Abstract
Anaplasma phagocytophila, an obligately intracellular bacterium of granulocytes, causes human granulocytic ehrlichiosis. Within 2 h after addition of A. phagocytophila, interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), and IL-6 mRNAs are induced in human peripheral blood leukocytes (PBLs) or monocytes in vitro. However, neutrophils generate only IL-1β mRNA. In the present study, signaling pathways for induction of these three cytokines were examined. TNF-α and IL-6 mRNA expression by PBLs was inhibited with SB 203580 (a p38 mitogen-activated protein kinase [MAPK] inhibitor), MG-132 (a proteasome inhibitor), and SN-50 (an NF-κB inhibitor). Activation of p38 MAPK and NF-κB mRNAs in monocytes was detectable within 15 to 30 min after addition of A. phagocytophila. Expression of these two cytokine mRNAs in PBLs and monocytes was also dependent on protein kinase C (PKC), protein kinase A (PKA), and protein tyrosine kinase (PTK). IL-1β mRNA expression by neutrophils was not dependent on p38 MAPK, and p38 MAPK was not activated in neutrophils incubated with A. phagocytophila. IL-1β mRNA induction by PBLs, monocytes, and neutrophils was dependent on PKC and PKA. Neutrophil expression of IL-1β mRNA was dependent on transglutaminase, phospholipase C, and PTK, all of which are also required for internalization of A. phagocytophila. However, monocyte expression of IL-1β mRNA was less dependent on these enzymes. These results suggest that A. phagocytophila transduces different signals between its host neutrophils and monocytes for proinflammatory cytokine generation.
Human granulocytic ehrlichiosis (HGE), an acute febrile systemic disease first described in 1994, has been increasingly recognized in the United States (5, 8, 33) and various parts of Europe (23, 30, 38). HGE is characterized by fever, chills, headache, myalgia, and hematological abnormalities such as thrombocytopenia and leukopenia, as well as increased serum aminotransferase activities (4, 25). HGE may be fatal in elderly and/or immunocompromised patients or when antibiotic treatment is delayed. The etiological agent of HGE is a strain of Anaplasma phagocytophila (8, 11). A. phagocytophila, an obligately intracellular gram-negative bacterium, propagates in the membrane-bound inclusions of granulocytes. Because very few organisms are usually detected in patients' blood even at the height of illness, we and other workers have speculated that the illness is not caused directly by A. phagocytophila but rather is caused by proinflammatory cytokines generated by the host in response to A. phagocytophila infection (19).
Using human peripheral blood leukocytes (PBLs), we recently found that A. phagocytophila HZ isolated from an acute-stage HGE patient (42) and the recombinant 44-kDa major outer membrane protein (rP44) cloned from this isolate (51) induce rapid, strong proinflammatory cytokine (interleukin-1β [IL-1β], tumor necrosis factor alpha [TNF-α], and IL-6) mRNA expression within 2 h and protein secretion within 24 h in vitro (19). Within the 2-h period after incubation with A. phagocytophila, IL-8, IL-10, gamma interferon, IL-2, and transforming growth factor β mRNAs were not consistently upregulated in PBLs, suggesting that expression of these cytokines either is not induced or is induced at later time points in vitro. Although human promyelocytic leukemia cell line HL-60 cells are widely used for analysis of cytokine gene expression, IL-1β, TNF-α, or IL-6 mRNA was not upregulated in HL-60 cells within 2 h of incubation (Kim and Rikihisa, unpublished data). Using HL-60 cells, other researchers have also reported an absence of IL-1β, TNF-α, and IL-6 induction, and IL-8 mRNA is not detectable at 12 h postinfection (2, 20). Since A. phagocytophila is granulocyte tropic, the monocyte responses to this bacterium have been neglected. However, when we separated neutrophils and monocytes in human PBLs prior to addition of A. phagocytophila or 2 h after stimulation with A. phagocytophila, we found that monocytes are the cells that express mRNAs of IL-1β, TNF-α, and IL-6, whereas neutrophils express only IL-1β mRNA. Thus, collectively, PBLs produce all three of these cytokines in response to A. phagocytophila. This means that A. phagocytophila has the ability to selectively activate monocytes to induce proinflammatory cytokine generation, but in neutrophils this activity may be suppressed (19). We previously reported an analogous difference between human monocytes and neutrophils; A. phagocytophila inhibits generation of superoxide in neutrophils but not in monocytes in response to various stimuli (34). Differences in the signals transduced by A. phagocytophila in these two types of primary host defensive cells may be critical in understanding the mechanism of its selective survival in granulocytes and HGE pathogenesis. For example, lesions found in the liver in HGE patients are lymphohistiocytic rather than granulocytic infiltrates (25).
Transcription of IL-1β, TNF-α, and IL-6 mRNAs is regulated by at least two different mechanisms. One mechanism involves nuclear translocation of cytoplasmic latent transcription factors, such as activator protein 1 (AP-1)/c-Jun, c-Fos, NF-κB, or NF-IL-6, and binding of these factors to the appropriate enhancer elements present in the promoter regions of IL-1β, TNF-α, and IL-6 genes (10, 27, 31, 37, 39, 40, 46). Another mechanism involves activation of mitogen-activated protein kinase (MAPK) family members that modulate the activity of transcription factors by phosphorylation (10, 45). In the present study, we examined the involvement of NF-κB or other transcription factors and the roles of MAPK and other protein kinases in the rapid induction of proinflammatory cytokines in human PBLs in order to understand responses by the total mixed leukocyte population and in separated monocytes and neutrophils in order to understand cell type-specific responses to A. phagocytophila.
MATERIALS AND METHODS
Cultures.
A. phagocytophila HZ isolated from an HGE patient (42) was propagated in human promyelocytic leukemia cell line HL-60 (American Type Culture Collection, Manassas, Va.) as described elsewhere (51).
Preparation of host-cell-free A. phagocytophila.
When >90% of the HL-60 cells were infected, as determined by Diff-Quik staining (Baxter Scientific Products, Obetz, Ohio), a cell suspension (107 cells in 5 ml of RPMI 1640 medium) was sonicated by using an ultrasonic processor (model W-380; Heat Systems, Farmingdale, N.Y.) and the predetermined minimum damaging conditions for A. phagocytophila (setting 2, 20 kHz for 7 s) and was centrifuged at 500 × g for 5 min. The supernatant containing host-cell-free A. phagocytophila was centrifuged at 10,000 × g for 10 min at 4°C. Because A. phagocytophila is small and multiplies as microcolonies (morulae) in the cytoplasm of granulocytes, it is impractical to accurately count individual organisms. Therefore, the number of host-cell-free A. phagocytophila cells was estimated as described previously (19).
Preparation of human PBLs, neutrophils, and monocytes.
Human PBLs, neutrophils, and monocytes were isolated from buffy coats from healthy human immunodeficiency virus-negative donors as described previously (19). Briefly, peripheral blood buffy coats were centrifuged at 500 × g for 5 min. Erythrocytes were lysed in a sterile 0.83% NH4Cl solution for 5 min at room temperature, and PBLs were washed twice in phosphate-buffered saline (PBS) (137 mM NaCl, 10 mM Na2HPO4, 2.7 mM KCl, 1.8 mM KH2PO4; pH 7.2). To separate neutrophils, buffy coats diluted 1:2 in PBS were layered on Ficoll-Paque Plus (Pharmacia, Uppsala, Sweden) and centrifuged at 750 × g for 15 min at room temperature. The pellet was washed twice in PBS with centrifugation at 400 × g for 5 min and suspended in RPMI 1640 medium containing 10% fetal bovine serum (FBS). The cell suspension was layered on top of a 62% Percoll (Pharmacia) solution and centrifuged at 400 × g for 15 min at room temperature, and the band of neutrophils was collected. The percentage of neutrophils in the preparation was >95%, as assessed by morphological examination of Diff-Quik-stained cells. To obtain adherent monocytes, the interface resulting from Ficoll-Paque Plus (Pharmacia) gradient centrifugation was collected, washed twice in PBS, and incubated in RPMI 1640 medium containing 10% FBS in 150-mm-diameter culture dishes (Corning, Corning, N.Y.) at 37°C for 2 h; floating lymphocytes were discarded, and adherent monocytes were used for the study. The viabilities of PBLs, neutrophils, and monocytes were >98%, as assessed by trypan blue dye exclusion tests. All sets of experiments were independently repeated two or more times by using human PBLs, monocytes, and neutrophils derived from different blood donors. Donor cells were never mixed, and each donor leukocyte assay included positive and negative controls to ensure the quality of both leukocyte and A. phagocytophila preparations. Data obtained with blood specimens from 23 anonymous blood donors were included in this study.
Treatment of cells.
Human PBLs, neutrophils, and monocytes (107 cells each in 1 ml of RPMI 1640 medium in a well of a 24-well plate) or host-cell-free A. phagocytophila cells were separately preincubated at 37°C with the inhibitors at the concentrations and for the time periods shown in Table 1. After the preincubation, cells were incubated for an additional 2 h with the host-cell-free A. phagocytophila (100 bacteria/cell) in the presence of inhibitors. Host-cell-free A. phagocytophila was preincubated with 10 μg of oxytetracycline (Sigma) per ml for 30 min and was then incubated with cells. As controls, cells in RPMI 1640 medium were incubated with 10 μl of the same medium supplemented with 10% FBS containing either no lysate or the lysate derived from 107 uninfected HL-60 cells. The viabilities of PBLs, neutrophils, and monocytes were >98%, as assessed by trypan blue dye tests at the end of an experiment.
TABLE 1.
Inhibitors used for investigation of signals of proinflammatory cytokine mRNA induction by A. phagocytophila
| Inhibitor | Concn useda | Cells pretreated | Length of preincubation at 37°C (min)a | Cellular target or mechanism
|
Source | ||
|---|---|---|---|---|---|---|---|
| Cellular target or mechanism | 50% Inhibitory concnb | Refer- ence | |||||
| MG-132 | 50 μM | PBLs, monocytes | 60, 90 | Proteasome inhibitor, inhibits NF-κB acti- vation by preventing IκBd degradation | 3 μM | 17 | Biomol |
| SN-50 | 50 or 100 μg/ml | PBLs, monocytes | 30, 60, 90 | NF-κB nuclear translocation inhibitor | 50 μg/mlc | 29 | Biomol |
| SB 203508 | 10 μM | PBLs, monocytes, neutrophils | 60, 90 | p38 MAPK inhibitor | 600 nM | 24 | Calbiochem- Novabiochem |
| PD 098059 | 20 μM | PBLs, monocytes | 60, 90 | ERK/MEK inhibitor | 2 μM | 3 | Biomol |
| H-7 | 25 μM | PBLs, monocytes, neutrophils | 30 | PKC inhibitor | 6 μM | 15 | Biomol |
| H-89 | 50 μM | PBLs, monocytes, neutrophils | 30 | PKA inhibitor | 48 nM | 9 | Biomol |
| Genistein | 100 μM | PBLs, monocytes, neutrophils | 30 | PTK inhibitor | 2.6 μM | 1 | Sigma |
| MDC | 100 μM | PBLs, monocytes, neutrophils | 30 | Transglutaminase inhibitor, receptor- mediated endocytosis inhibitor | 15 μM | 26 | Sigma |
| Verapamil | 100 μM | PBLs, monocytes, neutrophils | 30 | Ca2+ channel blocker | 60 μM | 52 | Sigma |
| W-7 | 30 μM | PBLs, monocytes, neutrophils | 30 | Ca2+-calmodulin kinase antagonist | 25 μM | 16 | Sigma |
| Neomycin sulfate | 20 μM | PBLs, monocytes, neutrophils | 30 | PLC inhibitor | 1-10 μM | 48 | Sigma |
| CHX | 20 μg/ml | PBLs, monocytes, neutrophils | 30 | Eukaryote protein synthesis inhibitor | ∼0.1 μg/ml | 6 | Sigma |
| Oxytetracycline | 10 μg/ml | A. phagocytophila | 30 | Prokaryote protein synthesis inhibitor | ∼0.1 μg/ml | 18 | Sigma |
When two or more concentrations or incubation times were used, the concentration or time in boldface type was the concentration or time used in the experiments whose results are shown in the figures.
The values were obtained from the references and from the catalogs of Calbiochem-Novabiochem (La Jolla, Calif.) and Biomol Research Lab (Plymouth Meeting, Pa.).
Concentration that resulted in ∼85% inhibition.
IκB, an inhibitor protein of NF-κB.
RNA isolation and RT-PCR.
Total RNA was extracted from human PBLs, monocytes, and neutrophils (107 cells each) by using TRIzol reagent (GIBCO-BRL, Gaithersburg, Md.) as previously described (19). For reverse transcription (RT)-PCR, total cellular RNA (2 μg) was reverse transcribed in a 30-μl reaction mixture containing 1× reaction buffer (50 mM Tris-HCl [pH 8.3], 75 mM KCl, 3 mM MgCl2), deoxynucleoside triphosphates (0.5 mM each), 1 U of an RNase inhibitor (GIBCO-BRL), 1.5 μM oligo(dT) primer, and 10 U of Moloney murine leukemia virus reverse transcriptase (GIBCO-BRL) at 42°C for 1 h. The reaction was terminated by heating the mixture at 94°C for 5 min, and the cDNA (2 μl) was amplified in a 50-μl reaction mixture containing 1× PCR buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 1.5 mM MgCl2), deoxynucleoside triphosphates (0.2 mM each), and 0.4 μM (each) 3′ and 5′ primers (Clontech Laboratories, Inc., Palo Alto, Calif.) in a DNA thermal cycler (model 9700; Perkin-Elmer Corp., Norwalk, Conn.). Positive controls for three proinflammatory cytokines were obtained from Clontech. To reduce nonspecific priming, all PCRs were performed by the hot-start method. Taq DNA polymerase (2 U; GIBCO-BRL) was added after incubation of the mixture at 94°C for 5 min. One PCR cycle consisted of denaturation at 94°C for 45 s, annealing at 60°C for 45 s, and extension at 72°C for 2 min. The PCR was conducted for 25 cycles in all experiments. After a final extension for 7 min, 10 μl of PCR products was electrophoresed in a 1.5% agarose gel containing ethidium bromide (final concentration, 0.5 μg/ml). DNA size markers (HaeIII fragments of φX174 replicative-form DNA [GIBCO-BRL]) providing bands at 1,353 to 72 bp were run in parallel. The amounts of the target PCR products were analyzed by using a gel video system (Gel Print 2000i; BioPhotonics Corp., Ann Arbor, Mich.) and image analysis software (ImageQuant; Molecular Dynamics, Sunnyvale, Calif.), and values were normalized against glyceraldehyde-3-phosphate dehydrogenase (G3PDH) mRNA in a corresponding sample.
p38 immunoblotting.
Monocytes or neutrophils (2 × 107 cells) were incubated for 15, 30, and 60 min with A. phagocytophila (100 bacteria/cell). Cells were lysed by adding 100 μl of sodium dodecyl sulfate (SDS) sample buffer (62.5 mM Tris-HCl [pH 6.8], 2% SDS, 10% glycerol, 50 mM dithiothreitol [DTT], 0.1% bromophenol blue) and were transferred to a microcentrifuge tube. The extract was sonicated on ice for 2 s and then boiled at 100°C for 5 min, cooled on ice, and centrifuged for 5 min. Equal amounts (20 μl per lane) of supernatants were subjected to SDS-12% polyacrylamide gel electrophoresis, in which 10 μl of prestained broad-range molecular weight standards (Bio-Rad, Hercules, Calif.) and 15-μl portions of control p38 MAPK cell extracts (Cell Signaling Technology, Beverly, Mass.) were included, and then transferred to nitrocellulose membranes. Protein blots were incubated in blocking buffer (5% nonfat dry milk in Tris-buffered saline-Tween 20 [TBST] buffer [50 mM Tris-HCl, 150 mM NaCl, 0.1% Tween 20; pH 7.6]) for 1 h at room temperature and washed three times (5 min each) with 15 ml of TBST buffer. The membranes were incubated overnight at 4°C with rabbit anti-p38 (pTpY180/182) MAPK antibody (Biosource International, Inc., Camarillo, Calif.) or rabbit anti-p38 MAPK antibody (Cell Signaling Technology) at a dilution of 1:1,000 in TBST buffer-5% bovine serum albumin (Sigma). Horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) was added at a dilution of 1:2,000 in TBST buffer-5% bovine serum albumin and incubated for 3 h at room temperature. The peroxidase-positive bands were detected by immersing the blots in a developing solution (73 mM sodium acetate, pH 6.2) containing 0.3% diaminobenzidine tetrahydrochloride (Nacalai Tesque, Inc., Kyoto, Japan) and 0.04% H2O2 at room temperature for 5 min. The enzyme reaction was terminated by washing the blots in 0.1 M H2SO4.
To investigate the dependence of activation of p38 MAPK on other kinases, monocytes (107 cells) incubated for 30 min with genistein, H-89, and H-7 and exposed to A. phagocytophila (100 bacteria/cell) were lysed for 30 min in 500 μl of ice-cold radioimmunoprecipitation assay buffer (1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS in 1× PBS) containing several inhibitors (1 mM phenylmethylsulfonyl fluoride [PMSF], 1 μM aprotinin, 14 μM leupeptin, 1 μM pepstatin, 1 μM NaVO3, and 1 μM NaF). The peroxidase-positive bands were detected by using an enhanced chemiluminesence Western blot detection system according to the manufacturer's protocol (Cell Signaling Technology).
Nuclear extract preparation.
Human PBLs (108 cells/ml in each well) were incubated for 2 h at 37°C, and adherent monocytes were used. Nuclear extract was prepared at each time point (0, 30, 60, 120, and 240 min) after A. phagocytophila infection. Briefly, cells were centrifuged and washed with Tris-buffered saline (pH 7.9) and then were suspended in 400 μl of cold buffer A, which contained 10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA [ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid], 1 mM DTT, 0.5 mM PMSF, 1 μM aprotinin, 14 μM leupeptin, 1 μM pepstatin, and 80 μg of benzamidine per ml. After incubation on ice for 15 min, the cells were lysed by adding 24 μl of 10% NP-40 (final concentration, 0.6%). Lysis was completed by vigorous vortexing for 10 s. The homogenate was centrifuged at 13,000 × g for 30 s in a microcentrifuge, and the nuclear pellet was resuspended in 50 μl of cold buffer B, which contained 20 mM HEPES (pH 7.9), 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM PMSF, 1 μM aprotinin, 14 μM leupeptin, 1 μM pepstatin, and 80 μg of benzamidine per ml. After centrifugation at 13,000 × g for 30 s at 4°C, the resulting supernatant of nuclear extract was stored at −85°C.
EMSA.
Electrophoretic mobility shift assays (EMSA) were performed by using a gel shift assay system (Promega Corp., Madison, Wis.) according to the manufacturer's protocol. NF-κB (22 bp) and AP-1 (21 bp) consensus oligonucleotides provided in the kit (Promega) were end labeled with [γ-32P]ATP by using T4 nucleotide kinase, and 50,000 cpm of the probe per lane was used to assess binding. The reaction mixture (final volume, 25 μl) for the DNA-protein interaction experiment contained 2 μl of the nuclear extract in 10 mM Tris-HCl (pH 7.5), 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM DTT, 50 mM NaCl, and 0.05 μg of poly(dI-dC) per μl in 4% glycerol. The radioactive DNA probe was added to this mixture and incubated at room temperature for 20 min. After incubation, equal amounts of samples were loaded onto a 4% polyacrylamide gel that had been prerun in 0.5× TBE buffer (44 mM Tris, 44 mM borate, 1 mM EDTA; pH 8.3) for 30 min and then were electrophoresed at 100 V for 3 h. After the gel was exposed to X-ray Hyperfilm (Amersham-Pharmacia Biotech, Inc., Piscataway, N.J.) for 16 h at −85°C overnight, the film was developed. For the supershift assay, 2 μl of rabbit polyclonal immunoglobulin G to p65 (C-20) (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.) was added to the extract before incubation with the labeled NF-κB oligonucleotide.
Effect of anti-PSGL-1 on IL-1β mRNA induction in human neutrophils.
Neutrophils at a concentration of 107 cells per ml of RPMI 1640 medium were preincubated with anti-PGSL-1 monoclonal antibody (MAb) (PL-1; 20 μg/ml; Ancell Corp., Bayport, Minn.) for 40 min at 4°C and then incubated with host-cell-free A. phagocytophila (100 organisms/cell) for 2 h at 37°C. After incubation, total RNA was isolated and subjected to RT-PCR.
RESULTS
Involvement of p38 MAPK, NF-κB, and protein kinase C (PKC) in induction of IL-1β, TNF-α, and IL-6 mRNAs in human PBLs, neutrophils, or monocytes in response to A. phagocytophila.
To determine whether p38 MAPK and/or extracellular-signal-regulated kinase (ERK)/MAPK kinase (MEK) and NF-κB and/or AP-1 are required for proinflammatory cytokine gene expression in response to A. phagocytophila, the effects of several inhibitors on cytokine gene expression by human PBLs were examined by RT-PCR at the linear range determined previously based on our dose- and time-dependent study in which competitive RT-PCR was used (19). Constitutively expressed G3PDH mRNA levels were used to normalize the amount of input RNA across the samples, and stimulation with the medium or the HL-60 cell lysate was used as a control for the basal and background cytokine gene expression by each donor blood specimen. The contamination of RNA with genomic DNA was negligible because the PCR products from reverse transcriptase-minus controls were not detected in any specimens. A densitometric analysis of the PCR products was performed, and Table 2 shows the percentages of inhibition of IL-1β, TNF-α, and IL-6 mRNA levels by each inhibitor in PBLs, monocytes, and neutrophils from several different donor blood specimens.
TABLE 2.
Inhibition of IL-1β, TNF-α, and IL-6 mRNAs in human PBLs, monocytes, and neutrophils in response to A. phagocytophila
| Cytokin mRNA | Inhibitor | % Inhibitiona
|
||
|---|---|---|---|---|
| PBLs | Monocytes | Neutrophils | ||
| IL-1β | SN-50 | 50 ± 12 | 8, 19 | NDb |
| MG-132 | 44 ± 7 | 23, 61 | ND | |
| SB 203580 | 60 ± 34 | 26, 32 | 0, 0 | |
| PD 098059 | 17 ± 10 | 9, 11 | ND | |
| H-7 | 95 ± 4 | 84 ± 8 | 75 ± 20 | |
| H-89 | 41, 64 | 73 ± 2 | 69 ± 11 | |
| Genistein | 49, 50 | 30, 52 | 90 ± 7 | |
| MDC | 19, 29 | 35, 54 | 64 ± 3 | |
| Verapamil | 0, 0 | 17, 27 | 12, 23 | |
| W-7 | 21, 33 | 5, 3 | 8, 16 | |
| Neomycin | 0, 0 | 16, 24 | 68 ± 12 | |
| TNF-αc | SN-50 | 74 ± 34 | 25, 53 | |
| MG-132 | 91 ± 25 | 32, 46 | ||
| SB 203580 | 93 ± 36 | 70 ± 2 | ||
| PD 098059 | 55 ± 16 | 25, 44 | ||
| H-7 | 94 ± 4 | 82 ± 5 | ||
| H-89 | 44, 72 | 68 ± 9 | ||
| Genistein | 79 ± 6 | 75 ± 13 | ||
| MDC | 32, 43 | 49, 57 | ||
| Verapamil | 67 ± 9 | 81 ± 7 | ||
| W-7 | 46, 60 | 47, 66 | ||
| Neomycin | 63 ± 7 | 0, 0 | ||
| IL-6c | SN-50 | 59 ± 24 | 42, 78 | |
| MG-132 | 95 ± 1 | 34, 82 | ||
| SB 203580 | 97 ± 1 | 94 ± 2 | ||
| PD 098059 | 44, 54 | 37, 54 | ||
| H-7 | 94 ± 3 | 90 ± 7 | ||
| H-89 | 93 ± 3 | 75 ± 3 | ||
| Genistein | 91 ± 1 | 39, 67 | ||
| MDC | 33, 34 | 11, 23 | ||
| Verapamil | 39, 42 | 18, 19 | ||
| W-7 | 38, 40 | 0, 0 | ||
| Neomycin | 37, 39 | 16, 17 | ||
Percentages of inhibition were calculated with the following formula: [1 − (band density in the presence of inhibitor/band density of G3PDH) ÷ (band density without inhibitor/band density of G3PDH)] × 100. The values are means ± standard deviations from three independent experiments or actual values from two independent experiments. Values that consistently were >50% are in boldface type.
ND, not determined.
Neutrophils were not induced in response to A. phagocytophila.
SB 203580, a selective inhibitor of p38 MAPK, binds with high affinity to p38 MAPK near the ATP-binding site, thus rendering p38 MAPK inactive (24). SB 203580 blocked induction of TNF-α and IL-6 mRNAs in PBLs in response to A. phagocytophila, while IL-1β mRNA was partially blocked with SB 203580 (Fig. 1; Table 2). PD 098059, a specific inhibitor that binds inactive forms of MEK and prevents their activation and phosphorylation, resulting in inhibition of ERK/MEK (3), had consistently weaker inhibitory effects than SB 203580 upon induction of any of the three proinflammatory cytokine mRNAs (Fig. 1; Table 2).
FIG. 1.
IL-1β, TNF-α, and IL-6 mRNA induction in human PBLs preincubated with several inhibitors and exposed to A. phagocytophila. PBLs (107 cells/ml) were preincubated for 1 h with SN-50 (100 μg/ml), MG-132 (50 μM), SB 203580 (10 μM), PD 098059 (20 μM), and H-7 (25 μM) and exposed to A. phagocytophila (100 bacteria/cell) for 2 h. Total RNA was extracted and subjected to RT-PCR. The amounts of cDNAs were normalized against the amounts of G3PDH mRNA in corresponding samples. The PCR products (10 μl each) were resolved on agarose gels containing ethidium bromide. Molecular size markers (HaeIII fragments of φX174 replicative-form DNA) were included. The data (donor 3 data) are representative of the data from two independent experiments (donors 1 and 3) that gave similar results.
At a concentration of 50 μM, MG-132, a cell-permeable peptide-aldehyde protease inhibitor that blocks NF-κB activation via its effect on the proteasome (17, 37), prevented induction of TNF-α and IL-6 mRNAs by PBLs, but IL-1β mRNA was less affected by this inhibitor than TNF-α and IL-6 mRNAs (Fig. 1; Table 2). SN-50, a cell-permeable peptide that inhibits nuclear translocation of NF-κB (29), at a concentration of 50 or 100 μg/ml inhibited cytokine mRNA expression by PBLs, but it was less effective than MG-132 (Fig. 1; Table 2). These results suggest that activated p38 MAPK and a proteasome-NF-κB pathway for induction of TNF-α and IL-6 mRNAs in PBLs are involved in the response to A. phagocytophila. p38 MAPK and a proteasome-NF-κB pathway are less involved in IL-1β mRNA expression than in TNF-α and IL-6 mRNA expression. In contrast, when the same donor PBLs were used, H-7, which selectively inhibits PKC activity via direct interaction with the catalytic site of the enzyme (15), showed significant inhibition of expression of all three proinflammatory cytokine mRNAs (Fig. 1; Table 2).
In a previous study (19), we found that A. phagocytophila induces all three cytokine mRNAs in human monocytes but only IL-1β mRNA in neutrophils within 2 h of incubation. To confirm that SB 203580 inhibits TNF-α and IL-6 mRNA induction by monocytes and to examine the effect of SB 203580 on neutrophil generation of IL-1β, cytokine mRNA expression was examined separately in isolated monocytes and neutrophils. SB 203580 significantly blocked TNF-α and IL-6 mRNA induction and only weakly inhibited IL-1β mRNA expression in monocytes (Fig. 2A; Table 2). However, in neutrophils, SB 230580 had no effect on IL-1β mRNA induction (Fig. 2B; Table 2). These results suggest that TNF-α and IL-6 mRNA expression in monocytes in response to A. phagocytophila requires p38 MAPK activation, and IL-1β mRNA induction in both monocytes and neutrophils in response to A. phagocytophila is less dependent on p38 MAPK. In contrast, H-7 inhibited induction of all three cytokine mRNAs in monocytes and IL-1β mRNA induction in neutrophils from the same donors (Fig. 2; Table 2).
FIG. 2.
Effects of SB 203580 and H-7 on proinflammatory cytokine mRNA induction in human monocytes and neutrophils exposed to A. phagocytophila. Monocytes or neutrophils (107 cells) were preincubated for 30 min with SB 203580 (10 μM) or H-7 (25 μM) and then incubated with host-cell-free A. phagocytophila (100 bacteria/cell) for 2 h. The total RNA was extracted and subjected to RT-PCR. The amounts of cDNAs were normalized against the amounts of G3PDH mRNA in corresponding samples. The PCR products were resolved on agarose gels containing ethidium bromide. Molecular size markers (HaeIII fragments of φX174 replicative-form DNA) were included. The data (donor 8 data) are representative of the data from three independent experiments (donors 6, 7, and 8) that gave similar results.
p38 MAPK is activated in monocytes but not in neutrophils in response to A. phagocytophila.
To verify the SB 230580 inhibition results (Fig. 1 and 2; Table 2), we examined p38 MAPK activation in both monocytes and neutrophils after A. phagocytophila stimulation by immunoblotting. Activation of p38 MAPK requires phosphorylation on Thr-180 and Tyr-182 (24) and can be assayed directly by using specific antibodies to the phosphorylated form of p38 MAPK. The data showed that phosphorylation of p38 MAPK in monocytes peaked at 15 min and was maintained for up to 60 min after A. phagocytophila stimulation (Fig. 3A). However, p38 MAPK was not activated in neutrophils incubated with HL-60 cell lysate alone during the same time period (Fig. 3B). These results confirmed the results of experiments with SB 230580 which indicated that the p38 MAPK activation at earlier time points is critical for TNF-α and IL-6 generation in human monocytes within 2 h after exposure to A. phagocytophila. These results also confirmed that IL-1β mRNA induction in neutrophils in response to A. phagocytophila is not dependent on the p38 MAPK pathway.
FIG. 3.
Western blot analysis of activation and phosphorylation of p38 MAPK in human monocytes (A) and neutrophils (B) in response to A. phagocytophila. A total of 2 × 107 cells were incubated for different times (0, 15, 30, and 60 min) with A. phagocytophila (100 bacteria/cell) or HL-60 cell lysate (negative control), lysed, blotted on a nitrocellulose membrane, and probed with anti-phospho-p38 MAPK antibody (pp 38) or with anti-p38 MAPK antibody (p 38). As controls, nonphosphorylated (N) and phosphorylated (P) p38 MAPK extracts from C-6 glioma cells prepared without and with anisomycin (Cell Signaling Technology) treatment, respectively, were used. The positive bands were detected by immersing the blots in a DAB developing solution. The data (donor 12 data) are representative of the data from two independent experiments (donors 9 and 12).
NF-κB but not AP-1 was activated in human monocytes in response to A. phagocytophila.
Because induction of TNF-α and IL-6 mRNAs in human PBLs in response to A. phagocytophila was inhibited by MG-132 or SN-50 and monocytes are the primary sources of cells generating TNF-α and IL-6 (19), activation of transcription factors NF-κB and AP-1 was examined by EMSA. A time course study of monocytes responding to A. phagocytophila showed that NF-κB was activated 30 min after stimulation (the earliest time point examined), but AP-1 was not (Fig. 4). Thus, the EMSA data supported the results obtained with MG-132 and SN-50 showing that A. phagocytophila rapidly induces NF-κB activation for expression of TNF-α and IL-6 mRNAs in monocytes.
FIG. 4.
Time course of NF-κB activation (A) and comparison of NF-κB activation and AP-1 activation (B) in human monocytes exposed to A. phagocytophila, examined by EMSA. Human monocytes (donor 5; 107 cells/ml in each well) were incubated with A. phagocytophila (100 bacteria/cell) or E. coli LPS (1 μg/ml) (positive control) for 30 min. Nuclear extracts were prepared at different times (0, 0.5, 1, 2, and 4 h) and used for EMSA. The data (donor 5 data) are representative of the data from three independent experiments (donors 5, 6, and 7). N, uninfected monocytes.
Effects of kinase inhibitors other than H-7 on induction of IL-1β, TNF-α, and IL-6 mRNAs.
Protein tyrosine kinase (PTK) activities are required for proinflammatory cytokine gene expression through NF-κB in human peripheral blood monocytes in response to lipopolysaccharide (LPS) (13). Therefore, genistein, which inhibits the binding of ATP to PTK (1), and H-89, a selective inhibitor of protein kinase A (PKA) (9), were examined. Both genistein and H-89 inhibited IL-1β, TNF-α, and IL-6 mRNA induction in PBLs (Fig. 5A; Table 2) and in monocytes (Fig. 2 and 5B; Table 2). Both also inhibited IL-1β mRNA induction in neutrophils (Fig. 5C; Table 2). However, H-7 most strongly inhibited induction of all three cytokine mRNAs (Fig. 1 and 2; Table 2).
FIG. 5.
Effects of several inhibitors on induction of proinflammatory cytokine mRNAs in PBLs (A), monocytes (B), and neutrophils (C) in response to A. phagocytophila. Cells (107 cells/ml) were preincubated for 30 min with CHX (20 μg/ml), MDC (100 μM), H-89 (50 μM), verapamil (100 μM), W-7 (30 μM), neomycin (20 μM), and genistein (100 μM) and exposed to host-cell-free A. phagocytophila (100 bacteria/cell) for 2 h. For the oxytetracycline (OTC) (10 μg/ml) treatment, host-cell-free A. phagocytophila (100 bacteria/cell) was preincubated for 30 min. Total RNA was extracted and subjected to RT-PCR. The amounts of cDNAs were normalized against the amounts of G3PDH mRNA in corresponding samples. The PCR products were resolved on agarose gels containing ethidium bromide. Molecular size markers (HaeIII fragments of φX174 replicative-form DNA) were included. (A) PBL data (donor 3 data) representative of the data from three independent experiments (donors 2, 3, and 10) that gave similar results. (B) Monocyte data (donor 11 data) representative of the data from two independent experiments (donors 11 and 13). (C) Neutrophil data (donor 12 data) representative of the data from two independent experiments (donors 12 and 13).
We have shown that internalization and infection, but not binding of members of the family Anaplasmataceae (Neorickettsia risticii in P388D1 cells [43] and Ehrlichia chaffeensis and A. phagocytophila in THP-1 cells and neutrophils [7, 35]), are inhibited by monodansylcadaverine (MDC), a transglutaminase inhibitor (26). Transglutaminase catalyzes the formation of ɛ-(γ-glutamyl)-lysine between protein molecules by coupling amines and diamines to the γ-carboxyl residue of glutamine (26). MDC significantly blocked IL-1β mRNA expression by neutrophils in response to A. phagocytophila, suggesting that clustering and/or internalization through binding to neutrophil receptors of A. phagocytophila is required for IL-1β mRNA induction (Fig. 5C; Table 2), whereas MDC had less effect on cytokine mRNA expression in monocytes or PBLs. Oxytetracycline, a prokaryotic protein synthesis inhibitor (18) known to inhibit A. phagocytophila at the concentration used (49), had no effect on expression of the three cytokine mRNAs in PBLs, monocytes, or neutrophils (Fig. 5). Thus, a newly synthesized protein of A. phagocytophila was not required, confirming our previous observation made with recombinant major surface protein rP44 of A. phagocytophila (19) that preformed proteins of A. phagocytophila are sufficient for expression of these cytokine genes.
An influx of Ca2+, calmodulin, and activation of phospholipase C (PLC) are required for A. phagocytophila, N. risticii, and E. chaffeensis infection (28, 35, 44). The involvement of these signals in induction of IL-1β, TNF-α, and IL-6 mRNAs in PBLs and monocytes or in induction of IL-1β mRNA in neutrophils in response to A. phagocytophila was examined. Verapamil (a phenyalkylamine Ca2+ channel blocker) (52) had an inhibitory effect on TNF-α but not on IL-1β and had less effect on IL-6 mRNA expression in PBLs or monocytes (Fig. 5A and B; Table 2). Neomycin (a PLC inhibitor) (48) had an inhibitory effect on IL-1β mRNA expression by neutrophils (Fig. 5C; Table 1). Cycloheximide (CHX) blocks eukaryotic protein synthesis (6). CHX enhanced IL-1β and TNF-α mRNA expression but not IL-6 mRNA expression in PBLs, TNF-α mRNA expression in monocytes, and IL-1β mRNA expression in neutrophils (Fig. 5; Table 2), indicating that preexisting host proteins are sufficient for expression of the genes for IL-1β and TNF-α and newly synthesized host proteins are rather suppressive (inflammation turn-off signals) for induction of IL-1β and TNF-α mRNAs in monocytes and neutrophils, respectively.
Effects of protein kinase and other kinase inhibitors on activation of p38 MAPK and NF-κB.
To determine whether the protein kinases are upstream signals required for p38 MAPK and NF-κB activation in monocytes, the effects of the inhibitors on p38 MAPK and NF-κB activation were examined. Because H-7 did not block p38 MAPK activation in monocytes in response to A. phagocytophila (Fig. 6), PKC-independent p38 MAPK activation by monocytes in response to A. phagocytophila is involved in induction of TNF-α and IL-6 mRNAs. However, with genistein or H-89, p38 MAPK activation was reduced, suggesting that PTK and PKA may be partially involved in p38 MAPK activation in monocytes in response to A. phagocytophila.
FIG. 6.
Western blot analysis of phosphorylated p38 MAPK. Human monocytes preincubated for 30 min with kinase inhibitors were incubated with A. phagocytophila (100 bacteria/cell) for 30 min. The same amount of protein was electrophoresed, blotted on a nitrocellulose membrane, and probed with anti-phospho-p38 MAPK antibody (pp38) and with anti-p38 MAPK antibody (p38). Horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G was added, and the positive bands were detected by using the enhanced chemiluminescence method. The data (donor 16 data) are representative of the data from five independent experiments (donors 15, 16, 20, 21, and 22) that gave similar results.
Because NF-κB activation was not reduced by A. phagocytophila after 45 min of preincubation of human monocytes with H-7 and was significantly reduced with H-89, genistein, or SB 203580, the EMSA data obtained with these inhibitors supported PKC-independent NF-κB activation and involvement of other cytoplasmic kinase enzyme cascades, such as PTK, PKA, and p38 MAPK, leading to NF-κB activation for expression of TNF-α and IL-6 mRNAs in monocytes (Fig. 7).
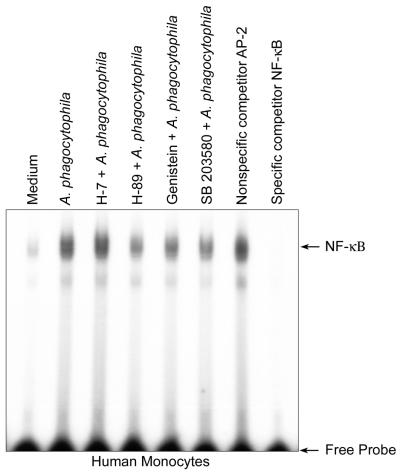
FIG. 7.
NF-κB activation in human monocytes preincubated with several inhibitors and exposed to A. phagocytophila. Human monocytes (107 cells/ml in each well) were preincubated with H-7, H-89, genistein, and SB 203580 for 45 min and then exposed to A. phagocytophila (100 bacteria/cell) for 30 min. Nuclear extracts were prepared and used for EMSA. The data (donor 19 data) are representative of the data from five independent experiments (donors 15, 16, 17, 18, and 19) that gave similar results. The nonspecific competitor AP-2 and the specific competitor NF-κB were included in this assay to verify the effects of these competitors on binding of the NF-κB complex.
Effect of anti-PSGL-1 MAb on the A. phagocytophila-induced IL-1β mRNA expression by human neutrophils.
Herron et al. (14) showed that MAb PL-1 against PSGL-1 at a concentration of >8 μg/ml almost completely blocks binding of A. phagocytophila to HL-60 cells. PL-1 at a concentration of 20 μg/ml did not inhibit IL-1β mRNA induction in response to A. phagocytophila in human neutrophils (data not shown), suggesting that a receptor other than PSGL-1 is involved in this induction.
DISCUSSION
Using Escherichia coli LPS as a control, we previously demonstrated that A. phagocytophila rapidly induces production of major proinflammatory cytokines by human PBLs at a level similar to the level induced by LPS (19). A. phagocytophila cannot survive outside neutrophils or other granulocytes. Since signals transduced by LPS activate the microbicidal activities of neutrophils, A. phagocytophila may use signals different from LPS to generate these cytokines. The present study revealed that the signaling pathways for induction of these cytokines in response to A. phagocytophila are indeed distinct from those known for LPS and are also different in neutrophils and monocytes.
A. phagocytophila activated in human PBLs and monocytes some signals (p38 MAPK and NF-κB) involved in TNF-α and IL-6 production in response to LPS. LPS activates ERK1/2, p38, c-Jun N-terminal kinase-stress-activated protein kinases, and both AP-1 and NF-κB (10). Because NF-κB activation by A. phagocytophila was partially blocked by SB 203580, p38 MAPK activation is upstream of NF-κB activation in human monocytes. There are four distinct isoforms of p38 MAPK, termed p38α, p38β, p38γ/SAPK3, and p38δ/SAPK4. Because SB 203580 inhibits p38α and p38β but not p38γ or p38δ (21), it seems that p38α and/or p38β is responsible for induction of TNF-α and IL-6 mRNAs in human monocytes in response to A. phagocytophila. We found that both p38 MAPK activation and NF-κB activation in human monocytes by A. phagocytophila were PTK and PKA dependent. NF-κB activation by LPS has also been reported to be PTK dependent (13, 46, 50), suggesting that A. phagocytophila may share with LPS the same PTK-dependent pathway upstream of p38 MAPK activation. PKA activation was reported to increase DNA binding of NF-κB in T cells (22), and PKA activates p38 MAPK in endocrine cells (41). The overall responses of PBLs and monocytes were similar in the present study. However, a few differences were seen in sensitivities to some inhibitors. Whether these differences are due to individual donor variation, the monocyte isolation procedure, or a requirement of the mixed or additional cell population for a PBL-type response remains to be clarified.
In contrast to what happens in monocytes, A. phagocytophila did not activate p38 MAPK in neutrophils. This does not seem to be due to a universal lack of responsiveness of neutrophil p38 MAPK, since p38 MAPK has been reported to be activated in human neutrophils by LPS (36). Whether NF-kB is activated and required for IL-β mRNA induction in neutrophils in response to A. phagocytophila was not directly examined in the present study. However, since IL-β mRNA induction in PBLs in response to A. phagocytophila was less sensitive to MG-132 or SN-50 treatment than TNF-α or IL-6 mRNA induction, NF-kB may not play a major role in neutrophil IL-β mRNA induction in response to A. phagocytophila. This also does not seem to be due to a universal inability of neutrophil NF-kB to be activated, since at least two reports have shown NF-kB activation in neutrophils in response to LPS (32, 36).
Proinflammatory cytokine gene expression in response to A. phagocytophila was sensitive to H-7 in PBLs, monocytes, and neutrophils. The site of PKC action appeared to be either independent from or downstream of p38 MAPK and NF-κB activation, because activation of either MAPK or NF-κB in monocytes in response to A. phagocytophila was not inhibited by H-7. Of note, LPS-induced TNF-α release in human monocytes has been reported to be PKC independent (47). Furthermore, neutrophil IL-1β mRNA expression was dependent on transglutaminase and PLC, suggesting that cross-linking of proteins, subsequent activation of PLC, and an increase in the intracellular Ca2+ level are required (28). The signaling pathway is somewhat similar to that described in a recent report showing that IL-1β synthesis by human neutrophils in response to granulocyte-macrophage colony-stimulating factor requires PKC activation and an increase in the intracellular Ca2+ level (12).
Leukocytes derived from 23 human immunodeficiency virus-negative donors were included in the present study. The blood donors were anonymous, and we could not obtain age, sex, or other information from most of these donors. Elderly and/or immunocompromised patients have more severe clinical signs of HGE than other patients (4). It remains to be determined whether and how the immune status of patients influences the signaling induced in PBLs, monocytes, and neutrophils in response to A. phagocytophila and thus the levels of parasitemia and proinflammatory cytokines in the blood.
In summary, the present data suggest (i) a key role for p38 MAPK and NF-κB in the induction of TNF-α and IL-6 mRNAs in human PBLs in response to A. phagocytophila, (ii) the involvement of PKC either downstream of or independent from p38 MAPK and NF-κB activation in IL-1β, TNF-α, and IL-6 mRNA expression in monocytes, (iii) the involvement of PKA and PTK upstream of p38 MAPK and NF-κB in TNF-α and IL-6 mRNA expression in human monocytes in response to A. phagocytophila, and (iv) the involvement of PKC and intracellular Ca2+ in IL-1β mRNA expression in neutrophils. The cell type-specific signaling may be important for understanding the granulocyte-specific survival of A. phagocytophila and pathological changes such as the liver damage and thrombocytopenia seen in HGE.
Acknowledgments
This research was supported by grants R01AI30010 and R01AI40934 from the National Institutes of Health.
Editor: R. N. Moore
REFERENCES
- 1.Akiyama, T., and H. Ogawara. 1991. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 201:362-370. [DOI] [PubMed] [Google Scholar]
- 2.Akkoyunlu, M., S. E. Malawista, J. Anguita, and E. Fikrig. 2001. Exploitation of interleukin-8-induced neutrophil chemotaxis by the agent of human granulocytic ehrlichiosis. Infect. Immun. 69:5577-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alessi, D. R., A. Cuenda, P. Cohen, D. T. Dudley, and A. R. Saltiel. 1995. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 270:27489-27494. [DOI] [PubMed] [Google Scholar]
- 4.Bakken, J. S., J. Krueth, C. Wilson-Nordskog, R. L. Tilden, K. Asanovich, and J. S. Dumler. 1996. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA 275:199-205. [PubMed] [Google Scholar]
- 5.Bakken, J. S., J. Krueth, R. L. Tilden, J. S. Dumler, and R. E. Kristansen. 1996. Serologic evidence of human granulocytic ehrlichiosis in Norway. Eur. J. Clin. Microbiol. Infect. Dis. 15:829-832. [DOI] [PubMed] [Google Scholar]
- 6.Baliga, B. S., A. W. Pronczuk, and H. N. Munro. 1969. Mechanism of cycloheximide inhibition of protein synthesis in a cell-free system prepared from rat liver. J. Biol. Chem. 244:4480-4489. [PubMed] [Google Scholar]
- 7.Barnewall, R. E., N. Ohashi, and Y. Rikihisa. 1999. Ehrlichia chaffeensis and E. sennetsu, but not the human granulocytic ehrlichiosis agent, colocalize with transferrin receptor and up-regulate transferrin receptor mRNA by activating iron-responsive protein 1. Infect. Immun. 67:2258-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, S.-M., J. S. Dumler, J. S. Bakken, and D. H. Walker. 1994. Identification of a granulocytotrophic Ehrlichia species as the etiological agent of human disease. J. Clin. Microbiol. 32:589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chijiwa, T., A. Mishima, M. Hagiwara, M. Sano, K. Hayashi, T. Inoue, K. Naito, T. Toshioka, and H. Hidaka. 1990. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino) ethyl] 5-isoquinolinesulfonamide(H-89), of PC12D pheochromocytoma cells. J. Biol. Chem. 265:5267-5272. [PubMed] [Google Scholar]
- 10.Downey, J. S., and J. Han. 1998. Cellular activation mechanisms in septic shock. Frontiers Biosci. 3:468-476. [DOI] [PubMed] [Google Scholar]
- 11.Dumler, J. S., A. F. Barbet, C. P. J. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales; unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia, and Ehrlichia with Neorickettsia; description of six new species combinations; and designation of Ehrlichia equi and “HGE agent” as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. E vol. Microbiol. 51:2145-2165. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez, M. C., P. T. Marucha, I. G. Rojas, and J. D. Walters. 2000. The role of protein kinase C and calcium in induction of human polymorphonuclear leukocyte IL-1β gene expression by GM-CSF. Cytokine 12:445-449. [DOI] [PubMed] [Google Scholar]
- 13.Geng, Y., B. Zhang, and M. Lotz. 1993. Protein tyrosine kinase activation is required for lipopolysaccharide induction of cytokines in human blood monocytes. J. Immunol. 151:6692-6700. [PubMed] [Google Scholar]
- 14.Herron, M. J., C. M. Nelson, J. Larson, K. R. Snapp, G. S. Kansas, and J. L. Goodman. 2000. Intracellular parasitism by human granuolocytic ehrlichiosis bacterium through the P-selectin ligand, PSGL-1. Science 288:1653-1656. [DOI] [PubMed] [Google Scholar]
- 15.Hidaka, H., M. Inagaki, S. Kawamoto, and Y. Sasaki. 1984. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry 23:5036-5041. [DOI] [PubMed] [Google Scholar]
- 16.Hidaka, H., Y. Sasaki, T. Tanaka, T. Endo, S. Ohno, Y. Fujii, and T. Nagata. 1981. N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide, a calmodulin antagonist, inhibits cell proliferation. Proc. Natl. Acad. Sci. USA 78:4354-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen, T. J., M. A. Loo, S. Pind, D. B. Williams, A. L. Goldberg, and J. R. Riordan. 1995. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell 83:129-135. [DOI] [PubMed] [Google Scholar]
- 18.Jimenez, A. 1976. Inhibitors of translation. Trends Biochem. Sci. 1:28-30. [Google Scholar]
- 19.Kim, H.-Y., and Y. Rikihisa. 2000. Expression of interleukin-1β, tumor necrosis factor alpha, and interleukin-6 in human peripheral blood leukocytes exposed to viable human granulocytic ehrlichiosis agent or recombinant major surface protein P44. Infect. Immun. 68:3394-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein, M. B., S. Hu, C. C. Chao, and J. L. Goodman. 2000. The agent of human granulocytic ehrlichiosis induces the production of myelosuppressing chemokines without induction of proinflammatory cytokines. J. Infect. Dis. 182:200-205. [DOI] [PubMed] [Google Scholar]
- 21.Kumar, S., P. C. McDonnell, R. J. Gum, A. T. Hand, J. C. Lee, and P. R. Young. 1997. Novel homologues of CSBP/p38 MAP kinase: activation, substrate specificity and sensitivity to inhibition by pyridinyl imidazoles. Biochem. Biophys. Res. Commun. 235:533-538. [DOI] [PubMed] [Google Scholar]
- 22.Lahdenpohja, N., T. Henttinen, and M. Hurme. 1996. Activation of the protein kinase A increases the DNA-binding and transcriptional activity of c-Rel in T cells. Scand. J. Immunol. 43:640-645. [DOI] [PubMed] [Google Scholar]
- 23.Lebech, A. M., K. Hansen, P. Pancholi, L. M. Sloan, J. M. Magera, and D. H. Persing. 1998. Immunoserologic evidence of human granulocytic ehrlichiosis in Danish patients with Lyme neuroborreliosis. Scand. J. Infect. Dis. 30:173-176. [DOI] [PubMed] [Google Scholar]
- 24.Lee, J. C., J. T. Laydon, P. C. Mcdonnel, T. F. Gallagher, S. Kumary, D. Green, D. McNulty, M. J. Blumenthal, J. R. Heys, S. W. Landvatter, J. E. Strickler, M. M. McLaughlin, I. R. Siemens, S. M. Fisher, G. P. Livi, J. R. White, J. L. Adams, and P. R. Young. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372:739-746. [DOI] [PubMed] [Google Scholar]
- 25.Lepidi, H., J. E. Bunnell, M. E. Martin, J. E. Madigan, S. Stuen, and J. S. Dumler. 2000. Comparative pathology, and immunohistology associated with clinical illness after Ehrlichia phagocytophila-group infections. Am. J. Trop. Med. Hyg. 62:29-37. [DOI] [PubMed] [Google Scholar]
- 26.Levitzki, A., M. Willingham, and I. Pastan. 1980. Evidence for participation of transglutaminase in receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA 77:2706-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Libermann, T. A., and D. Baltimore. 1990. Activation of interleukin-6 gene expression through the NF-κB transcription factor. Mol. Cell. Biol. 10:2327-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin, M., M. X. Zhu, and Y. Rikihisa. 2002. Rapid activation of protein tyrosine kinase and phospholipase C-γ2 and increase in cytosolic free calcium are required by Ehrlichia chaffeensis for internalization and growth in THP-1 cells. Infect. Immun. 70:889-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin, Y.-Z., S. Y. Yao, R. A. Veach, T. R. Torgerson, and J. Hawiger. 1995. Inhibition of nuclear translocation of transcription factor NF-κB by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J. Biol. Chem. 270:14255-14258. [DOI] [PubMed] [Google Scholar]
- 30.Lotric-Furlan, S., T. Avsic-Zupanc, M. Petrovec W. L. Nicholson, J. W. Sumner, J. E. Childs, and F. Strle. 2001. Clinical and serological follow-up of patients with human granulocytic ehrlichiosis in Slovenia. Clin. Diagn. Lab. Immunol. 8:899-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsusaka, T., K. Fujikawa, Y. Nishio, N. Mukaida, K. Matsushima, T. Kishmoto, and S. Akira. 1993. Transcription factors NF-IL6 and NF-κB synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc. Natl. Acad. Sci. USA 90:10193-10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDonald, P. P., A. Bald, and M. A. Cassatella. 1997. Activation of the NF-κB pathway by inflammatory stimuli in human neutrophils. Blood 89:3421-3433. [PubMed] [Google Scholar]
- 33.McQuiston, J. H., C. D. Paddock, R. C. Holman, and J. E. Childs. 1999. The human ehrlichioses in the United States. Emerg. Infect. Dis. 5:635-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mott, J., and Y. Rikihisa. 2000. Human granulocytic ehrlichiosis agent inhibits superoxide anion generation by human neutrophils. Infect. Immun. 68:6697-6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mott, J., Y. Rikihisa, and S. Tsunawaki. 2002. Effects of Anaplasma phagocytophila on NADPH oxidase components in human neutrophils and HL-60 cells. Infect. Immun. 70:1359-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nick, J. A., N. J. Avdi, S. K. Young, L. A. Lehman, P. P. McDonald, S. C. Frasch, M. A. Billstrom, P. M. Henson, G. L. Johnson, and G. S. Worthen. 1999. Selective activation and functional significance of p38α mitogen-activated protein kinase in lipopolysaccharide-stimulated neutrophils. J. Clin. Investig. 103:851-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palombella, V. J., O. J. Rando, A. L. Goldberg, and T. Maniatis. 1994. The ubiquitin-proteasome pathway is required for processing the NF-κB1 precursor protein and the activation of NF-κB. Cell 78:773-785. [DOI] [PubMed] [Google Scholar]
- 38.Petrovec, M., S. Lotric-Furlan, T. Avsic-Zupanc, F. Strle, P. Brouqui, V. Roux, and J. S. Dumler. 1997. Human disease in Europe caused by a granulocytic Ehrlichia species. J. Clin. Microbiol. 35:1556-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rawadi, G., J. Garcia, B. Lemercier, and S. Roman-Roman. 1999. Signal transduction pathways involved in the activation of NF-κB, AP-1, and c-fos by Mycoplasma fermentans membrane lipoproteins in macrophages. J. Immunol. 162:2193-2203. [PubMed] [Google Scholar]
- 40.Rhoades, K. L., S. H. Golub, and J. S. Economou. 1992. The regulation of human tumor necrosis factor α promoter region in macrophages, T cells, and B cell lines. J. Biol. Chem. 267:22102-22107. [PubMed] [Google Scholar]
- 41.Richards, J. S. 2001. New signaling pathways for hormones and cyclic adenosine 3′,5′-monophosphate action in endocrine cells. Mol. Endocrinol. 15:209-218. [DOI] [PubMed] [Google Scholar]
- 42.Rikihisa, Y., N. Zhi, G. P. Wormser, B. Wen, H. W. Horowitz, and K. E. Hechemy. 1997. Ultrastructural and antigenic characterization of a granulocytic ehrlichiosis agent directly isolated and stably cultivated from a patient in New York State. J. Infect. Dis. 175:210-213. [DOI] [PubMed] [Google Scholar]
- 43.Rikihisa, Y., Y. Zhang, and J. Park. 1994. Inhibition of infection of macrophages with Ehrlichia risticii by cytochalasins, monodansylcadaverine, and taxol. Infect. Immun. 62:5126-5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rikihisa, Y., Y. Zhang, and J. Park. 1995. Role of Ca2+ and calmodulin in ehrlichial infection in macrophages. Infect. Immun. 63:2310-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schulze-Osthoff, K., D. Ferrari, K. Riehemann, and S. Wesselborg. 1997. Regulation of NF-κB activation by MAP kinase cascades. Immunobiology 198:35-49. [DOI] [PubMed] [Google Scholar]
- 46.Shakhov, A. N., M. A. Collart, P. Vassali, S. A. Nedospasov, and C. V. Jongeneel. 1990. κB-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor α gene in primary macrophages. J. Exp. Med. 171:35-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shames, B. D., C. H. Selzman, E. J. Pulido, X. Meng, D. R. Meldrum, R. C. McIntyre, Jr., A. H. Harken, and A. Banerjee. 1999. LPS-induced NF-κB activation and TNF-α release in human monocytes are protein tyrosine kinase dependent and protein kinase C independent. J. Surg. Res. 83:69-74. [DOI] [PubMed] [Google Scholar]
- 48.Streb, H., J. P. Heslop, R. F. Irvine, I. Schulz, and M. J. Berridge. 1985. Relationship between secretagogue-induced Ca2+ release and inositol polyphosphate production in permeabilized pancreatic acinar cells. J. Biol. Chem. 260:7309-7415. [PubMed] [Google Scholar]
- 49.Yoshiie, K., H.-Y. Kim, J. Mott, and Y. Rikihisa. 2000. Intracellular infection by the human granulocytic ehrlichiosis agent inhibits human neutrophil apoptosis. Infect. Immun. 68:1125-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoza, B. K., J. Y. Q. Hu, and C. E. McCall. 1996. Protein-tyrosine kinase activation is required for lipopolysaccharide induction of interleukin-1 beta and NF-κB activation, but not NF-κB nuclear translocation. J. Biol. Chem. 271:18306-18309. [DOI] [PubMed] [Google Scholar]
- 51.Zhi, N., N. Ohashi, Y. Rikihisa, G. P. Wormser, H. W. Horowitz, and K. E. Hechemy,. 1998. Cloning and expression of 44-kilodalton major outer membrane protein gene of the human granulocytic ehrlichiosis agent and application of the recombinant protein to serodiagnosis. J. Clin. Microbiol. 36:1666-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zobrist, R. H., K. M. Giacomini, W. L. Nelson, and J. C. Giacomini. 1986. The interaction of phenylalkylamine calcium channel blocked with the 1,4-dihydropyridine binding site. J. Mol. Cell. Cardiol. 18:963-974. [DOI] [PubMed] [Google Scholar]