
Each value is the mean ± standard deviation (n = 4).
The phagocytosis and respiratory burst activity and DNA content are expressed as the geometric means of the fluorescence.
Viability of toxin-treated leukocytes.
Granulocytes were more susceptible to killing by F. necrophorum leukotoxin than mononuclear cells, as determined by an analysis of membrane integrity based on exclusion of PI. The average doses that permeabilized 50% of the cells were 110 and 817 U/ml for granulocytes and lymphocytes, respectively.
Simultaneous phagocytosis and respiratory burst assays.
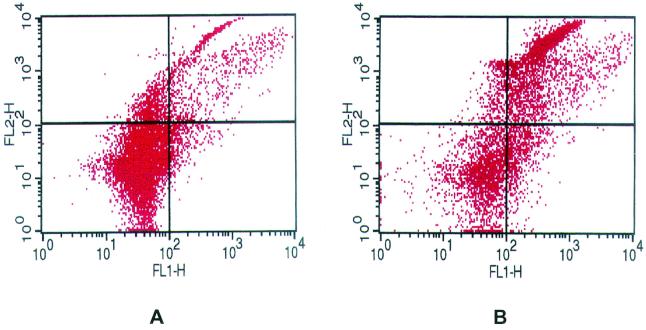
Based on PI uptake, F. necrophorum leukotoxin stimulated phagocytosis and oxidative burst activities of bovine PMNs (Table 1). Bovine PMNs were analyzed for any change in their ability to phagocytize PI-stained S. aureus (red fluorescence) or to produce reactive oxygen intermediates (ROI; conversion of DHR to green fluorescent rhodamine). At low concentrations of leukotoxin (0.2 to 20 U/ml), a slight increase in the numbers of PMNs undergoing both phagocytosis and respiratory burst occurred. Peak phagocytosis and respiratory burst activities occurred following exposure of PMNs to 20 to 40 U of leukotoxin (Table 1). At 40 U, the mean number of PMNs phagocytizing PI-stained S. aureus and producing ROIs increased to 442 compared to 215 with untreated cells (Table 1; Fig. 2). As expected, there were no differences in phagocytosis and respiratory burst between control and leukotoxin-treated lymphocytes (Table 2).
FIG. 2.
Two-color dot plot analysis of simultaneous phagocytosis and oxidative burst activity of untreated granulocytes (A) and granulocytes treated with 40 U of leukotoxin/ml (B). Lower left quadrant, cells negative for phagocytosis and ROI; upper left quadrant, cells with phagocytosed PI-labeled S. aureus, but negative for ROI; lower right quadrant, cells reactive for ROI but negative for phagocytosis; upper right quadrant, cells with simultaneous phagocytosis and ROI.
Analysis of hypodiploid nuclei by fluorescence-activated cell sorting.
Unlike lymphocytes, PMNs are terminally differentiated and are expected to be in G0 of the cell cycle. The PMNs and lymphocytes appeared as two distinct populations of cells in FSC/SSC plots. The fluorescence of cells (FL-3A) (x axis) was plotted against the number of cell events (y axis), and the geometric mean fluorescence intensity was determined (Tables 1 and 2). When the peripheral leukocytes were exposed to low concentrations of leukotoxin (0.2 to 2 U/ml for PMNs and 0.4 to 4 U/ml for lymphocytes), the mean fluorescence intensity dropped (43% for PMNs and 63% for lymphocytes), indicating a decrease in DNA content compared to that of untreated cells. Interestingly, as the concentration of leukotoxin was increased, the mean fluorescence intensity increased significantly, possibly due to DNA lysis and release of nuclear DNA in ethanol-fixed cells.
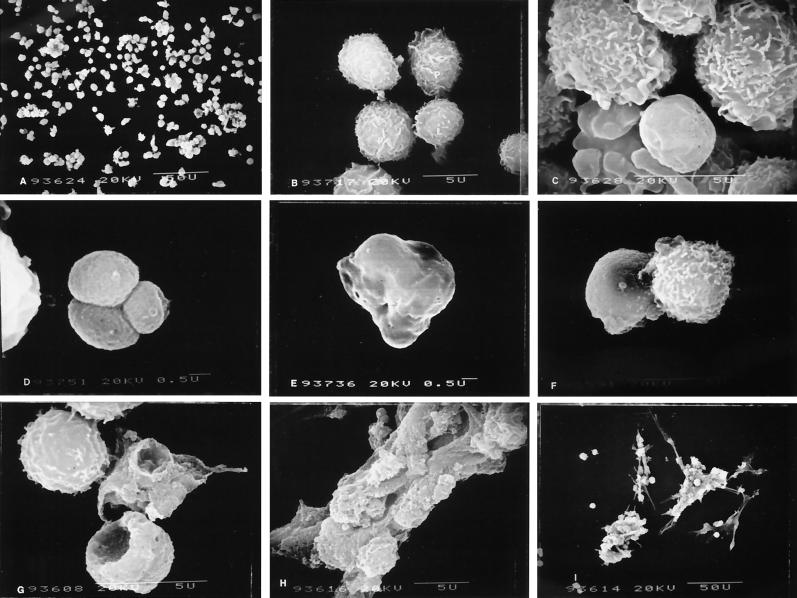
Ultrastructural changes of leukotoxin-treated cells by SEM.
PMNs not treated with leukotoxin appeared as large cells approximately 4 to 6 μm in diameter, with surfaces covered by characteristic ruffles. Lymphocytes appeared as smaller cells, 3.5 to 4.5 μm in diameter, with surface structures shaped more like villi (Fig. 3B). Leukocytes not treated with leukotoxin were more uniformly distributed, with minimal clumping (Fig. 3A). On exposure to low levels of leukotoxin (0.02 to 0.2 U/ml), the first detectable change in surface structures was loss of surface projections and overall smoothening of the cells. At 0.2 U/ml, approximately 20% of the PMNs showed membrane blebbing, which appeared as small surface projections (zeiosis). The affected cells lacked surface projections and appeared smooth but did not show any increase in size or ballooning, a characteristic effect of pore-forming toxins on target cells (Fig. 3C and D). In fact, the affected cells decreased in size to an average diameter of approximately 3 μm. Low levels of leukotoxin (0.02 or 0.2 U/ml) failed to produce any detectable changes in the surface structure of lymphocytes.
FIG. 3.
SEM of leukotoxin-treated and untreated bovine peripheral blood leukocytes. Untreated cells appear single with minimal clumping (A and B). (B) Normal PMNs and mononuclear cells. At low concentrations of leukotoxin, PMNs showed decrease in cell size and clumping (C and D). Few cells at 0.02 to 2 U of toxin/ml showed surface lesions resembling pores (E) or small craters (F). At 20 U of leukotoxin/ml, huge craters appeared on the surfaces of PMNs (G). At high concentration (over 200 U/ml) cells agglutinated (H) and failed to separate even after DNase treatment (I).
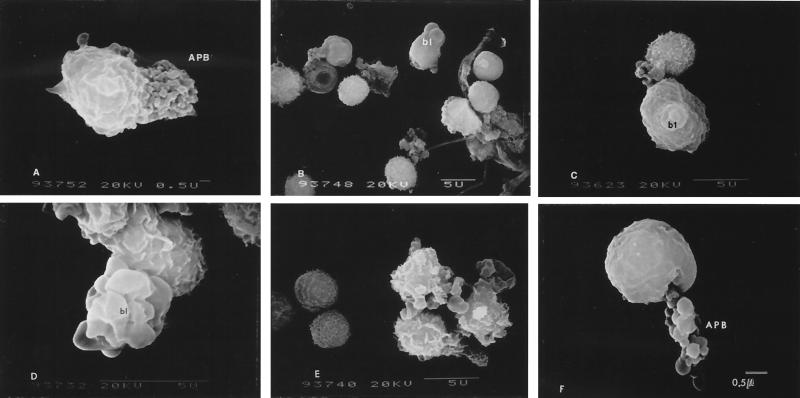
After exposure to 2 U of leukotoxin/ml, neutrophils lost their surface structures and appeared as smaller, smoother cells with numerous surface anomalies (Fig. 3E and F). More than 50% of the PMNs showed plasma membrane blebbing and/or formation of small apoptotic bodies that looked like a bunch of grapes (Fig. 4A through D). At 20 U of leukotoxin/ml, a predominant feature of PMNs was huge craters on the surfaces of the cells (Fig. 3G). Although the affected cells still appeared smaller than normal cells, their surfaces were roughened by formation of craters and numerous surface lesions. At least 70% of the neutrophils showed characteristics of cells undergoing apoptosis, such as zeiosis or formation of apoptotic bodies. After exposure to 2 to 20 U of leukotoxin/ml, lymphocytes gradually lost villous projections from their surfaces and appeared slightly smaller than the control cells but failed to show any characteristic features of cells undergoing apoptosis.
FIG. 4.
Apoptotic changes of PMNs (A to D) and lymphocytes (E and F). Plasma membrane blebbing (bl; B to E) and apoptotic bodies (APB; A and F) are shown. Leukotoxin concentrations, 2 (A to C) and 20 U/ml (D to F).
At 200 U of leukotoxin/ml, most PMNs were lysed, and any remaining PMNs appeared to be in the late stages of apoptosis. Lysed PMNs aggregated to form clumps of cells that lacked any characteristic morphology, and the aggregates remained following DNase I treatment (Fig. 3H and I). Almost all of the nonaggregated cells showed no surface blebbing and were lymphocytes. About 20% of these lymphocytes lost their surface projections and appeared as small, smoothened cells. At 625 U of leukotoxin/ml, there were no discernible PMNs present in the preparations. However, approximately one-half of the lymphocytes in a field appeared normal at this toxin concentration. Of the remaining lymphocytes, many of the cells appeared to have lost their cellular surface architecture and were attached to agglutinated masses of lysed cells. Few of the lymphocytes showed characteristic features of cells undergoing apoptosis, such as membrane blebbing and formation of grape-like clustered apoptotic bodies (Fig. 4E and F).
At a higher concentration of leukotoxin (1,250 U/ml), there were very few unagglutinated cells (predominantly lymphocytes), of which more than 50% showed apoptotic changes. At the highest concentration of leukotoxin tested (2,000 U/ml), more than 95% of the cellular mass was formed by agglutinated cells showing various stages of lysis and clumping, which occurred within the first 5 min of exposure. Among the cells that were not clumped, most were apoptotic bodies of 0.5 to 0.75 μm in diameter.
Ultrastructural features of leukotoxin-treated cells under TEM.
Control bovine neutrophils appeared as cells with multilobulated nuclei and numerous intracellular primary and secondary granules and constituted approximately 30% of the cells in the peripheral leukocyte preparation (Fig. 5A). Very few eosinophils with their characteristic primary and secondary granules (containing the characteristic rod-shaped central core and the surrounding matrix) were found. Lymphocytes (approximately 60% of total cells) appeared as smaller cells with single large nuclei and a thin cytoplasm. Monocytes, rarely seen in our preparations, appeared as cells containing large centrally placed kidney-shaped interphase nuclei each with abundant cytoplasm containing numerous mitochondria, the endoplasmic reticulum, the Golgi apparatus, and other organelles. The ratio of individual cell types in our peripheral leukocyte preparations was consistent with immunophenotyping results from flow cytometry.
FIG. 5.
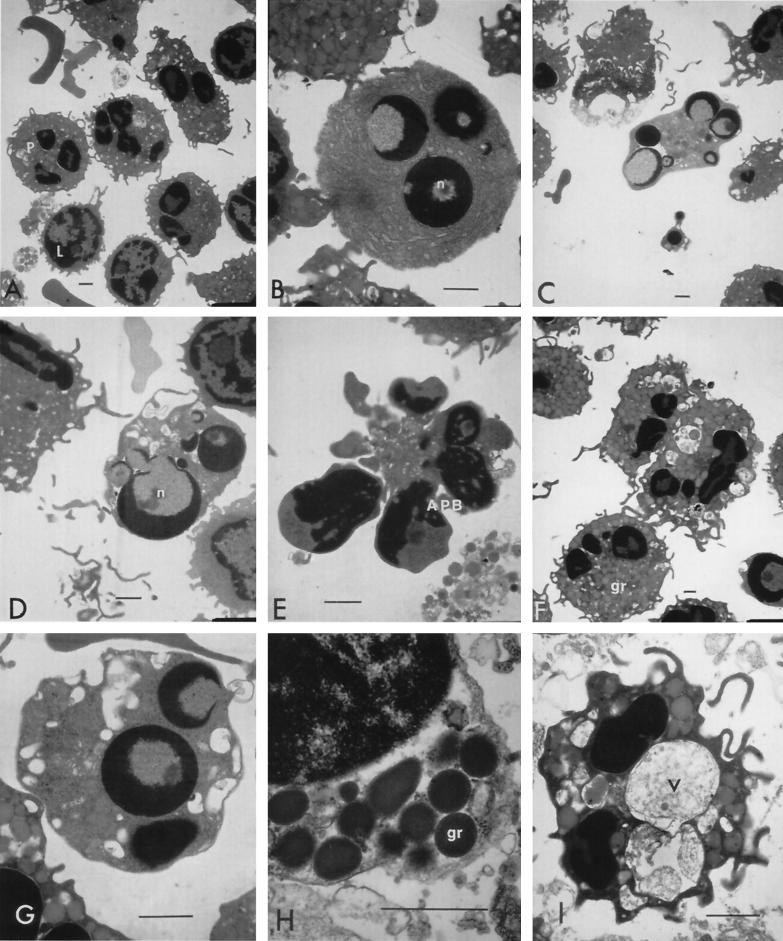
TEM of bovine peripheral leukocytes treated with low concentrations of leukotoxin. Normal PMNs (P) and lymphocytes (L) appeared among untreated cells (A). Chromatin condensation was noticed in single or multiple nuclei (n), marginated as rounded or crescent-shaped structures in monocytes (B) and neutrophils (C and D). Apoptotic bodies (APB) were found with their characteristic condensed nuclei surrounded by a thin cytoplasm (E). Cytoplasm of phagocytes showed peripheral translocation of granules (gr; H) and vacuolation (F and G), with vacuoles (v) sometimes appearing large and containing material similar to the extracellular contents (I). (A) Untreated cells; (B to C and F to H) cells treated with 0.2 U of leukotoxin/ml; (D) cells treated with 0.02 U/ml; (E and I) cells treated with 20 U/ml. Bars (all panels), 1 μm.
At low concentrations of toxin (0.02 to 0.2 U/ml), monocytes and lymphocytes seemed unaffected. However, the nuclei and cytoplasmic organelles of PMNs showed many changes consistent with cells undergoing apoptosis. Nuclear collapse was the first disturbance in the overall architecture of PMNs exposed to leukotoxin detected. This was characterized by extremely condensed chromatin (pyknotic nuclei) that marginated into crescent- or horseshoe-shaped structures or that completely collapsed to one or more spheres (Fig. 5C and D). Cytoplasmic organelles, such as mitochondria, the Golgi apparatus, and the endoplasmic reticulum, appeared condensed and dark and had discernible internal features. Mitochondria in apoptotic cells appeared distinct with thick, electron-dense cristae and inner membranes (Fig. 6A to D). There was a substantial decrease in the size of the cytoplasmic layer, but the organelles, such as primary and secondary granules, were intact although closely packed. At a leukotoxin concentration of 0.02 U/ml, eosinophils appeared normal, with their nuclei and cytoplasmic organelles resembling those of untreated cells. At 0.2 U of leukotoxin/ml, more of the neutrophils showed pyknotic nuclei with or without loss of continuity in the nuclear membrane.
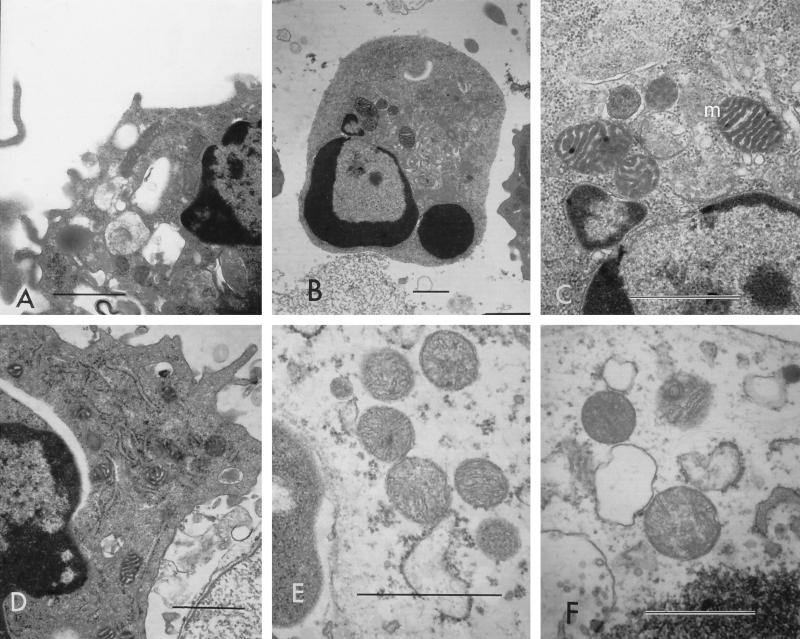
FIG. 6.
Cytoplasmic organelles from cells undergoing apoptosis versus cells undergoing necrosis. Mitochondria (m) and endoplasmic reticulums (er) appeared as condensed, electron-dense structures with discernible internal architecture (A to D), whereas mitochondria in cells undergoing necrosis appeared as light-staining, swollen structures with minimal internal structures (E and F). (A to C) Cells treated with 2 U of leukotoxin/ml; (D) cells treated with 20 U/ml; (E) cells treated with 625 U/ml; (F) cells treated with 2,000 U/ml. Bars (all panels), 1 μm.
A few neutrophils seemed to have lost a majority of their cytoplasmic granules and had very minimal cytoplasmic structures. More than 50% of neutrophils showed pyknotic nuclei and apoptotic bodies when the leukotoxin concentration was increased to 2 U/ml. Apoptotic bodies appeared as small cellular remnants with circular electron-dense nuclei surrounded by very thin layers of condensed cytoplasm containing no organelles and appeared to lack intact membranes. Most PMNs showed cytoplasmic hypervacuolation and translocation of azurophilic granules to the peripheral cytoplasmic layers (Fig. 5F and G). The percentage of PMNs showing condensed nuclei increased to more than 80% when cells were treated with 20 U of toxin/ml. Many cells showed multiple apoptotic bodies separated from each other by a central hypervacuolated cytoplasm (Fig. 5E). Separation of the inner and outer layers of the nuclear membrane and the resultant perinuclear vacuolation were found in many PMNs that were affected by leukotoxin. On rare occasions, nuclear contents from the apoptotic bodies seemed to spill out into the extracellular space. However, the predominant feature was the apparent fusion of many azurophilic granules to the cytoplasmic membrane of the cell and hypervacuolation of the cytoplasm (Fig. 5F through H). It is interesting that leukotoxin at 20 U/ml enhanced phagocytosis and respiratory burst activities of PMNs (flow-cytometric assay), and numerous invaginations resembling huge craters on the surface of PMNs were seen under SEM. At leukotoxin concentrations of 2 to 20 U/ml, a few monocytes showed multiple condensed chromatin bodies in their nuclei. Golgi apparatus and mitochondria found in the cytoplasm of these monocytes, however, appeared unaffected. Although approximately 10% of the lymphocytes lost their surface projections and showed condensation of chromatin, the majority of the lymphocytes appeared normal in size and cellular architecture.
The predominant feature of cells exposed to leukotoxin at concentrations of 200 U/ml was the loss of integrity of the plasma membrane and the release of cellular contents. Very few PMNs were present in the cell preparation, and these appeared as significantly smaller cells featuring a light-staining cytoplasm and intact plasma membrane but lacking any primary and secondary granules. These cells also showed huge cytoplasmic vacuoles that seemed to contain material similar to the extracellular fluid, which, at a higher concentration of leukotoxin, consisted of cytoplasm with dark-staining glycogen granules released from lysed cells (Fig. 4I). Lymphocytes were the most prevalent intact cells with apparently normal nuclei and slightly vacuolated cytoplasm. They showed pyknotic nuclei, perinuclear vacuolation, and dark-staining mitochondria that lacked discernible details such as inner membranes or cristae. Eosinophils showed light-staining cytoplasm with peripherally translocated cytoplasmic granules and condensed nuclear material. Perinuclear vacuolation was noticed in these cells as well.
At a toxin concentration of 625 U/ml, no PMNs were present. A very few apoptotic bodies, whose origin was unknown, were present throughout the milieu formed by spilled cytoplasmic contents. Lymphocytes were the only intact cells at this high leukotoxin concentration (Fig. 7). Approximately 50% of the lymphocytes appeared normal, with intact nuclei and cytoplasm, 10% of the lymphocytes showed apoptotic changes, and the rest showed very lightly stained nuclei and cytoplasm and were found among the agglutinated mass of lysed cells. Most cells seemed to undergo necrosis at this leukotoxin concentration (Fig. 7). However, a few lymphocytes showed morphological changes characteristic of cells undergoing apoptosis (Fig. 7C). Mitochondria in necrotic cells were distinct from those in cells undergoing apoptosis and appeared as very-light-staining structures, showing considerable ballooning and loss of internal architecture (Fig. 6E and F). The predominant feature of the bovine peripheral leukocyte preparation treated with 1,250 U of leukotoxin/ml was the formation of a milieu of cytoplasmic contents with various organelles such as vacuoles, granules, nuclei, and apoptotic bodies at different stages of degeneration and degradation. A few lymphocytes were the only discernible type of cells present, and these appeared to be undergoing apoptosis (pyknotic nuclei and dark-staining condensed cytoplasm), whereas most lymphocytes and PMNs showed features of necrotic death and were found as a mass of dead or dying cells (Fig. 7).
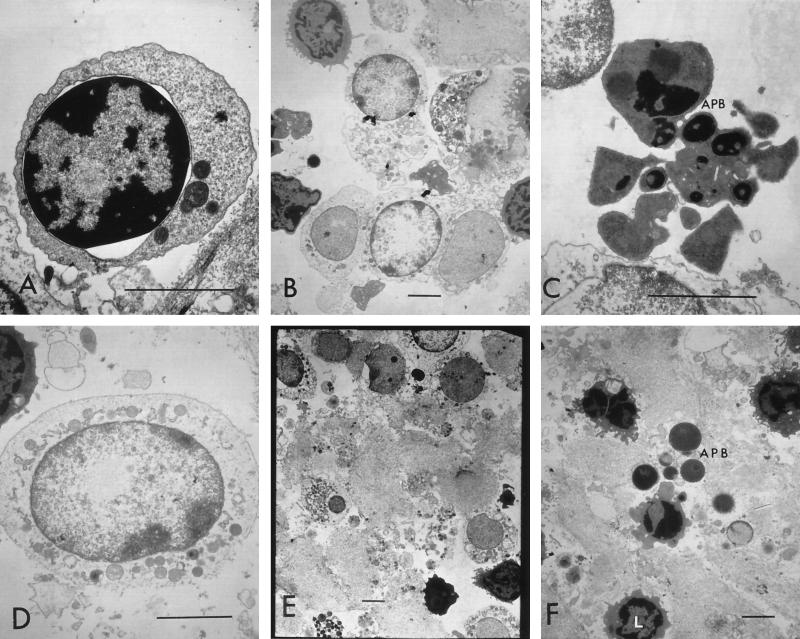
FIG. 7.
TEM of bovine peripheral lymphocytes treated with high concentrations of leukotoxin. Cells show perinuclear vacuolation (A) and various stages of necrosis (B, D, and E) or apoptosis (C and F). Lymphocytes appeared normal (L) or as apoptotic bodies (APB) in a milieu made of cytoplasmic contents from dead cells. (A) Cells treated with 200 U of leukotoxin/ml; (B to D and F) cells treated with 625 U/ml; (E) cells treated with 1,250 U/ml. Bars (all panels), 3 μm.
At the highest concentration of leukotoxin used for treating bovine peripheral leukocytes (2,000 U/ml), very few cells were present after 45 min of treatment. These cells were predominantly lymphocytes that showed pyknosis and perinuclear vacuoles. These lymphocytes were found among a mixture of cytoplasmic contents, organelles, vacuoles, and a few intact apoptotic bodies. As mentioned earlier, exposing leukocytes to high concentrations of toxin caused apparent lysis and aggregation of cells within the first few minutes of exposure.
LDH released from toxin-treated leukocytes.
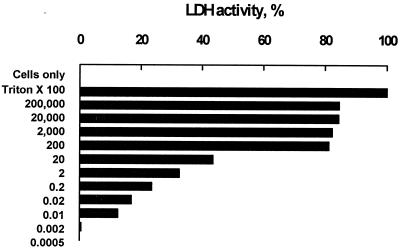
Leakage of cytoplasmic LDH into the extracellular fluid indicated loss of plasma membrane integrity. At toxin concentrations below 0.002 U/ml, there was no detectable LDH activity in the supernatants (Fig. 8). However, at a toxin concentration of 0.01 U/ml, approximately 12% of intracellular LDH had leaked into the supernatant, suggesting membrane damage. There was a dose-dependent increase in LDH activity (12.2 to 82.8%) when the toxin concentrations were increased from 0.01 to 200 U/ml. The LDH escaping from toxin-treated cells to the supernatant hit a plateau between 82 to 84% when the leukotoxin concentrations were raised from 200 to 200,000 U/ml. At a high concentration of leukotoxin, there were no intact cells found in the solution, suggesting formation of pockets by agglutinated cells that prevented complete leakage of intracellular LDH.
FIG. 8.
LDH activity of bovine leukocytes untreated (cells only) or treated with Triton X-100 (positive control) or leukotoxin at various concentrations (200,000 to 0.0005 U/ml).
DISCUSSION
In this study, we treated bovine peripheral leukocytes with various concentrations of leukotoxin, from a very low concentration (0.02 U/ml) that had no effect on the ability of treated cells to reduce the MTT dye to formazan to a high concentration (2,000 U/ml) that caused rapid lysis of most of the exposed target cells. Bovine PMNs were more susceptible to the effects of F. necrophorum leukotoxin than PBMC, based on the dose of the toxin necessary to kill the cells. Also, the ultrastructural changes in PMNs were detected at concentrations of leukotoxin lower than those at which changes in PBMC were detected, and the lymphocytes were the only cells that prevailed at concentrations of leukotoxin over 625 U/ml. This differential toxicity among bovine peripheral leukocytes, along with the fact that F. necrophorum leukotoxin has lower toxicity for horse PMNs and no toxicity for swine and rabbit PMNs (45), suggested a specificity of F. necrophorum leukotoxin for ruminant PMNs. This specificity could be a function of the presence of high-affinity receptors or an increased number of receptors on the surfaces of ruminant PMNs.
At very low concentrations of F. necrophorum leukotoxin (0.02 to 0.2 U/ml), leukocytes (PMNs or PBMC) showed very little surface damage, except for loss of surface structures, but there was a noticeable increase in the extracellular LDH. Loss of surface structures and smoothening of the cell surface have been reported with ruminal epithelial cells and bovine kidney cells treated with F. necrophorum and their extracts (15, 30). Leukotoxin produced some surface anomalies that appeared as pores on the target cell membranes at concentrations over 2 U/ml. However, exposed cells showed a decrease in size as opposed to an increase in the overall size of the cells (ballooning), even at high concentrations of leukotoxins (200 to 2,000 U/ml). Increase in cell size (ballooning) associated with a hypotonic shock is a characteristic feature of target cells affected by pore-forming leukotoxins (5, 6, 17). Also, formation of surface anomalies, including craters at a leukotoxin concentration of 20 U/ml, was consistent with activation of PMNs but did not correspond to a decrease in the exclusion of or increased staining with PI. Toxin-mediated stimulation of PMN cell activities was evidenced by increased phagocytosis and oxidative burst in flow-cytometric assays and by an increase in the exclusion of primary and secondary granules into the extracellular space. These observations suggested that F. necrophorum leukotoxin could cause damage to the membranes of target cells, but pore formation, osmotic shock, and death did not appear to be the sequence of events in leukotoxin-mediated cytotoxicity.
Several pieces of evidence suggested that F. necrophorum leukotoxin induced apoptosis in the target cells. Evaluation of the light scatter properties of the toxin-treated cells revealed a decrease in FSC (both PMNs and PBMC), with a concurrent increase in SSC of viable PBMC. These changes in light scatter properties of toxin-treated cells suggested a decrease in cell volume and increase in cellular granularity due to organelle condensation, consistent with morphological changes of apoptotic cells (20, 42). Furthermore, the intensity and proportion of cells that showed changes in the FSC and SSC increased in a leukotoxin dose-dependent manner. Also, a decrease in the cellular DNA content, as determined by flow cytometry of ethanol-fixed cells, supports our observation that a low concentration of leukotoxin causes bovine peripheral PMNs and lymphocytes to undergo apoptosis. SEM confirmed a decrease in the size of toxin-treated cells compared to that of untreated cells and a noticeable loss of surface projections compared to the ruffled surface seen in control cells. Membrane blebbing or zeiosis, a plasma membrane deformation due to cytoskeletal reorganization that is characteristic of apoptotic cells, was observed in PMNs at low concentrations of leukotoxin and in PBMC at relatively high concentrations. Frequently, affected cells divided into multiple circular (apoptotic) bodies (0.5 to 0.75 μm in diameter) that appeared under SEM as grape-like clusters. Leukotoxin-treated bovine peripheral leukocytes exhibited functional and morphological signs of apoptosis and failed to take up vital stains (37). TEM revealed ultrastructural alterations in toxin-treated cells that are considered hallmarks of cells undergoing apoptosis (37, 41, 47). Cells showed nuclear alterations including chromatin collapse with subsequent chromatin margination in crescent-shaped masses around the periphery of the nucleus. Nuclear condensation with reduction in nuclear size was evident, as were a decrease in total cell volume, an increase in density of cytoplasm, and a compaction of cytoplasmic organelles. The numbers of cytoplasmic organelles including the endoplasmic reticulum, mitochondria, and Golgi apparatus, were reduced. Cells showed hypervacuolation of the cytoplasm, perinuclear vacuolation regions, leading to the separation of the inner and outer membranes of the nucleus, separation of the nucleus into multiple severely condensed circular structures surrounded by a thin condensed cytoplasm and intact membrane called apoptotic bodies, and progressive degeneration of residual nuclear and cytoplasmic structures.
Changes in mitochondrial structure and function have been shown in many systems to cause metabolic stress leading to apoptosis (4, 21, 31). In the present study, apart from the overall decrease in their numbers, mitochondria of cells undergoing apoptosis showed characteristic morphological changes, including condensation and intact inner and outer membranes with well-discernible cristae, following treatment with F. necrophorum leukotoxin. However, the role of mitochondria in inducing apoptosis in F. necrophorum leukotoxin treated-cells is not understood.
Toxin-treated PMNs had increased phagocytosis and respiratory burst activity. At 20 U of leukotoxin/ml, this activity increased twofold compared to that for untreated cells. Additionally, electron microscopy demonstrated surface craters (SEM) and translocation of primary and secondary granules to the peripheral layers of PMNs followed by expulsion of the granules to the exterior. Peripheral translocations of granules and release have been demonstrated among human PMNs treated with A. actinomycetemcomitans leukotoxin (14). Although the PI exclusion assay was valuable to evaluate the integrity of the surface membrane, it was not a reliable marker for nonviable cells. At concentrations of leukotoxin (0.02 to 2 MTT units) where most cells excluded this vital dye, exposed cells underwent apoptotic cell death. This observation supports the claim that having a relatively intact cell membrane, thus excluding vital dyes, does not necessarily mean that the cells are not undergoing apoptosis (21).
It appears that the primary mode of toxicity of F. necrophorum leukotoxin on target cells is induction of apoptosis and cellular activation. Cytoplasmic condensation (due to apoptosis), active degranulation of PMNs, and release of toxic ROI and catalytic enzymes into the adjacent medium could cause secondary necrosis of the nearby cells. However, F. necrophorum at higher concentrations can directly damage the cell, which leads to primary necrosis of the target cells.
Certain bacterial toxins are capable of inducing apoptosis in target cells (4). However, the mechanism(s) through which they induce apoptosis differs for each toxin. S. aureus alpha toxin, Escherichia coli hemolysin, A. actinomycetemcomitans leukotoxin, and Mannheimia (Pasteurella) haemolytica leukotoxin are pore-forming proteins that can directly destroy the cells at high concentrations; however, at lower concentrations they cause subtle plasma membrane changes which activate the central apoptotic pathway. Corynebacterium diphtheriae, Pseudomonas aeruginosa, Shigella dysenteriae, and other enterobacteria produce toxins that inhibit protein synthesis, a process associated with the induction of apoptosis (18, 25). The adenylate cyclase activity of Bordetella pertussis toxin elicits a rapid increase in cytoplasmic cyclic AMP (cAMP) of target cells, with a consequent induction of apoptosis via activation of cAMP-dependent protein kinase I (19). Further studies are necessary to delineate the mechanisms of induction of apoptosis by F. necrophorum leukotoxin.
In conclusion, our study provides evidence that F. necrophorum leukotoxin causes cellular activation and induction of apoptosis-mediated cell death in bovine peripheral leukocytes at lower concentrations and necrosis at higher concentrations. The ability of F. necrophorum leukotoxin to modulate the host immune system by its toxicity, including cellular activation of PMNs and apoptosis-mediated killing of phagocytes and immune effector cells, represents a potentially important mechanism of its pathogenesis.
Acknowledgments
We thank Sam Ives, Neil Wallace, and Bill Jackson for assistance in the laboratory, Melinda Dalby for help with flow cytometry, and Maureen Rider for help with the cell culture experiments.
The research was supported by the NRI Competitive Grants Program of the U.S. Department of Agriculture (grant no. 2002-35204-11662).
Editor: J. T. Barbieri
Footnotes
This paper is contribution no. 02-17-j from the Kansas Agricultural Experiment Station.
REFERENCES
- 1.Allen, R. C. 1986. Phagocytic leukocyte oxygenation activities and chemiluminescence: a kinetic approach to analysis. Methods Enzymol. 133:449-493. [DOI] [PubMed] [Google Scholar]
- 2.Allen, R. C., D. C. Dale, and F. B. Taylor, Jr. 2000. Blood phagocyte luminescence: gauging systemic immune activation. Methods Enzymol. 305:591-629. [DOI] [PubMed] [Google Scholar]
- 3.Brown, J. F., F. Leite, and C. J. Czuprynski. 1997. Binding of Pasteurella haemolytica leukotoxin to bovine leukocytes. Infect. Immun. 65:3719-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, Y., and A. Zychlinsky. 1994. Apoptosis induced by bacterial pathogens. Microb. Pathog. 17:203-212. [DOI] [PubMed] [Google Scholar]
- 5.Clinkenbeard, K. D., D. A. Mosier, and A. W. Confer. 1989. Effects of Pasteurella haemolytica leukotoxin on isolated bovine neutrophils. Toxicon 27:797-804. [DOI] [PubMed] [Google Scholar]
- 6.Clinkenbeard, K. D., D. A. Mosier, and A. W. Confer. 1989. Transmembrane pore size and role of cell swelling in cytotoxicity caused by Pasteurella haemolytica leukotoxin. Infect. Immun. 57:420-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coyle-Dennis, J. E., and L. H. Lauerman. 1979. Correlation between leukocidin production and virulence of two isolates of Fusobacterium necrophorum. Am. J. Vet. Res. 40:274-276. [PubMed] [Google Scholar]
- 8.Emery, D. L. 1989. Antigens of Fusobacterium necrophorum, p. 135-140. In J. R. Egerton, W. K. Yong, and G. G. Riffkin (ed.), Footrot and foot abscesses of ruminants. CRC Press, Boca Raton, Fla.
- 9.Emery, D. L., R. D. Edwards, and J. S. Rothel. 1986. Studies on purification of leukocidin of Fusobacterium necrophorum and its neutralization by specific antisera. Vet. Microbiol. 11:357-372. [DOI] [PubMed] [Google Scholar]
- 10.Emery, D. L., J. A. Vaughan, B. L. Clark, J. H. Duffy, and J. Stewart. 1985. Culture characteristics and virulence of strains of Fusobacterium necrophorum isolated from feet of cattle and sheep. Aust. Vet. J. 62:43-46. [DOI] [PubMed] [Google Scholar]
- 11.Hagelskjaer, K. J., and J. Prag. 2000. Human necrobacillosis, with emphasis on Lemierre's syndrome. Clin. Infect. Dis. 31:524-532. [DOI] [PubMed] [Google Scholar]
- 12.Iwase, M., H. M. Korchak, E. T. Lally, P. Berthold, and N. S. Taichman. 1992. Lytic effects of Actinobacillus actinomycetemcomitans leukotoxin on human neutrophil cytoplasts. J. Leukoc. Biol. 52:224-227. [DOI] [PubMed] [Google Scholar]
- 13.Jewett, A., W. R. Hume, H. Le, T. N. Huynh, Y. W. Han, G. Cheng, and W. Shi. 2000. Induction of apoptotic cell death in peripheral blood mononuclear and polymorphonuclear cells by an oral bacterium, Fusobacterium nucleatum. Infect. Immun. 68:1893-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson, A., R. Claesson, L. Hanstrom, G. Sandstrom, and S. Kalfas. 2000. Polymorphonuclear leukocyte degranulation induced by leukotoxin from Actinobacillus actinomycetemcomitans. J. Periodontal Res. 35:85-92. [DOI] [PubMed] [Google Scholar]
- 15.Kanoe, M., T. Ishii, and K. Kai. 1987. Effects of Fusobacterium necrophorum leukocidin on bovine ruminal cells in vitro. Microbios Lett. 35:119-123. [Google Scholar]
- 16.Kanoe, M., T. Ishii, K. Mizutani, and H. Blobel. 1986. Partial characterization of leukocidin from Fusobacterium necrophorum. Zbl. Bakteriol. Mikrobiol. Hyg. A 261:170-176. [DOI] [PubMed] [Google Scholar]
- 17.Karakelian, D., J. D. Lear, E. T. Lally, and J. C. Tanaka. 1998. Characterization of Actinobacillus actinomycetemcomitans leukotoxin pore formation in HL60 cells. Biochim. Biophys. Acta 1406:175-187. [DOI] [PubMed] [Google Scholar]
- 18.Keenen, K. P., D. D. Sharpnack, H. Collins, S. B. Formal, and A. D. O'Brien. 1986. Morphological evaluation of the effects of Shiga toxin and E. coli Shiga-like toxin on the rabbit intestine. Am. J. Pathol. 125:69-80. [PMC free article] [PubMed] [Google Scholar]
- 19.Khelef, N., A. Zychlinsky, and N. Guiso. 1993. Bordetella pertussis induces apoptosis in macrophages: role of adenylate cyclase-hemolysin. Infect. Immun. 61:4064-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korostoff, J., J. F. Wang, I. Kieba, M. Miller, B. J. Shenker, and E. T. Lally. 1998. Actinobacillus actinomycetemcomitans leukotoxin induces apoptosis in HL-60 cells. Infect. Immun. 66:4474-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korostoff, J., N. Yamaguchi, M. Miller, I. Kieba, and E. T. Lally. 2000. Perturbation of mitochondrial structure and function plays a central role in Actinobacillus actinomycetemcomitans leukotoxin-induced apoptosis. Microb. Pathog. 29:267-278. [DOI] [PubMed] [Google Scholar]
- 22.Langworth, B. F. 1977. Fusobacterium necrophorum: its characteristics and role as an animal pathogen. Bacteriol. Rev. 41:373-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maheswaran, S. K., D. J. Weiss, M. S. Kannan, E. L. Townsend, K. R. Reddy, L. O. Whiteley, and S. Srikumaran. 1992. Effects of Pasteurella haemolytica A1 leukotoxin on bovine neutrophils: degranulation and generation of oxygen-derived free radicals. Vet. Immunol. Immunopathol. 33:51-68. [DOI] [PubMed] [Google Scholar]
- 24.Marshall, M. J., G. A. Bohach, and D. F. Boehm. 2000. Characterization of Staphylococcus aureus beta-toxin induced leukotoxicity. J. Nat. Toxins 9:125-138. [PubMed] [Google Scholar]
- 25.Morimoto, H., and B. Bonavida. 1992. Diphtheria toxin- and Pseudomonas A toxin mediated apoptosis. ADP ribosylaion of elongation factor-2 is required for DNA fragmentation and cell lysis and synergy with tumor necrosis factor-alpha. J. Immunol. 149:2089-2094. [PubMed] [Google Scholar]
- 26.Mulligan, M. 1989. Ear, nose, throat, head and neck infections, p 263-288. In S. M. Finegold and W. L. George (ed.), Anaerobic infections in humans. Academic Press, New York, N.Y.
- 27.Nagaraja, T. G. 1998. Necrobacillosis associated with Fusobacterium necrophorum, p. 400-402. In J. L. Howard and R. A. Smith (ed.), Current veterinary therapy 4-food animal practice. W. B. Saunders Co., Philadelphia, Pa.
- 28.Nagaraja, T. G., and M. M. Chengappa. 1998. Liver abscesses in feedlot cattle: a review. J. Anim. Sci. 76:287-298. [DOI] [PubMed] [Google Scholar]
- 29.Narayanan, S., T. G. Nagaraja, M. M. Chengappa, and G. C. Stewart. 2001. Cloning, sequencing and expression of the leukotoxin gene from Fusobacterium necrophorum. Infect. Immun. 69:5447-5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okada, Y., M. Kanoe, K. Okamoto, K. Sakamoto, Y. Yaguchi, and T. Watanabe. 2000. Effects of Fusobacterium necrophorum subspecies on extracellular matrix of tissue-culture bovine kidney cells. Microbios 101:147-156. [PubMed] [Google Scholar]
- 31.Roberg, K., and K. Ollinger. 1998. Oxidative stress causes relocation of lysosomal enzyme cathepsin-D with ensuing apoptosis in neonatal rat cardiomyocytes. Am. J. Pathol. 152:1151-1156. [PMC free article] [PubMed] [Google Scholar]
- 32.Saginala, S., T. G. Nagaraja, K. F. Lechtenberg, M. M. Chengappa, K. E. Kemp, and P. M. Hine. 1997. Effect of Fusobacterium necrophorum leukotoxoid vaccine on susceptibility to experimentally induced liver abscesses in cattle. J. Anim. Sci. 75:1160-1166. [DOI] [PubMed] [Google Scholar]
- 33.Saginala, S., T. G. Nagaraja, Z. L. Tan, K. F. Lectenberg, M. M. Chengappa, and P. M. Hine. 1996. The serum neutralizing antibody response in cattle to Fusobacterium necrophorum leukotoxoid and possible protection against experimentally induced hepatic abscesses. Vet. Res. Commun. 20:493-504. [DOI] [PubMed] [Google Scholar]
- 34.Saginala, S., T. G. Nagaraja, Z. L. Tan, K. F. Lectenberg, M. M. Chengappa, K. E. Kemp, and P. M. Hine. 1996. The serum neutralizing antibody response and protection against experimentally induced liver abscesses in steers vaccinated with Fusobacterium necrophorum. Am. J. Vet. Res. 57:483-488. [PubMed] [Google Scholar]
- 35.Scanlan, C. M., J. N. Berg, and W. H. Fales. 1982. Comparative in vitro leukotoxin production of three strains of Fusobacterium necrophorum. Am. J. Vet. Res. 43:1329-1333. [PubMed] [Google Scholar]
- 36.Schinenti, K. J., and J. W. Jacobberger. 1992. Fixation of mammalian cells for flow cytometric evaluation of DNA content and nuclear immunofluorescence. Cytometry 13:48-59. [DOI] [PubMed] [Google Scholar]
- 37.Shenker, B. J., L. A. Vitale, I. Kieba, G. Harrison, P. Bethold, E. Golub, and E. T. Lally. 1994. Flow cytometric analysis of the cytotoxic effects of Actinobacillus actinomycetemcomitans leukotoxin on human natural killer cells. J. Leukoc. Biol. 55:153-160. [DOI] [PubMed] [Google Scholar]
- 38.Shinjo, T., T. Fujisawa, and T. Mitsuoka. 1991. Proposal of two subspecies of Fusobacterium necrophorum (Flugge) Moore and Holdeman: Fusobacterium necrophorum subsp. necrophorum subsp. nov., nom. rev. (ex Flugge 1886), and Fusobacterium necrophorum subsp. funduliforme subsp. nov., nom. rev. (ex Hallé 1898). Int. J. Syst. Bacteriol. 41:395-397. [DOI] [PubMed] [Google Scholar]
- 39.Smits, E., C. Burvenich, and R. Heyneman. 1997. Simultaneous flow cytometric measurement of phagocytotic and oxidative burst activity of polymorphonuclear leukocytes in whole bovine blood. Vet. Immunol. Immunopathol. 56:259-269. [DOI] [PubMed] [Google Scholar]
- 40.Stevens, P. K., and C. J. Czuprynski. 1996. Pasteurella haemolytica induces bovine leukocytes to undergo morphological changes consistent with apoptosis in vitro. Infect. Immun. 64:2687-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart, C. C., and S. J. Stewart. 1995. The use of directly and indirectly labeled monoclonal antibodies in flow cytometry. Methods Mol. Biol. 45:129-147. [DOI] [PubMed] [Google Scholar]
- 42.Sun, Y., K. D. Clinkenbeard, C. L. Ownby, L. Cudd, and S. K. Highlander. 2000. Ultrastructural characterization of apoptosis in bovine lymphocytes exposed to Pasteurella haemolytica leukotoxin. Am. J. Vet. Res. 61:51-56. [DOI] [PubMed] [Google Scholar]
- 43.Tan, Z. L., T. G. Nagaraja, and M. M. Chengappa. 1996. Fusobacterium necrophorum infections: virulence factors, pathogenic mechanism, and control measures Vet. Res. Commun. 20:113-140. [DOI] [PubMed] [Google Scholar]
- 44.Tan, Z. L., T. G. Nagaraja, and M. M. Chengappa. 1992. Factors affecting leukotoxin activity of Fusobacterium necrophorum. Vet. Microbiol. 33:15-28. [DOI] [PubMed] [Google Scholar]
- 45.Tan, Z. L., T. G. Nagaraja, M. M. Chengappa, and J. S. Smith. 1994. Biological and biochemical characterization of Fusobacterium necrophorum leukotoxin. Am. J. Vet. Res. 55:515-519. [PubMed] [Google Scholar]
- 46.Tan, Z. L., T. G. Nagaraja, M. M. Chengappa, and J. J. Staats. 1994. Purification and quantification of Fusobacterium necrophorum leukotoxin using monoclonal antibodies. Vet. Microbiol. 42:121-133. [DOI] [PubMed] [Google Scholar]
- 47.Wang, J. F., I. R. Kieba, J. Korostoff, T. L. Guo, N. Yamaguchi, H. Rozmiarek, P. C. Billings, B. J. Shenker, and E. T. Lally. 1998. Molecular and biochemical mechanisms of Pasteurella haemolytica leukotoxin-induced cell death. Microb. Pathog. 25:317-331. [DOI] [PubMed] [Google Scholar]