Abstract
Therapeutic vaccination is an attractive strategy to control infection and disease caused by Helicobacter pylori. In mice infected with H. pylori we have studied the protective effect of oral immunization with an H. pylori lysate preparation given together with the mucosal adjuvant cholera toxin (CT), both against the initial infection and against a later reinfection challenge. We have also examined the effects of treatment with the CT adjuvant alone on H. pylori infection and reinfection. Specific immunization with lysate was found to result in a sixfold reduction of the extent (bacterial load) of the primary infection and also to provide similar levels of protection against reinfection. However, these effects were associated with severe postimmunization gastritis. In contrast, oral treatment with CT alone at the time of initial infection, while unable to suppress the initial infection, gave rise to a 20-fold reduction in bacterial load upon reinfection without causing any associated gastric inflammation. Both the infected animals that were specifically immunized and those that were treated with CT only displayed increased in vitro proliferative responses of mononuclear cells to H. pylori antigens. Antibody levels in response to H. pylori were on the other hand only marginally increased after treatment with CT, whereas they were markedly elevated after immunization with lysate plus CT, with a rise in both (Th2-driven) immunoglobulin G1 (IgG1) and, especially, (Th1-driven) IgG2a antibodies. The results illustrate the complex balance between protection and harmful inflammation after postinfection vaccination against H. pylori as studied in a mouse model.
The first description of Helicobacter pylori in the stomachs of patients with gastritis and peptic ulceration was in 1984 (25); it is now known that one-half of the world's population is infected with this organism. Infection with H. pylori is the most important cause of both peptic ulcer disease and gastric adenocarcinoma, especially involving the distal stomach (2, 3, 8). Almost all patients with duodenal ulcers and 80 to 90% of gastric ulcer patients are infected with H. pylori in the stomach (32). The hallmark of H. pylori infection is the development of chronic inflammation in the gastric mucosa (9). Over the past decade several animal models which have provided opportunities to study host responses to infection and the effects of prophylactic as well as therapeutic immunization have been developed (21-23). In mice infected with H. pylori the gastric tissue shows an abundance of bacteria on the mucosal surface associated with an inflammatory infiltrate of T and B lymphocytes, macrophages, and neutrophils in the lamina propria (13). Progressively, the gastric infection and inflammation lead to a disturbance of the epithelial gland structure and function, with diminished mucus production, hyperplasia, and in severe cases atrophy of the stomach mucosa (22).
Recent clinical studies have convincingly shown that effective antimicrobial treatment leading to eradication of the infection is followed by rapid and complete remission of ulcers (40). Although recurrence rates after antimicrobial therapy were earlier reported to be low, this may be more due to the low exposure rates and opportunity for reinfection in the patient groups monitored than to the existence of effective immunity as a result of the previous infection. Indeed, in areas where there is a higher risk for H. pylori infection, true reinfections are found to be a serious problem (17, 30, 38, 41).
Given the known problems with poor compliance and the risk for resistance development associated with antibiotic treatment of H. pylori infection, vaccination is attractive as an approach that is either alternative or complementary to antibiotic treatment for controlling H. pylori infection and/or reinfection. Indeed, several studies with the mouse model have reported significant protection against H. pylori infection after oral vaccination with different H. pylori antigen preparations either before or after establishment of the infection; characteristically vaccination has reduced the bacterial burden by one or two log units although it usually has not been able to completely prevent or eradicate the infection (6, 16, 18, 20, 34, 36). However, some of these studies have also shown that protection in these mice appears to be associated with gastritis (18, 34). Since postimmunization gastritis is an undesired side effect of vaccination, the mechanisms behind it have attracted much recent attention (11, 34; A. A. Akhiani, K. Schön, L. E. Franzen, J. Pappo, and N. Lycke, abstracts from the 2nd Meet. Eur. Mucosal Immunol. Group 2000, abstr. D25 and D29).
It is known that in mice infected with Helicobacter a T-helper 1 (Th1) T-cell response leads to an enhanced gastritis, while a Th2 response favors protection (28). Since orally administered CT is known to stimulate Th2 immunity (24), we have investigated the effects of administering CT alone or combined with specific immunization with H. pylori lysate on bacterial colonization and gastritis in relation to both the initial infection and a reinfection. Our results point to a complex balance between protection against experimental H. pylori infection and tissue-damaging inflammation.
MATERIALS AND METHODS
Animals.
Six- to 8-week-old C57BL/6 mice were obtained from B&K Universal (Sollentuna, Stockholm, Sweden). They were housed in microisolators at the animal house at Göteborg University during the study. All experiments were approved by the National Board for Laboratory Animals (ethical permit 291/99).
Bacteria and culture conditions for infection.
Mouse-adapted H. pylori strain SS1, stored at −70°C and kept in Luria-Bertani medium containing 20% glycerol, was used as the stock culture for all experiments. The bacteria were cultured on Columbia isoagar plates for 2 days in an incubator at 37°C under microaerophilic conditions. On day 3 the bacteria were transferred to brucella broth (Difco Laboratories, Detroit, Mich.) supplemented with 5% newborn calf serum and antibiotics (vancomycin, 10 μg/ml; polymyxin B, 10 U/ml; trimethoprim, 10 μg/ml). The flask containing the culture was then flushed for 2 min with a mixture of gases containing 10% CO2, 6% O2, and 85% N2 (AGA Gas AB, Sundbyberg, Sweden) and incubated for 24 h in a shaking incubator (150 rpm). After centrifugation at 13,000 × g for 10 min bacteria were resuspended in brucella broth without antibiotics to a final concentration corresponding to an optical density at 595 nm of 1.5, a density of approximately 109 bacteria/ml. Serial dilutions of this suspension were plated on horse blood agar plates to determine the viable count of the infectious dose.
Preparation of H. pylori antigens. (i) Lysate SS1.
H. pylori SS1 bacteria from the −70°C stock culture were grown on Columbia isoagar plates as described above. The bacterial harvest from 25 plates was suspended in 5 ml of sterile phosphate-buffered saline (PBS). Each plate provided approximately 1010 bacteria. The bacteria were then pulse sonicated for 10 min at 50% capacity while kept in an ice bath. The sonicated bacteria were then centrifuged at 13,000 × g for 30 min at 4°C, and the supernatant was filtered through a 0.2-μm-pore-size filter. The protein content was determined with Bradford's reagent (Bio-Rad). The antigen preparation was stored in aliquots at −70°C until further use.
(ii) MP from strain SS1.
Whole-membrane proteins (MP) from H. pylori SS1 were prepared as described by Achtman et al. (1).
(iii) LPS.
Lipopolysaccharide (LPS) was purified from strain H. pylori SS1 by the hot phenol-water method of Westphal and Jann (39), followed by treatment with DNase II (2,000 U/mg; Boehringer Mannheim Scandanavia AB, Bromma, Sweden), RNase (40 U/mg; Boehringer Mannheim), and protease (type XIV; Sigma) and ultracentrifugation as described previously (19). The protein content in the LPS preparation was found to be less than 1% as determined (by the method of Lowry et al. [31]) by using the Sigma diagnostics protein assay kit. The low protein content was further confirmed by silver staining of sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels with the Bio-Rad (Richmond, Calif.) original silver staining kit.
(iv) Urease from strain E32.
Urease was purified from strain E32 by a combination of methods described by Dunn et al. (10) and Evans et al. (14). Briefly, H. pylori bacteria were harvested in PBS and centrifuged at 17,000 × g for 10 min. The pellet was then suspended in 1% N-octylglucose and left for 20 min at room temperature. After centrifugation at 26,000 × g for 15 min the supernatant was dialyzed against PBS overnight at 4°C. Further purification was obtained by size exclusion chromatography on a Sepharose CL 6B column (Pharmacia LKB, Uppsala, Sweden). The urease-containing fractions were identified, pooled, and dialyzed against PBS. After filtration through a 0.45-μm-pore-size filter the suspension was subjected to anion-exchange chromatography by fast protein liquid chromatography on a Resource Q column (Pharmacia). The purity of the preparations was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting with urease-specific monoclonal antibodies, and urease activity was confirmed by using a commercial urease test.
Primary infection and immunization of mice.
Mice were given 0.4 mg of omeprazole (Losec infusion substance; AstraZeneca, Sweden) intragastrically 3 h before infection to neutralize the stomach acid. The mice were then infected with approximately 109 H. pylori SS1 bacteria by using an intubation tube under anesthesia (Metofane; Shering Plough Inc., Madison, N.J.). Subgroups of mice were thus either left untreated (infection controls) or given 10 μg of cholera toxin (CT) (List Biological Laboratories Inc., Campbell, Calif.) together with the infecting dose followed by an identical CT dose 1 week later. Yet other groups of mice, starting 2 weeks after the initial infection, were vaccinated with four weekly doses of 400 μg of lysate antigen, each dose being given intragastrically in 3% sodium hydrogen carbonate buffer together with 10 μg of CT.
Eradication and reinfection model.
Mice infected with H. pylori SS1 and treated as described above were given a combination of metronidazole (Dumex; 1.35 mg), amoxicillin (Scand Pharm; 5 mg), and omeprazole (0.4 mg of Losec; AstraZeneca) intragastrically daily for a period of 5 days starting 7 weeks after infection, i.e., 1 week after the last immunization. A week after this treatment the mice were reinfected with the same dose of bacteria used for the initial infection. At this time point a control group of age-matched naive animals were also infected and are referred to as reinfection controls.
Quantitative culture of H. pylori SS1 from the stomachs of mice.
Mice were killed 6 weeks after the initial infection or 2 weeks after reinfection; the latter time point was chosen as it had been shown in our previous work that 2 weeks was the minimum time needed for stable bacterial colonization in the mouse stomach (S. Raghavan et al., unpublished results). One-half of the stomach was gently washed with PBS to remove any loose stomach contents, and then it was homogenized in 2 ml of brucella broth with a tissue homogenizer (Ultra Turrax; IKA Laboratory Technology, Staufen, Germany). Serial 10-fold dilutions of the homogenate were plated on Skirrow blood agar plates, which were incubated at 37°C under microaerophilic conditions for 6 days before a colony count was made. Colonies were counted on the basis of having typical H. pylori colony morphology, and in case of uncertainty their H. pylori identity was confirmed by the urease test and dot blot staining using monoclonal antibodies against a protein specific to H. pylori (4). The mean numbers of bacteria from two dilutions giving the easiest countable colonies, approximately 10 to 150 per dilution, were calculated as the basis for extrapolating the number of bacteria per stomach.
Specific serum antibody titer measurements.
Blood was collected from the axillary plexus immediately before the mice were killed. Serum antibody titers were determined by enzyme-linked immunosorbent assay (26) using MP of H. pylori SS1 for coating at room temperature overnight. Levels of immunoglobulin G (IgG) antibodies were measured in each group by using 1:10-prediluted sera followed by horseradish peroxidase (HRP)-coupled goat anti-mouse IgG (Jackson ImmunoResearch). Levels of IgG subtypes were further analyzed against MP from H. pylori SS1 by using 1:10-prediluted sera and HRP-coupled IgG1 or IgG2a antibodies (Southern Biotechnology Associates Inc.). The antibody titers are defined as the reciprocal sample dilution giving an absorbance of 0.4 above the background.
H. pylori antigen-specific proliferation assay. (i) Preparation of cells.
Spleen and mesenteric lymph node (MLN) lymphocytes were isolated by passing the tissues through a nylon net. Spleen erythrocytes were lysed with hypotonic Tris-ammonium chloride, and the resulting pellet of spleen or MLN cells was washed and resuspended to an appropriate cell density.
(ii) Cell culture in vitro.
Spleen and MLN cells were cultured at 105 cells/well in Iscove's medium (Biochrom KG, Berlin, Germany), supplemented with 5% heat-inactivated fetal calf serum (Biochrom), 50 μM 2-mercaptoethanol (Sigma), 1 mM l-glutamine (Biochrom), and 50 μg of gentamicin (Sigma)/ml, in 96-well flat-bottom plates (Nunc A/S, Roskilde, Denmark) at 37°C in the presence or absence of restimulating H. pylori antigens (lysate from SS1, 20 μg/ml; urease from E32, 15 μg/ml; LPS from SS1, 15 μg/ml) or concanavalin A (2.5 μg/ml; Sigma).
(iii) Proliferation assay.
Proliferation in cell cultures was determined by addition of 1 μCi of tritiated thymidine (Amersham International) per well 6 h before ending the culture. The cells were then collected with a cell harvester (Skatron) onto glass fiber filters, and incorporated radioactivity was determined in a scintillation counter (Beckman, LKB, Bromma, Sweden). The results are expressed as mean counts per minute ± standard deviations (SD) of triplicate cultures.
Histology.
Strips of the entire longer curvature of the stomach were cut and fixed in 4% phosphate-buffered formalin and then embedded in paraffin. Sections 5 μm thick were cut and stained with hematoxylin and eosin. The slides were then examined by light microscopy (×100), and the extent of gastritis was graded using the Sydney gastritis scoring system (9).
Statistical analysis.
The Mann-Whitney test was used for comparisons between groups with the help of the Prism software system (GraphPad Software Inc.).
RESULTS
Therapeutic immunization with H. pylori lysate vaccine protects against primary infection.
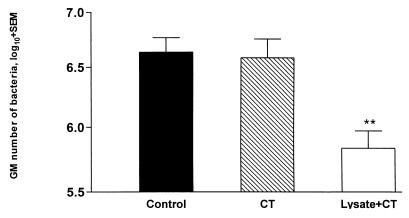
The effect of intragastric vaccination with an H. pylori SS1 lysate preparation administered together with a CT adjuvant on the extent of an already-initiated infection was evaluated. C57BL/6 mice were infected with the H. pylori SS1 strain, and, starting 2 weeks later, when the infection had been well established in the stomach and had reached a steady-state bacterial load of approximately 1 × 106 to 3 × 106 bacteria (S. Raghavan et al., submitted for publication), the mice were given four weekly intragastric doses of 400 μg of lysate plus 10 μg of CT. In another group of mice, the effect of administering 10 μg of CT together with the infecting dose of the bacteria, followed 1 week later by another 10-μg CT dose, was also tested. Animals were sacrificed 6 weeks after the start of infection, and the effects of different treatments on the extent of infection (bacterial load) were determined by comparison with a concomitantly infected untreated group. As shown in Fig. 1, the treatment with CT alone had no demonstrable effect on the course of the infection. In contrast, the specific therapeutic immunization with lysate plus CT resulted in a statistically significant (P < 0.01) mean sixfold reduction in the number of bacteria in the mouse stomach.
FIG. 1.
Protective effect by therapeutic immunization with an H. pylori lysate preparation. Groups of mice were infected with H. pylori SS1 and either given CT alone at the time and again 1 week after the infection (hatched bar) or immunized four times with lysate plus CT after the establishment of infection (open bar). Infected untreated mice served as controls (filled bar). Results show geometric mean numbers (plus standard errors of the means) of bacteria cultured from the mouse stomach 6 weeks after start of infection. Mice immunized with lysate plus CT were protected significantly from a primary infection (P < 0.01). Results represent the composite of two consecutive experiments with eight mice per group, the results of which were so similar and overlapping that data were pooled for the graphic representation. The Mann-Whitney test was used for statistical analysis of differences between groups. ∗∗, P < 0.01 compared to the control group.
Treatment with CT at the time of initial infection protects against reinfection.
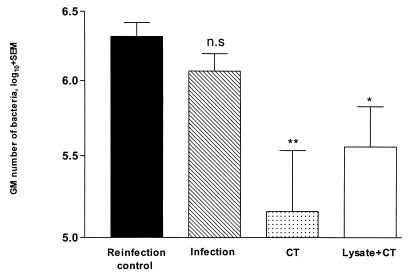
We were also interested in determining the effects of the treatments described above on protection against reinfection, after complete eradication of the initial infection with the aid of antibiotic treatment. Three groups of C57BL/6 mice were infected and treated as described above. Six weeks after infection, all mice were given 5 days of antibiotic treatment, and 1 week later they were infected in the same way as for the initial infection. At this time point a fourth group of naive age-matched mice were also infected (reinfection controls). Additionally, mice initially infected and treated with antibiotics but not subsequently reinfected served as controls to confirm complete eradication of the infection. Interestingly, for the mice administered CT alone at the time of infection, quantitative culture of gastric samples 2 weeks after reinfection revealed a 20-fold decrease in the number of bacteria (Fig. 2), in contrast to the lack of effect of this treatment on the primary infection. The animals that had been immunized with lysate plus CT also showed a significant sixfold reduction in bacterial load after reinfection, compared with reinfection controls. A previous infection in the absence of specific immunization did not protect appreciably against reinfection (Fig. 2).
FIG. 2.
Treatment with CT or immunization with lysate plus CT during a primary infection leads to protection against reinfection. Groups of mice were infected with H. pylori SS1 and treated with CT or immunized with lysate plus CT as indicated in the legend for Fig. 1. The primary infection was eradicated after 6 weeks with antibiotics, and the animals were reinfected with H. pylori SS1. Reinfection controls were naive mice infected with H. pylori SS1. Results represent the composite of two consecutive experiments with eight mice per group, the results of which were so similar and overlapping that the data were pooled for the graphic representation. Statistical evaluation of differences between treatment groups was done by the Mann-Whitney test. ∗∗, P < 0.01; ∗, P < 0.04; n.s, P > 0.05.
Immune responses after infection and therapeutic immunization.
To study the immune responses to infection and therapeutic immunization, sera, spleen, and MLN from groups of mice were collected 6 weeks after infection with H. pylori SS1 and specific antibody titers and cell proliferation were examined.
Serum antibodies.
High antibody titers against H. pylori were seen in the infected controls, and these titers were not changed significantly by treatment with CT. However repeated immunization with lysate plus CT resulted in significantly higher (P < 0.01) antibody titers in response to MP of H. pylori SS1 than those for the infected control group (Table 1). Analyses of IgG isotypes for the same antigen revealed that, while treatment with CT alone tended to enhance both Th2-driven IgG1 and Th1-driven IgG2a titers against H. pylori slightly, there was a much more marked rise in titers resulting from specific immunization with lysate plus CT, especially in the IgG2a response, which was significantly (P < 0.01) increased over the IgG1 responses in the infection controls and in the infected mice treated with CT only (Table 1).
TABLE 1.
Immune responses after infection and vaccination in the various treatment groups 6 weeks after initial infection
| Group | Antibody titers in response to MPa | Titers of:
|
Proliferationb of cells from:
|
||
|---|---|---|---|---|---|
| IgG1 | IgG2a | Spleen | MLN | ||
| Control | 4,400 + 1,934 | 778 + 216 | 58 + 9 | 4,530 ± 712 | 2,896 ± 448 |
| CT | 4,000 + 1,605 | 594 + 1,050 | 67 + 94 | 7,775 ± 1,317 | 13,652 ± 191 |
| Lysate + CT | 12,000 + 2,113d | 1,783 + 1,139 | 648 + 306c | 8,934 ± 2,551 | 9,815 ± 663 |
Serum antibody titers in response to whole-membrane antigen preparation of H. pylori SS1 were measured by enzyme-linked immunosorbent assay as described in Materials and Methods. Results are expressed as geometric mean antibody titers of individual mice in a group plus standard errors of the means.
Proliferation of cells from spleen or MLN in response to lysate preparation of H. pylori SS1 was measured by radioactive thymidine uptake after 4 days in culture. Values shown are means ± SD of stimulated triplicate cultures after subtraction of the means of unstimulated control cultures.
Value significantly higher than those for both the infected-control (P < 0.01) and the CT-treated groups.
Significantly higher than value for infection controls.
Proliferative responses.
Mononuclear cells from the spleen and MLN of mice from the differently treated groups were stimulated with H. pylori SS1 lysate antigen, and their proliferative responses were measured after 4 days of in vitro culture. In infected mice treated with CT, the proliferative responses to antigen shown by cells from both the spleen and, to a greater degree, the MLN were higher than that shown by cells from infected untreated controls (Table 1). A similar increase in the proliferative response to the same H. pylori antigens was seen in the group immunized with lysate plus CT (Table 1). The proliferative response to purified urease antigen was found to be lower than that achieved with the lysate preparation, and also, in an apparent difference from the responses to lysate, the responses to urease in infected controls and the two treatment groups were not different (data not shown). Proliferation in response to H. pylori SS1 LPS was very low, with values similar to the unstimulated background levels in both spleen and MLN cells (data not shown).
Protection after specific vaccination is associated with gastric inflammation.
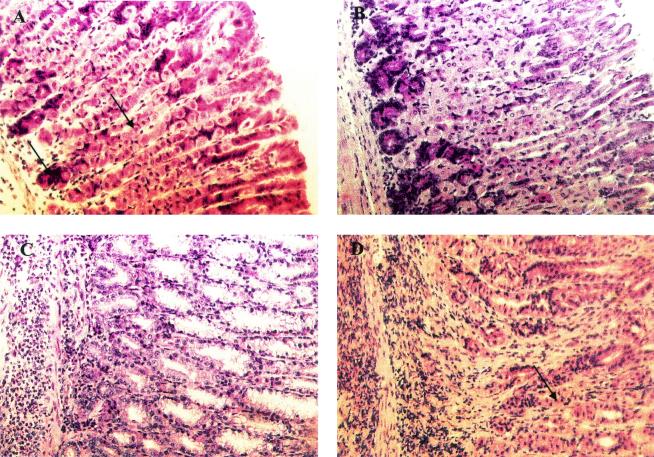
Previous work using prophylactic vaccination with H. pylori antigens showed a correlation between the extent of inflammation and the vaccine-induced protection (13). We therefore evaluated the gastric pathology of all mice both after therapeutic immunization and after reinfection. As shown in Table 2 and Fig 3A, infected control mice did not show any overt gastric inflammation for the duration of the experiment. In contrast, infection followed by therapeutic vaccination with lysate plus CT, which resulted in significant protection against infection, also led to severe gastritis with both atrophy, defined as the loss of chief cells and parietal cells, and mucus cell hyperplasia, together with intense inflammation (Table 2; Fig. 3B). Following immunization during the primary infection and eradication of any residual infection by antibiotic treatment over 5 days, there was a progressive resolution of immunization-induced gastritis over the next few weeks (data not shown). A different picture was seen, however, in similarly treated groups that were reinfected with H. pylori. In animals which had been previously infected but not vaccinated, the reinfection gave rise to mild inflammation but no detectable atrophy (Table 2). However, in the groups that had previously been both infected and vaccinated, the reinfection gave rise to mucosal inflammation with a severity similar to that of postimmunization gastritis after the primary infection, although with a tendency to less-severe atrophy (Table 2 and Fig. 3D).
TABLE 2.
Histopathology in the corpus mucosas of H. pylori-infected vaccinated mice in relation to immunization status
| Group | Score for:
|
Protection factorsc | |
|---|---|---|---|
| Atrophya | Inflammationb | ||
| Therapeutic immunization model | |||
| Infected Control | 0 ± 0 | 0.4 ± 0.4 | 1.6 ± 1.9 |
| CT | 1 ± 1.8 | 1 ± 1 | 1.0 ± 0.7 |
| Lysate + CT | 5 ± 2d | 5. ± 1.6d | 10 ± 12 |
| Reinfection model | |||
| Reinfection control | 0 ± 0 | 0.25 ± 0.2 | 1.5 ± 1.5 |
| Infection | 0 ± 0 | 1.3 ± 1 | 4 ± 4.3 |
| CT | 0 ± 0e | 0.8 ± 1e | 18 ± 21 |
| Lysate + CT | 4 ± 1 | 4 ± 0.3 | 40 ± 46 |
Atrophy of the corpus and fundus is defined in the Sydney system of grading gastritis as the loss of acid-secreting parietal cells and zymogen-secreting chief cells in the stomach, graded on a scale of 0 to 6. Individual mice were scored, and the mean score of each group is presented.
Inflammation is defined as infiltration of leukocytes in the lamina propria of gastric tissue, graded on a scale of 0 to 6. Individual mice were scored, and the mean score of each group is presented.
Protection factors were calculated as the ratio of the geometric mean number of bacteria in infected control mice to the number of bacteria in individual vaccinated mice ± SD.
P < 0.001 compared to value for infected control group.
P < 0.001 compared to value for the group immunized with lysate plus CT after reinfection.
FIG. 3.
Histopathology of mice infected with H. pylori. (A) Oxyntic mucosa of an infected mouse (6 to 8 weeks old) displaying normal morphology. Chief cells and parietal cells in the oxyntic mucosa can be seen (arrows). (B) Mucosa of a mouse treated with CT and examined after reinfection challenge, showing a normal morphology comparable to that of infected mice except for mild focal inflammation. (C) Mucosa of an infected mouse vaccinated with lysate plus CT showing massive lymphocyte infiltration and destruction of chief cells and parietal cells, which are replaced by undifferentiated mucus-secreting cells. (D) Mice immunized with lysate plus CT after eradication and reinfection showing severe inflammation but also the reappearance of some parietal cells (arrow).
Protection against reinfection after treatment with CT is not associated with gastric inflammation.
The gastric pathology in the CT-treated groups, both after primary infection and after reinfection, was also studied. Six weeks after the initial infection, the CT-treated mice, which then had no change in the colonization level compared to the control infected mice, had no gastritis either, except in two out of six mice where atrophy and inflammation were observed in a small region close to the fore stomach. Interestingly, even though a stronger protection against reinfection was seen in the CT-only-treated group than in the group immunized with lysate plus CT, the CT treatment was not associated with any gastritis, in sharp contrast to the severe gastritis after reinfection in the group immunized with lysate plus CT. The epithelium had normal morphology except for a small focal inflammation at the base of the oxyntic glands (Table 2 and Fig. 3C).
DISCUSSION
Based on the findings that prophylactic oral immunization with various antigen preparations could suppress H. pylori infection in a mouse model, recent efforts have largely been focused on testing whether immunization could also protect against an already-established infection (7, 16, 18). In areas where H. pylori infection is endemic, a situation found in many developing and transitional countries, the populations are at a high risk of acquiring reinfections (17, 30, 38, 41). This makes it important to also determine protection against reinfection after antibiotic treatment of the primary infection, either as a result of an immunizing effect of the primary infection or after specific vaccination. In this study we show that oral immunization of H. pylori-infected mice with lysate was able to reduce the primary infection sixfold and offered similar partial protection against reinfection. An enhanced humoral as well as cellular immune response but also postimmunization gastritis were seen after therapeutic immunization which was associated with measurable protection. In contrast, oral administration of CT at the time of the primary infection, while having no clear effect on the extent of the initial infection, had a marked effect against reinfection, with a 20-fold reduction in bacterial load without causing any gastritis. Infection alone, in the absence of vaccination or CT treatment, did not confer any significant protection against a later reinfection.
Our findings that therapeutic immunization with a lysate preparation could both suppress an established primary infection and offer partial protection against reinfection are consistent with previous reports for an H. pylori mouse model (16). The vaccination-induced reduction of bacterial load by 1 to 2 log units is similar to the level of protection after vaccination reported by others using the highly virulent SS1 strain of H. pylori, which is known to be much more difficult to suppress by specific immunotherapy than H. pylori strains used in the previous reports (18, 29, 34, 36). It should also be emphasized that this level of protection most likely is fully compatible with the protection data reported by others that were based on histological detection of bacteria (5, 36). Thus Sutton et al. (36) found, when comparing the sensitivities of different methods, that histology had a detection limit of 105 bacteria/g of gastric tissue and that lesser levels of infection may not be detected. A key question is then whether the levels of vaccine-induced immune responses reported here could if extended to therapeutic vaccination in humans be sufficient to tip the balance of infection from persistence toward eradication. Although this remains unclear in our study, data from animal studies are consistent with such an effect. Mohammadi et al. (27) have, in the Helicobacter felis infection model, compared the kinetics of infection and gastritis in infected or immunized challenged mice. While infection with H. felis persisted and increased the severity of gastritis over a period of 16 weeks, the immunized challenged mice eradicated their infection and, more interestingly, resolved their postimmunization gastritis over the same 16-week period.
It is known that protection against H. pylori infection by specific immunization can be achieved in mice deficient in B cells or CD8+ but not CD4+ T cells (13, 29, 35). This indicates a critical role for CD4+ T-cell-mediated immunity in protection. In accordance with the important role of cell-mediated immunity for protection after vaccination, we found that T cells from mice immunized with lysate or given CT together with the infection had a much higher proliferative response to H. pylori antigens than those from the untreated infected controls. In addition the specific antibody levels in response to H. pylori were elevated as a result of the immunization, and analyses of isotypes of H. pylori-specific serum antibodies showed increased levels of both Th2-driven IgG1 and Th1-driven IgG2a antibodies, with the increases in the latter levels being more pronounced than the increases in the former.
It is noteworthy that repeated intragastric immunization with whole bacterial lysate antigen from H. pylori could protect against both the primary infection and reinfection, while the coadministration of CT and the infectious dose had no detectable effect on the primary infection even though it was strongly protective against reinfection. We speculate that this difference is mainly due to the markedly different amounts of antigen reaching the inductive sites for generating protective immunity in the two types of immunization. The locations of these inductive sites for evoking protective immunity against H. pylori infection are not well defined, but recent evidence from the immunization with a model vaccine of H. pylori-infected volunteers suggests an important role for the upper part of the small intestine as an inductive site. In this regard, it was shown that administration of a cholera vaccine through intubation into the small intestine (with prevention of any retrograde reflux of antigen) stimulated a strong vaccine-specific immune response in the stomach mucosa. Thus, in H. pylori-infected individuals, the inflamed stomach mucosa itself may also serve as an inductive site (33). In the mouse model used in this study, the repeated intragastric immunizations with lysate clearly provide much higher antigen amounts for stimulating an immune response than are provided by the infection itself. The important effect of CT, compared with the infection-only situation, is then most likely to act as a strong mucosal adjuvant. As such CT allows the limited amounts of H. pylori antigen from the infection to become sufficiently immunogenic to generate an effective memory immune response, although the enhancement of the acute response is not strong enough for controlling the initial infection.
A finding in this study, and perhaps a consequence of the elevated T-cell response to H. pylori SS1 lysate immunization, was that the vaccine-induced protection against both the primary infection and reinfection was associated with atrophic gastritis and inflammation in the corpus mucosa of the stomach, a phenomenon often referred to as postimmunization gastritis (12). This contrasts with a recent study by Sutton et al. in which they reported a reduction in bacterial load after prophylactic immunization of C57BL/6 mice with H. pylori SS1 lysate antigen in the absence of any postimmunization gastritis (34). Reasons for the discrepancies between the studies could be a difference between prophylactic and therapeutic vaccination regimens and the differences in environmental conditions and/or flora of the mice with which these experiments were performed (28). However, neither of these explanations are plausible in view of the fact that Akhiani et al. (2nd Meet. Eur. Mucosal Immunol. Group, abstr. D25 and D29) have recently also described severe postimmunization gastritis after prophylactic immunization in experiments carried out in the same animal facility as our study.
It has been suggested that a vaccine-induced Th1 T-cell response leads to aggravation of gastritis in Helicobacter-infected mice, while a Th2 response instead favors protection (15, 28). Our findings of an increased cellular immune response to H. pylori after immunization and evidence of enhanced Th1 as well as Th2 activity are therefore well consistent with the combined effects of gastritis and protection achieved by the vaccination. However perhaps the most interesting finding in this study was that CT had a protective effect in the absence the any postimmunization gastritis. This might be explained by the observations that immunization with CT leads to longstanding immunological memory (37) and that the immune response induced is tilted toward a protective Th2 immunity, with lesser stimulation of proinflammatory Th1 activity, as supported both by the present data and by findings with other antigens (24).
In conclusion, we report here that immunization with an H. pylori lysate vaccine offers partial protection against both the primary infection and against a later reinfection. Treatment of infected animals with CT alone was able to markedly reduce the bacterial load by 20-fold upon reinfection without causing any postimmunization gastritis, in sharp contrast to immunization with lysate vaccine, which gave rise to a similar level of protection but then in combination with severe gastritis. While there is evidence that these effects may be explained by differential stimulation of protective Th2 versus gastritis-promoting Th1 activity by the different treatments, further studies to prove the role of specific T-cell subsets and perhaps secreted cytokines in protection and gastritis are needed. This would have an impact on efforts to design a protective vaccine against H. pylori infection in humans that does not cause gastritis.
Acknowledgments
We thank the Swedish Medical Research Council for project support (projects 6x-3382 and 6x-9084) and The Knut and Alice Wallenberg Foundation for financial support to GUVAX.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Achtman, M., G. Morelli, and S. Schwuchow. 1978. Cell-cell interactions in conjugating Escherichia coli: role of F pili and fate of mating aggregates. J. Bacteriol. 135:1053-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaser, M. J. 1990. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J. Infect. Dis. 161:626-633. [DOI] [PubMed] [Google Scholar]
- 3.Blaser, M. J., and J. Parsonnet. 1994. Parasitism by the “slow” bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J. Clin. Investig. 94:4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolin, I., H. Lonroth, and A. M. Svennerholm. 1995. Identification of Helicobacter pylori by immunological dot blot method based on reaction of a species-specific monoclonal antibody with a surface-exposed protein. J. Clin. Microbiol. 33:381-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corthesy-Theulaz, I., N. Porta, M. Glauser, E. Saraga, A. C. Vaney, R. Haas, J. P. Kraehenbuhl, A. L. Blum, and P. Michetti. 1995. Oral immunization with Helicobacter pylori urease B subunit as a treatment against Helicobacter infection in mice. Gastroenterology 109:115-121. [DOI] [PubMed] [Google Scholar]
- 6.Corthesy-Theulaz, I. E., S. Hopkins, D. Bachmann, P. F. Saldinger, N. Porta, R. Haas, Y. Zheng-Xin, T. Meyer, H. Bouzourene, A. L. Blum, and J. P. Kraehenbuhl. 1998. Mice are protected from Helicobacter pylori infection by nasal immunization with attenuated Salmonella typhimurium phoPc expressing urease A and B subunits. Infect. Immun 66:581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuenca, R., T. G. Blanchard, S. J. Czinn, J. G. Nedrud, T. P. Monath, C. K. Lee, and R. W. Redline. 1996. Therapeutic immunization against Helicobacter mustelae in naturally infected ferrets. Gastroenterology 110:1770-1775. [DOI] [PubMed] [Google Scholar]
- 8.Dixon, M. F. 1991. Helicobacter pylori and peptic ulceration: histopathological aspects. J. Gastroenterol. Hepatol. 6:125-130. [DOI] [PubMed] [Google Scholar]
- 9.Dixon, M. F., R. M. Genta, J. H. Yardley, and P. Correa. 1996. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 20:1161-1181. [DOI] [PubMed] [Google Scholar]
- 10.Dunn, B. E., G. P. Campbell, G. I. Perez-Perez, and M. J. Blaser. 1990. Purification and characterization of urease from Helicobacter pylori. J. Biol. Chem. 265:9464-9469. [PubMed] [Google Scholar]
- 11.Eaton, K. A., S. R. Ringler, and S. J. Danon. 1999. Murine splenocytes induce severe gastritis and delayed-type hypersensitivity and suppress bacterial colonization in Helicobacter pylori-infected SCID mice. Infect. Immun. 67:4594-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ermak, T. H., R. Ding, B. Ekstein, J. Hill, G. A. Myers, C. K. Lee, J. Pappo, H. K. Kleanthous, and T. P. Monath. 1997. Gastritis in urease-immunized mice after Helicobacter felis challenge may be due to residual bacteria. Gastroenterology 113:1118-1128. [DOI] [PubMed] [Google Scholar]
- 13.Ermak, T. H., P. J. Giannasca, R. Nichols, G. A. Myers, J. Nedrud, R. Weltzin, C. K. Lee, H. Kleanthous, and T. P. Monath. 1998. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J. Exp. Med. 188:2277-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans, D. J., Jr., D. G. Evans, L. Engstrand, and D. Y. Graham. 1992. Urease-associated heat shock protein of Helicobacter pylori. Infect. Immun. 60:2125-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox, J. G., P. Beck, C. A. Dangler, M. T. Whary, T. C. Wang, H. N. Shi, and C. Nagler-Anderson. 2000. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat. Med. 6:536-542. [DOI] [PubMed] [Google Scholar]
- 16.Ghiara, P., M. Rossi, M. Marchetti, A. Di Tommaso, C. Vindigni, F. Ciampolini, A. Covacci, J. L. Telford, M. T. De Magistris, M. Pizza, R. Rappuoli, and G. Del Giudice. 1997. Therapeutic intragastric vaccination against Helicobacter pylori in mice eradicates an otherwise chronic infection and confers protection against reinfection. Infect. Immun 65:4996-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurel, S., F. Besisk, K. Demir, Z. Mungan, S. Kaymakoglu, G. Boztas, Y. Cakaloglu, O. Yeginsu, and A. Okten. 1999. After the eradication of Helicobacter pylori infection, relapse is a serious problem in Turkey. J. Clin. Gastroenterol. 28:241-244. [DOI] [PubMed] [Google Scholar]
- 18.Ikewaki, J., A. Nishizono, T. Goto, T. Fujioka, and K. Mifune. 2000. Therapeutic oral vaccination induces mucosal immune response sufficient to eliminate long-term Helicobacter pylori infection. Microbiol. Immunol. 44:29-39. [DOI] [PubMed] [Google Scholar]
- 19.Jonson, G., A. M. Svennerholm, and J. Holmgren. 1989. Vibrio cholerae expresses cell surface antigens during intestinal infection which are not expressed during in vitro culture. Infect. Immun. 57:1809-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keenan, J., J. Oliaro, N. Domigan, H. Potter, G. Aitken, R. Allardyce, and J. Roake. 2000. Immune response to an 18-kilodalton outer membrane antigen identifies lipoprotein 20 as a Helicobacter pylori vaccine candidate. Infect. Immun 68:3337-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, A., J. G. Fox, G. Otto, and J. Murphy. 1990. A small animal model of human Helicobacter pylori active chronic gastritis. Gastroenterology 99:1315-1323. [DOI] [PubMed] [Google Scholar]
- 22.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 23.Marchetti, M., B. Arico, D. Burroni, N. Figura, R. Rappuoli, and P. Ghiara. 1995. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science 267:1655-1658. [DOI] [PubMed] [Google Scholar]
- 24.Marinaro, M., H. F. Staats, T. Hiroi, R. J. Jackson, M. Coste, P. N. Boyaka, N. Okahashi, M. Yamamoto, H. Kiyono, H. Bluethmann, et al. 1995. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J. Immunol. 155:4621-4629. [PubMed] [Google Scholar]
- 25.Marshall, B. J., and J. R. Warren. 1984. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet i:1311-1315. [DOI] [PubMed] [Google Scholar]
- 26.Mattsson, A., A. Tinnert, A. Hamlet, H. Lonroth, I. Bolin, and A. M. Svennerholm. 1998. Specific antibodies in sera and gastric aspirates of symptomatic and asymptomatic Helicobacter pylori-infected subjects. Clin. Diagn. Lab. Immunol. 5:288-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohammadi, M., S. Czinn, R. Redline, and J. Nedrud. 1996. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J. Immunol. 156:4729-4738. [PubMed] [Google Scholar]
- 28.Mohammadi, M., J. Nedrud, R. Redline, N. Lycke, and S. J. Czinn. 1997. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology 113:1848-1857. [DOI] [PubMed] [Google Scholar]
- 29.Pappo, J., D. Torrey, L. Castriotta, A. Savinainen, Z. Kabok, and A. Ibraghimov. 1999. Helicobacter pylori infection in immunized mice lacking major histocompatibility complex class I and class II functions. Infect. Immun. 67:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patchett, S., S. Beattie, E. Leen, C. Keane, and C. O'Morain. 1992. Helicobacter pylori and duodenal ulcer recurrence. Am. J. Gastroenterol. 87:24-27. [PubMed] [Google Scholar]
- 31.Peterson, G. L. 1977. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 83:346-356. [DOI] [PubMed] [Google Scholar]
- 32.Peterson, W. L. 1991. Helicobacter pylori and peptic ulcer disease. N. Engl. J. Med. 324:1043-1048. [DOI] [PubMed] [Google Scholar]
- 33.Quiding-Jarbrink, M., H. Lonroth, I. Ahlstedt, J. Holmgren, and A. M. Svennerholm. 2001. Human gastric B cell responses can be induced by intestinal immunisation. Gut 49:512-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutton, P., S. J. Danon, M. Walker, L. J. Thompson, J. Wilson, T. Kosaka, and A. Lee. 2001. Post-immunisation gastritis and Helicobacter infection in the mouse: a long term study. Gut 49:467-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutton, P., J. Wilson, T. Kosaka, I. Wolowczuk, and A. Lee. 2000. Therapeutic immunization against Helicobacter pylori infection in the absence of antibodies. Immunol. Cell Biol. 78:28-30. [DOI] [PubMed] [Google Scholar]
- 36.Sutton, P., J. Wilson, and A. Lee. 2000. Further development of the Helicobacter pylori mouse vaccination model. Vaccine 18:2677-2685. [DOI] [PubMed] [Google Scholar]
- 37.Vajdy, M., and N. Y. Lycke. 1992. Cholera toxin adjuvant promotes long-term immunological memory in the gut mucosa to unrelated immunogens after oral immunization. Immunology 75:488-492. [PMC free article] [PubMed] [Google Scholar]
- 38.Veenendaal, R. A., A. S. Pena, J. L. Meijer, H. P. Endtz, M. M. van der Est, W. van Duijn, F. Eulderink, J. Kreuning, and C. B. Lamers. 1991. Long term serological surveillance after treatment of Helicobacter pylori infection. Gut 32:1291-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westphal, O., and K. Jann. 1965. Bacterial lipopolysaccharide: extraction with phenol-water and further applications of the procedure, p. 83-92. In R. Whitler (ed.), Methods in carbohydrate chemistry. Academic Press, New York, N.Y.
- 40.Wong, B. C., S. K. Lam, K. C. Lai, W. H. Hu, C. K. Ching, J. Ho, S. T. Yuen, C. K. Chan, G. K. Lau, and C. L. Lai. 1999. Triple therapy for Helicobacter pylori eradication is more effective than long-term maintenance antisecretory treatment in the prevention of recurrence of duodenal ulcer: a prospective long-term follow-up study. Aliment. Pharmacol. Ther. 13:303-309. [DOI] [PubMed] [Google Scholar]
- 41.Xia, H. X., N. J. Talley, C. T. Keane, and C. A. O'Morain. 1997. Recurrence of Helicobacter pylori infection after successful eradication: nature and possible causes. Dig. Dis. Sci. 42:1821-1834. [DOI] [PubMed] [Google Scholar]