Abstract
We tested a cytokine-enhanced, multiantigen, DNA priming and poxvirus boosting vaccine regimen for prevention of malaria in the Plasmodium knowlesi-rhesus macaque model system. Animals were primed with a mixture of DNA plasmids encoding two preerythrocytic-stage proteins and two erythrocytic-stage proteins from P. knowlesi and combinations of the cytokines granulocyte-macrophage colony-stimulating factor, interleukin-4, and tumor necrosis factor alpha and were boosted with a mixture of four recombinant, attenuated vaccinia virus strains encoding the four P. knowlesi antigens. Two weeks after boosting, the geometric mean immunofluorescence titers in the immunized groups against sporozoites and infected erythrocytes ranged from 160 to 8,096 and from 1,810 to 5,120, respectively. The geometric mean anti-P. knowlesi circumsporozoite protein (PkCSP) titers ranged from 1,761 to 24,242. Peripheral blood mononuclear cells (PBMC) from the immunized monkeys produced gamma interferon (IFN-γ) in response to incubation with pooled peptides from the PkCSP at frequencies of 10 to 571 spot-forming cells/106 PBMC. Following challenge with 100 infectious P. knowlesi sporozoites, 2 of 11 immunized monkeys were sterilely protected, and 7 of the 9 infected monkeys resolved their parasitemias spontaneously. In contrast, all four controls became infected and required treatment for overwhelming parasitemia. Early protection was strongly associated with IFN-γ responses against a pool of peptides from the preerythrocytic-stage antigen, PkCSP. These findings demonstrate that a multistage, multiantigen, DNA priming and poxvirus boosting vaccine regimen can protect nonhuman primates from an otherwise lethal malaria sporozoite challenge.
Each year, malaria parasites infect 270 to 350 million people and kill 1.5 to 2.7 million people, mostly children in sub-Saharan Africa (29); drug resistance is spreading rapidly, and there is currently no licensed vaccine. In a mammalian host Plasmodium sporozoites injected by a mosquito move within minutes to hepatocytes, in which they develop during several days before emerging to infect circulating erythrocytes. Two models suggest that immune control of malaria is possible. First, in mice (15), monkeys (10), and humans (3), immunization with radiation-attenuated sporozoites can provide sterile protection against sporozoite challenge, mediated by CD8+ T cells and gamma interferon (IFN-γ) directed at the intrahepatocytic stage of the parasite (6). Adults in areas where malaria is endemic develop partial clinical immunity, which is largely mediated by antibodies directed against blood-stage antigens (19, 21). An effective malaria vaccine will likely need to induce both T-cell responses against infected hepatocytes and antibodies against blood-stage parasites. While DNA vaccines represent a flexible vaccine technology, well adapted to simultaneous delivery of multiple antigens, they have been less than optimally immunogenic in human trials, inducing modest T-cell responses and small amounts of antibodies or no antibodies (20, 27). Recent studies have shown that heterologous priming and boosting vaccination regimens in which priming doses of DNA are followed by boosting with recombinant virus can be highly immunogenic and have induced protection against human immunodeficiency virus (1, 17) and Ebola virus (26) in rhesus macaques. In murine malaria models, heterologous priming and boosting regimens are more effective than DNA vaccination alone (23, 24), and regimens in which the priming DNA is supplemented with a plasmid encoding murine granulocyte-monocyte colony-stimulating factor (GM-CSF) are more effective still (25). We recently tested a multiantigen, heterologous DNA priming and canarypox virus boosting regimen in the Plasmodium knowlesi-rhesus monkey model, a system in which both reliable challenge with sporozoites and partial protection after immunization with irradiated sporozoites have been demonstrated (10). The priming immunogens consisted of plasmids encoding two preerythrocytic-stage proteins, the P. knowlesi circumsporozoite protein (PkCSP) and P. knowlesi sporozoite surface protein 2 (PkSSP2), and two erythrocytic-stage proteins, P. knowlesi apical membrane antigen 1 (PkAMA1) and the 42-kDa carboxy-terminal fragment of P. knowlesi merozoite surface protein 1 (PkMSP1p42). A cocktail of recombinant canarypox viruses encoding the four P. knowlesi antigens was used for boosting. Although the regimen induced both antibodies and IFN-γ responses, 11 of 12 immunized monkeys became infected, and all but one infected monkey required treatment for overwhelming parasitemia (18). In the present study we made two modifications to the original regimen. First, recombinant, attenuated vaccinia virus (COPAK) (14), rather than canarypox virus, was used for boosting. Second, we tested several different cytokine mixtures to see if any of them enhanced immune responses to the vaccine plasmids. In murine malaria DNA vaccine experiments inclusion of a plasmid encoding GM-CSF substantially improved immunogenicity and protective efficacy (25, 28), possibly by enhancing recruitment of dendritic cells to the injection site (11). However, in preliminary studies we found no effect of the rhesus macaque GM-CSF plasmid on the immunogenicity of the P. knowlesi DNA vaccine in macaques (unpublished data). In mice, in vitro culture of immature dendritic cells from bone marrow precursors requires recombinant GM-CSF protein; however, human dendritic cells grow best when both GM-CSF and interleukin-4 (IL-4) are added (2). We therefore asked whether addition of both GM-CSF and IL-4 enhanced immunogenicity. Immature dendritic cells take up antigen efficiently but present it inefficiently, while mature dendritic cells present antigen efficiently but take it up inefficiently (2). Tumor necrosis factor alpha (TNF-α) is one of several inflammatory signals that cause dendritic cells to mature (22). We therefore asked if inclusion of a TNF-α plasmid enhanced immunogenicity.
MATERIALS AND METHODS
Immunogens.
DNA vaccine plasmids expressing four P. knowlesi antigens were constructed and characterized as described previously (18). Plasmids encoding rhesus macaque cytokines in the expression vector VR1012 (12) were kind gifts from Richard Hedstrom (GM-CSF) and Francois Villinger (IL-4 and TNF-α). To construct the recombinant COPAK viruses expressing the four P. knowlesi antigens, it was necessary to modify the occurrence of the sequence TTTTTNT, which serves as an early transcriptional terminator in vaccinia virus. A single occurrence of this sequence in PkAMA1 was mutagenized to alter the nucleotide sequence without changing the encoded amino acid by using a QuickChange mutagenesis kit (Stratagene, Inc., La Jolla, Calif.) and primers Pkmut1 (GTCTCATTAATGACAAAAATTTCTTTGCAACAACAGCGTTATCTC) andPkmut2 (GAGATAACGCTGTTGTTGCAAAGAAATTTTTGTCATTAATGAGAC) according to the manufacturer's instructions. The remaining three P. knowlesi genes contain no instances of the sequence TTTTTNT. Each P. knowlesi gene was fused to the modified H6 vaccinia virus promoter (9, 16) by insertion into an intermediate vector, pC6L/H6 (18). The restriction fragments containing the H6-P. knowlesi gene fusions were then excised and cloned into donor plasmid pSD553VC (Virogenetics, Troy, N.Y.), which contains a cloning site adjacent to the vaccinia virus host range gene, K1L, flanked by 0.4- and 0.3-kb sequences from the A24R and A27L regions of the vaccinia virus genome. Recombination and host range selection to produce P. knowlesi recombinant vaccinia virus (COPAK) were performed as described previously (16). Each donor plasmid was used to generate a recombinant COPAK virus (COPAK-PkCSP, COPAK-PkSSP2, COPAK-PkAMA1, and COPAK-PkMSP1p42). The mixture of the four P. knowlesi antigen-encoding COPAK viruses was designated COPAK-4.
Animals and immunizations.
Animals were used under protocols approved by institutional animal care and use committees at facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. The experiments described here were conducted according to the principles set forth in the Guide for the Care and Use of Laboratory Animals (12). Thirteen male and three female rhesus macaques (Macaca mulatta; Three Springs Scientific, Inc., Perkasie, Pa.) that were 18 to 33 months old and weighed 3 to 4 kg were divided into four groups of four animals and immunized with the mixtures of the four P. knowlesi antigen plasmids and rhesus macaque cytokine plasmids shown in Table 1 (500 μg/plasmid or 2 mg of VR1020 [empty vector]). DNA immunizations were given at weeks 0, 4, 8, and 40 by intramuscular injection in the tibialis anterior with a needle and syringe (weeks 0, 4, and 8) or with a needleless injection device (Biojector) (week 40). At week 44 the animals were boosted by intramuscular injection (with a needle and syringe) of either 2.5 × 108 PFU of each of the four recombinant COPAK viruses expressing the four P. knowlesi antigens (groups 2 to 4) or 1 × 109 PFU of parental COPAK (group 1).
TABLE 1.
Immunization regimens and schedules
| Group | Plasmid antigen(s) | Plasmid cytokine(s) | DNA priming weeks | Recombinant vaccinia virus (COPAK) | Boosting week | Challenge week |
|---|---|---|---|---|---|---|
| 1 | VR1020 | GM-CSF, IL-4, TNF-α | 0, 4, 8, 40 | Parental | 44 | 46 |
| 2 | PkCSP, PkSSP2, PkAMA1, PkMSP1p42 | GM-CSF | 0, 4, 8, 40 | PkCSP, PkSSP2, PkAMA1, PkMSP1p42 | 44 | 46 |
| 3 | PkCSP, PkSSP2, PkAMA1, PkMSP1p42 | GM-CSF, IL-4 | 0, 4, 8, 40 | PkCSP, PkSSP2, PkAMA1, PkMSP1p42 | 44 | 46 |
| 4 | PkCSP, PkSSP2, PkAMA1, PkMSP1p42 | GM-CSF, IL-4, TNF-α | 0, 4, 8, 40 | PkCSP, PkSSP2, PkAMA1, PkMSP1p42 | 44 | 46 |
Parasites and challenge.
At week 46 sporozoites of P. knowlesi strain H were dissected from the salivary glands of infected Anopheles dirus mosquitoes in E199 medium supplemented with 10% heat-inactivated, random-source, normal rhesus macaque serum. For challenge, 100 sporozoites were injected into the saphenous vein. Monkeys were challenged in groups of four, chosen so that the last monkey challenged in each group was a control and the other three were one monkey from each experimental group in random order; the time between challenge of the first animal and challenge of the last animal was 1 h. Beginning on day 6 after challenge, peripheral thick and thin blood films were examined to determine parasitemia. Parasitemia in thick films was determined by the method of Earle and Perez (8), and parasitemia in thin films was determined by separate enumeration of infected and total erythrocytes. In order to prevent unnecessary deaths, when the level of parasitemia reached 2% or higher, animals were treated by daily intramuscular injection of chloroquine hydrochloride (Sanofi Winthrop Pharmaceuticals, New York, N.Y.) by using 20 mg/kg on day 1 and 10 mg/kg on days 2 to 4 of treatment.
Recombinant PkCSP.
A 981-bp fragment of the gene encoding amino acids H-21 to V-347 of PkCSP from the H strain of P. knowlesi was cloned into bacterial expression plasmid pET11a (Novagen, Madison, Wis.). In this plasmid expression of the foreign gene is under control of the T7 promoter. The resultant recombinant plasmid, designated pPkCSP, was transformed into Escherichia coli strain BL21 (λDE3) containing a T7 RNA polymerase gene under control of the lacZ promoter. pPkCSP-transformed BL21 cells were grown in super broth (pH 7.2) containing 100 μg of ampicillin per ml at 37°C with shaking. At an optical density at 600 nm of 1.0, the cells were induced with 2 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and 4 h later they were harvested by centrifugation at 4,000 × g for 20 min. The cell pellet was gently resuspended in 20% sucrose in 50 mM Tris (pH 7.4) and incubated on ice for 10 min. The resuspended cells were centrifuged at 8,000 × g for 20 min at 4°C, and the pellet was resuspended in chilled distilled water. After incubation on ice for 10 min, the cell suspension was centrifuged at 15,000 × g for 10 min at 4°C. The supernatant, containing the periplasmic fraction, was brought to 25 mM Tris (pH 7.4) and loaded onto a heparin Sepharose affinity column equilibrated in the same buffer. The recombinant PkCSP protein was sequentially eluted with a step gradient by using 0.125, 0.25, and 0.5 M NaCl in 25 mM Tris (pH 7.4) with a fast protein liquid chromatography system. Fractions containing PkCSP were pooled, concentrated, and purified to homogeneity by gel filtration chromatography.
Immunologic assays.
Immunofluorescence assays (IFA) against air-dried P. knowlesi sporozoites or infected erythrocytes and enzyme immunoassays (EIA) against recombinant PkCSP were carried out as described previously (18). The IFN-γ ELISPOT assay was performed as described previously (13), except that the following peptide pools were used: pool 1, amino acids 1 to 20 (MKNFILLAVSSILLVDLLPT), 11 to 30 (SILLVDLLPTHFEHNVDLSR), 21 to 40 (HFEHNVDLSRAINVNGVSFN), 41 to 60 (NVDTSSLGAQQVRQSASRGR), and 51 to 70 (QVRQSASRGRGLGEKPKEGA); pool 2, amino acids 61 to 70 (GLGEKPKEGADKEKKKEKGK2), 81 to 100 (EKEEEPKKPNENKLKQPNEG), 91 to 110 (ENKLKQPNEGQPQAQGDGAN0), 101 to 120 (QPQAQGDGANAGQPQAQGDG), and 234 to 253 (QAQGDGANVPRQGRNGGGA); pool 3, amino acids 244 to 263 (PRQGRNGGGAPAGGNEGNKQ), 254 to 273 (PAGGNEGNKQAGKGQGQNNQ), 264 to 283 (AGKGQGQNNQGANAPNEKVV), 274 to 293 (GANAPNEKVVNDYLHKIRSS), and 294 to 313 (VTTEWTPCSVTCGNGVRIRR); and pool 4, amino acids 304 to 323 (TCGNGVRIRRKAHAGNKKAE), 314 to 333 (KAHAGNKKAEDLTMDDLEVE), 284 to 303 (NDYLHKIRSSVTTEWTPCSV), and 334 to 353 (ACVMDKCAGIFNVVSNSLGL).
Statistical analyses.
Differences between immune responses in multiple groups were assessed by one-way analysis of variance of log-transformed data, followed by Tukey's honestly significant difference test to identify which groups differed. Parasitemia curves for several days were compared by two-way repeated-measures analysis of variance; Dunnett's test was used to address multiple comparisons with a single control group. Comparisons between two groups were made by using a two-tailed Student's t test. To look for associations between protection early in infection and immune responses, we calculated an early parasitemia index (EPI) as follows. On days 8 to 10 of challenge, during which parasitemia was rising exponentially, we divided the log10 parasitemia of each monkey by the average log10 parasitemia of all of the monkeys on that day. Values greater or less than 1 indicate parasitemias higher or lower than the group average on that day. For each monkey, we then averaged the scores from days 8 to 10 to calculate the EPI. The EPI thus is a quantitative measure of the extent to which the parasitemias of individual monkeys differ during the early phase of parasitemia. Finally, we used simple and multiple linear regression to determine to what extent any of the measured immune responses predicted the EPI. All statistical calculations were performed with SigmaStat, version 2.03 for Windows (SPSS, Inc., Chicago, Ill.).
RESULTS
Antibody responses.
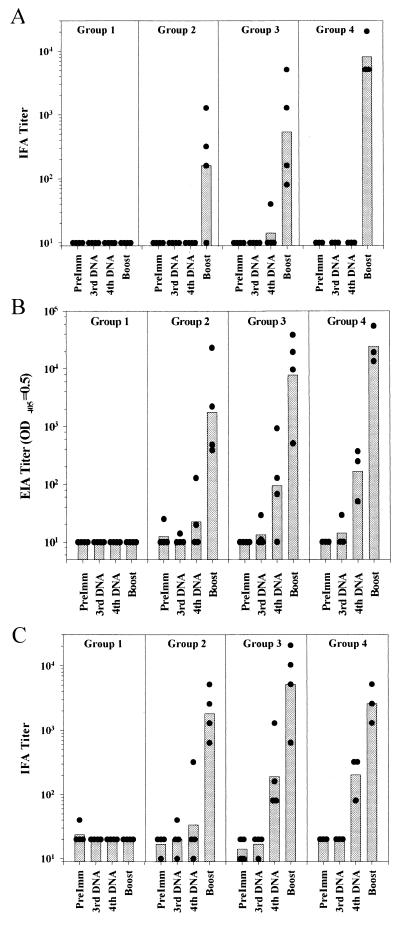
We assessed antibody responses against air-dried sporozoites (Fig. 1A) and P. knowlesi-infected erythrocytes by IFA (Fig. 1C) and antibody responses against PkCSP by EIA (Fig 1B). Virtually no antibodies were induced by the initial three doses of DNA. Following the fourth dose very modest anti-PkCSP and anti-infected erythrocyte titers were detectable. Two weeks after boosting with recombinant COPAK, however, moderately high antibody titers against both sporozoites and blood-stage parasites and against PkCSP were induced (Fig. 1; Table 2). In the anti-sporozoite IFA titers and the anti-PkCSP EIA titers there was a weak trend for higher geometric mean titers in groups 3 (GM-CSF, IL-4) (IFA titer, 538; EIA titer, 7,789) and 4 (GM-CSF, IL-4, TNF-α) (IFA titer, 8,096; EIA titer, 24,242) than in group 2 (GM-CSF) (IFA titer, 160; EIA titer, 1,761); however, the differences were not statistically significant. It is formally possible that the observed antibody titers represent a primary response to the boosting with recombinant vaccinia virus; however, in an earlier study we found that immunization of macaques with one or two doses of a mixture of four recombinant ALVAC viruses (5 × 108 PFU/recombinant) failed to induce detectable titers to P. knowlesi in naïve rhesus monkeys but could boost antibody responses in monkeys primed by DNA vaccination (unpublished data).
FIG. 1.
Antibody responses: IFA titers against P. knowlesi sporozoites (A) EIA titers corresponding to an optical density at 405 nm (OD405) of 0.5 against recombinant PkCSP (B), and IFA titers against P. knowlesi-infected rhesus monkey erythrocytes (C). The bars indicate the geometric mean titer for each group. The solid circles indicate values for individual animals. Serum samples were obtained before immunization (PreImm), 2 weeks following the third dose of DNA (3rd DNA), after the fourth dose of DNA (4th DNA), and after the viral boost (Boost).
TABLE 2.
Immune responses, parasitemia, and protection
| Monkey | Group | Titer
|
Spot-forming cells/106 PBMCc
|
EPId | Protectione | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sporozoite IFAa | PKCSP EIAb | Infected erythrocyte IFAa | Pool 1 | Pool 2 | Pool 3 | Pool 4 | ||||
| TWD | 1 | 10 | 10 | 20 | −9 | −10 | −14 | −10 | 1.65 | No |
| JTX | 1 | 10 | 10 | 20 | 0 | 1 | 4 | 0 | 1.48 | No |
| JVT | 1 | 10 | 10 | 20 | 0 | 0 | 0 | 0 | 1.41 | No |
| TCP | 1 | 10 | 10 | 20 | 5 | 3 | 6 | 0 | 1.03 | No |
| VBW | 2 | 1,280 | 23,119 | 2,560 | 5 | 0 | 411 | 234 | 1.22 | Yes |
| TAB | 2 | 10 | 2,218 | 1,280 | 25 | 6 | 0 | 248 | 1.36 | Yes |
| JPT | 2 | 320 | 481 | 5,120 | −3 | 0 | 26 | 118 | 1.35 | Yes |
| TVK | 2 | 160 | 390 | 640 | 1 | 16 | 144 | 41 | 0.97 | Yes |
| TGB | 3 | 80 | 507 | 640 | 11 | 11 | 26 | 98 | 1.14 | Yes |
| KCA | 3 | 1,280 | 19,470 | 10,240 | 84 | 233 | 455 | 70 | 0.79 | No |
| KKG | 3 | 160 | 9,665 | 5,120 | 19 | 29 | 59 | 95 | 0.90 | Yes |
| TAA | 3 | 5,120 | 38,587 | 20,480 | 100 | 540 | 93 | 108 | 0.00 | Yes |
| TGD | 4 | 5,120 | 13,478 | 5,120 | 10 | 99 | 11 | 0 | 0.97 | Yes |
| JKA | 4 | 5,120 | 19,236 | 1,280 | 3 | −3 | 233 | 156 | 0.72 | No |
| TAJ | 4 | 20,240 | 54,952 | 2,560 | 85 | 571 | 5 | 10 | 0.00 | Yes |
Endpoint titer.
Titer corresponding to an optical density at 405 nm of 0.5.
Net numbers of IFN-γ spot-forming cells per 106 PBMC following incubation with different peptide pools, calculated as described in Materials and Methods. Boldface type indicates values that were more than the mean plus 2 standard deviations for the control animals.
Calculated as described in Materials and Methods.
Protection is defined as either an absence of detectable parasitemia or control of parasitemia without treatment.
T-cell responses.
We used an IFN-γ ELISPOT (13) assay to measure T-cell responses. Because of limitations in the volume of blood available from the animals and in available peptides and because the circumsporozoite protein is a well-established target of protective, preerythrocytic-stage immunity, we focused on responses to pools of 20-mer peptides derived from PkCSP. IFN-γ responses to the peptide pools were not detectable in preimmune samples or in samples taken 2 weeks after the fourth dose of DNA (data not shown). Table 2 shows the responses seen in peripheral blood mononuclear cells (PBMC) taken 2 weeks after the COPAK boosting. We considered positive those responses to each peptide which were greater than the mean response to the peptide in the control group plus 2 standard deviations. Cells from all immunized monkeys responded to at least one peptide pool with responses ranging from 11 to 571 spot-forming cells/106 PBMC. Although insufficient cells were available to demonstrate T-cell subset dependence of the responses, in earlier studies using a DNA priming and canarypox virus boosting regimen based on the same four P. knowlesi antigens tested here, this assay detected only CD4+-dependent IFN-γ responses (13).
Monkeys in group 2 (GM-CSF) responded well to peptide pools 3 and 4 but had minimal responses to peptide pools 1 and 2. All four monkeys in group 3 (GM-CSF plus IL-4) responded to all four peptide pools, and the responses to peptide pools 1 and 2 were greater than the responses of the monkeys in group 2 (P = 0.045 and P = 0.029, as determined by two-tailed t test on log-transformed data). In group 4 (GM-CSF plus IL-4 plus TNF-α) the T-cell responses were not improved by the addition of TNF-α and may have decreased slightly. However, the small number of animals in these studies and the multiple comparisons of measures of antibody and T-cell immunity mean that individual comparisons must be interpreted with caution. When all INF-γ spots for each peptide pool were added, there was no significant difference between immunization groups in the total number of INF-γ-producing cells. The results of cytokine EIA for supernatants of PBMC stimulated with the same peptide pools were comparable to the ELISPOT results, although in a number of cases responses detected by ELISPOT assays were not evident in the EIA (data not shown).
Protective immunity.
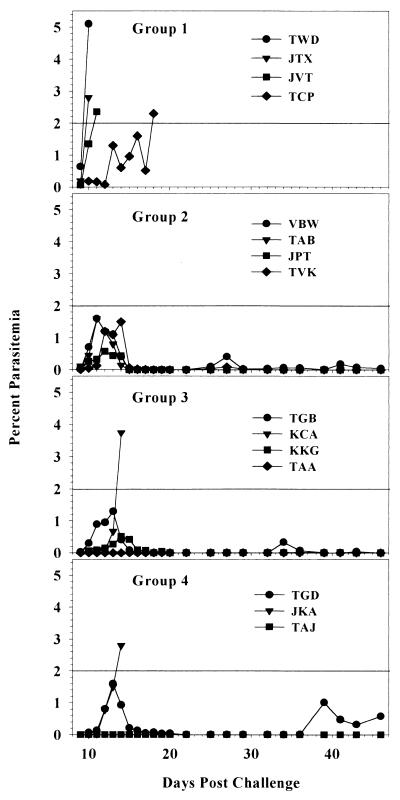
In order to assess the protective efficacy of the vaccine regimens, we challenged the animals by intravenous injection of 100 H strain P. knowlesi sporozoites. This is a very stringent challenge model. In an early experiment, sporozoite-induced P. knowlesi infection was lethal in all 17 monkeys tested (4). In several recent experiments in our laboratory, challenge with 100 P. knowlesi H strain sporozoites infected all 18 control monkeys and caused overwhelming infection requiring drug treatment in 17 of the 18 monkeys (18; unpublished data), as well as in 30 of 32 monkeys immunized with a variety of regimens. We established a treatment threshold of 2% parasitemia because untreated parasitemias above this level can increase 10-fold within 24 h, putting the animal at a high risk of death. We considered separately the parasitemia during the first 4 days of detectable parasitemia (days 7 to 10 postchallenge), in which residual effects of preerythrocytic-stage immunity may be seen, and the later course of parasitemia, which is likely influenced to a greater extent by immune responses directed at infected erythrocytes. Table 3 shows the parasitemias in individual monkeys in each of the groups from day 7, when the first parasitemias were detected, through day 10, when monkeys began to require treatment for overwhelming parasitemia. Two monkeys, one in group 3 and one in group 4, did not develop detectable parasitemia during the 46-day follow-up period after challenge. The mean parasitemias in the immunized groups were consistently 10- to 100-fold lower than those in the control group during the early phase of the challenge; the parasitemia curves were statistically different on days 8 to 10 (P = 0.044, as determined by two-way repeated-measures analysis of variance), and the parasitemia of group 4 monkeys was significantly lower than that of the controls on days 8 and 9 (P < 0.05, as determined by Dunnett's test). Overall, there was a 1- to 2-day delay in reaching any given level of parasitemia in the immunized monkeys compared to the controls. Both the delay in parasitemia and the failure of two immunized monkeys to become parasitemic suggest that a significant reduction of the parasite burden occurred in the preerythrocytic stage. As the parasitemia in both controls and vaccinees increased approximately 10-fold each 24 h during this phase (Table 3), a 1- to 2-day delay suggests that the burden of hepatic-stage merozoites released into the circulation at the end of the preerythrocytic stage may have been 10- to 100-fold lower in the vaccinees than in the controls and may thus have corresponded to elimination of 90 to 99% of the preerythrocytic-stage parasites. Figure 2 shows the course of parasitemia in the individual monkeys in each of the groups throughout the trial. All four controls required treatment for parasitemia of >2%. In contrast, seven of the nine immunized monkeys that developed detectable parasitemia resolved the parasitemia without drug treatment between days 20 and 30 after the peak parasitemia was reached between days 11 and 14. Of the seven monkeys that spontaneously controlled their parasitemias, five experienced brief, low-level recrudescences between days 30 and 45, which also resolved spontaneously. Five of the nine protected monkeys spontaneously controlled parasitemia following a second challenge 4 months after the first challenge; however, interpretation of repeat challenges is difficult in this model because infection and cure may induce partial immunity even in nonvaccinated animals (data not shown).
TABLE 3.
Early parasitemia
| Monkey | Group | Parasitomia (parasites/mm3)
|
|||
|---|---|---|---|---|---|
| Day 7 | Day 8 | Day 9 | Day 10 | ||
| TWD | 1 | 45 | 1,943 | 31,018 | 168,981 |
| JTX | 1 | 0 | 540 | 15,740 | 96,296 |
| JVT | 1 | 18 | 549 | 6,597 | 42,130 |
| TCP | 1 | 0 | 45 | 595 | 14,120 |
| VBW | 2 | 18 | 99 | 2,425 | 47,222 |
| TAB | 2 | 0 | 576 | 5,042 | 18,750 |
| JPT | 2 | 9 | 567 | 6,713 | 11,227 |
| TVK | 2 | 0 | 108 | 477 | 714 |
| TGB | 3 | 0 | 81 | 1,914 | 13,657 |
| KCA | 3 | 0 | 9 | 171 | 4,282 |
| KKG | 3 | 18 | 36 | 360 | 1,554 |
| TAA | 3 | 0 | 0 | 0 | 0 |
| TGD | 4 | 9 | 45 | 252 | 7,870 |
| JKA | 4 | 0 | 18 | 72 | 651 |
| TAJ | 4 | 0 | 0 | 0 | 0 |
FIG. 2.
Course of parasitemia: percentages of parasitemia in individual monkeys in each group following challenge based on examination of thin blood films.
Immune correlates of protection.
We next asked if any of the specific immune responses identified predicted protection (Table 2). To address possible preerythrocytic protection, we calculated a measure of parasitemia over days 8 to 10 of the challenge, the EPI, as described in Materials and Methods. In a simple linear regression analysis, several measures of preerythrocytic immunity predicted low EPIs, PkCSP EIA titers (R2 = 0.45, P = 0.024), sporozoite IFA titers (R2 = 0.53, P = 0.011), peptide pool 1 IFN-γ ELISPOT responses (R2 = 0.64, P = 0.003), and peptide pool 2 IFN-γ ELISPOT responses (R2 = 0.84, P = 0.00007). Neither the blood-stage IFA titers nor the ELISPOT responses to peptide pools 3 or 4 were associated with low EPIs. In a backwards stepwise multiple linear regression model, the ELISPOT response to pool 2 predicted 84% of the variation in the EPI; none of the other immune variables added significantly to the model's ability to predict the EPI. The finding that the best predictors of a low EPI, putatively a measure of the reduction in the preerythrocytic-stage parasite load, were IFN-γ ELISPOT responses to peptides from PkCSP is consistent with the critical role of IFN-γ responses to preerythrocytic-stage proteins like the circumsporozoite protein in murine models of preerythrocytic-stage immunity (7). It is certainly possible that if we had been able to assay for IFN-γ responses to the other preerythrocytic-stage antigen, PkSSP2, we might have identified a stronger predictor of a low EPI. It is interesting that even the two vaccinated monkeys which ultimately required treatment for high parasitemia (monkeys KCA and JKA) had lower EPIs than all four controls, suggesting that they had partially effective preerythrocytic protection but lacked an effective response to blood-stage parasites. Logistic regression failed to identify immunologic responses predictive of spontaneous resolution of blood-stage parasitemia; however, the immune responses were measured prior to challenge. It is possible that antibody levels measured at the time of peak parasitemia or treatment or the results of functional antibody assays, such as an in vitro growth inhibition assay, would better correlate with blood-stage protection.
DISCUSSION
We have demonstrated that a multiantigen DNA priming and poxvirus boosting vaccine targeting both preerythrocytic- and erythrocytic-stage vaccine targets can protect rhesus monkeys against a stringent, otherwise lethal challenge with P. knowlesi sporozoites. The protection induced by this multistage vaccine appears to act at both the preerythrocytic and erythrocytic stages of the parasite life cycle. Those animals which never developed parasitemia (monkeys TAA and TAJ) or which had very low early parasitemias but went on to require treatment (monkeys KCA and JKA) presumably had a substantial component of preerythrocytic-stage immunity, while animals which developed higher parasitemias early but spontaneously cleared the parasitemias (e.g., monkeys TAB and JPT) presumably had a more significant component of erythrocytic-stage immunity. Definitive identification of the stage at which protection occurs depends on ongoing experiments in which monkeys are immunized with either the preerythrocytic- or erythrocytic-stage components alone. Overall, 9 of 11 monkeys were protected from life-threatening parasitemia. This degree of protection is substantially greater than that seen in other sporozoite challenge trials of a variety of subunit vaccines for P. knowlesi. For example, immunization with soluble heat-stable P. knowlesi antigen induced only limited protection (5), and even immunization with irradiated sporozoites, which induces high levels of protection against sporozoite challenge in mice and humans, induced only incomplete protection in the P. knowlesi-rhesus monkey model (10).
A number of important questions about the protection seen here remain. First, because we lack reagents to assay antibody and T-cell reagents to all vaccine components separately, we do not know which antigens are the targets of protective immunity in this regimen. We do know that antibodies were induced against both sporozoites and blood-stage parasites and against a recombinant PkCSP, so that at a minimum, responses were induced to PkCSP and to either PkAMA1 or PkMSP1. In previous experiments, when mice were immunized with each of the four vaccine plasmids used here separately, antibodies were induced to each of the four antigens (18). Second, although the sterile protection in two monkeys, the reduction in early parasitemia in the immunized monkeys compared with the parasitemia in the controls, and the correlation of low EPI with IFN-γ secretion in response to PkCSP peptides strongly suggest that the vaccine induced preerythrocytic-stage immunity, the role of the vaccine in the blood-stage protection observed is less clear. We found no correlation between levels of antibody to infected erythrocytes on the day of challenge and spontaneous clearance of parasites; it is possible that preerythrocytic responses induced by the vaccine caused a delay in parasitemia sufficient to allow protective responses to blood-stage parasites to be induced by the infection, independent of the blood-stage components included in the vaccine. We are currently testing the protective efficacy of vaccine regimens incorporating single antigens or the preerythrocytic-stage antigens alone to determine which antigenic components are required for protection.
It is interesting that the regimen described here induced much greater protection than a similar priming and boosting regimen in which canarypox virus was used instead of attenuated vaccinia virus (18). The attenuated vaccinia virus used here is capable of limited replication in some mammalian cells, at least in vitro (16), while the canarypox virus is completely replication deficient in mammalian cells (15a) and may therefore be less immunogenic. Similar heterologous priming and boosting vaccination regimens have been shown to protect nonhuman primates from a variety of pathogens, including simian immunodeficiency virus (1, 17). In the simian immunodeficiency virus system, vaccine-induced immune responses do not control the initial viremia but do prevent later development of high circulating viremia and immunodeficiency. In some respects, the P. knowlesi system is an even more stringent challenge to vaccine development, because the untreated infection is rapidly fatal and there is little time for a vaccine-induced immune response to be boosted by infection.
The effect of cytokine-encoding plasmids in the priming DNA dose was probably minor. In mice, GM-CSF alone enhances antibody and T-cell responses to malaria plasmids and improves priming for plasmid boosts (28); however, it did not have measurable effects in previous rhesus macaque trials (unpublished data). In this study, addition of both IL-4 and GM-CSF to the malaria plasmids still did not produce measurable T-cell responses prior to the viral boosting. The addition of IL-4 may have improved T-cell priming, as some, but not all, peptide responses were enhanced after viral boosting. The addition of TNF-α had little effect. There was a trend toward lower early parasitemias in the monkeys in groups 3 and 4, the two groups incorporating GM-CSF and IL-4, compared with group 2 monkeys, which received GM-CSF alone; the differences, however, were relatively small and were not statistically significant. Whether the addition of any of the cytokine combinations tested enhanced immune responses to a small degree or not, it is clear that any effect of the cytokines in the priming phase of the regimen was minor compared to the dramatic enhancement caused by the viral boosting.
In brief, we demonstrated that a multiantigen DNA priming and poxvirus boosting vaccination regimen can provide protection against a stringent P. knowlesi sporozoite challenge. Although the regimen used here was very complex, it appears likely that it can be significantly simplified. Studies are under way to determine whether it may be possible to eliminate the cytokine plasmids, reduce the number of antigens used, and simplify the immunization schedule without a loss of efficacy. Such a simplified regimen could be readily adapted for human trials of a Plasmodium falciparum vaccine.
Acknowledgments
This work was supported by Naval Medical Research Center Work Unit STOF 6.2.622787A.0101.870.EFX(MIDRP), by World Health Organization/TDR project 960452, and by a cooperative research and development agreement between the Naval Medical Research Center and Aventis Pasteur, Inc.
We thank Daniel Carucci for assistance with the automated counting of the ELISPOT responses, William Collins for providing A. dirus mosquitoes, Martha Sedegah for assistance with dissection of mosquitoes and purification of sporozoites, Richard Stout (Bioject, Inc., Portland, Oreg.) for the gift of Biojector syringes, Francois Villinger for the gift of the rhesus macaque IL-4 and TNF-α plasmids, and the staff of the Division of Veterinary Medicine, Walter Reed Army Institute of Research, for assistance with care of the rhesus monkeys.
W.O.R and W.R.W. contributed equally to this work.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 2.Cella, M., F. Sallusto, and A. Lanzavecchia. 1997. Origin, maturation and antigen presenting function of dendritic cells. Curr. Opin. Immunol. 9:10-16. [DOI] [PubMed] [Google Scholar]
- 3.Clyde, D. F., H. Most, V. C. McCarthy, and J. P. Vanderberg. 1973. Immunization of man against sporozoite-induced falciparum malaria. Am. J. Med. Sci. 266:169-177. [DOI] [PubMed] [Google Scholar]
- 4.Coatney, G. R., W. E. Collins, M. Warren, and P. G. Contacos. 1971. The primate malarias. U.S. Department of Health, U.S. Government Printing Office, Bethesda, Md.
- 5.Collins, W. E., P. G. Contacos, A. J. Harrison, P. S. Stanfill, and J. C. Skinner. 1977. Attempts to immunize monkeys against Plasmodium knowlesi by using heat-stable, serum-soluble antigens. Am. J. Trop. Med. Hyg. 26:373-376. [DOI] [PubMed] [Google Scholar]
- 6.Doolan, D. L., and S. L. Hoffman. 1997. Pre-erythrocytic-stage immune effector mechanisms in Plasmodium spp. infections. Philos. Trans. R. Soc. Lond. B Biol. Sci. 352:1361-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doolan, D. L., and S. L. Hoffman. 2000. The complexity of protective immunity against liver-stage malaria. J. Immunol. 165:1453-1462. [DOI] [PubMed] [Google Scholar]
- 8.Earle, W. S., and M. Perez. 1932. Enumeration of parasites in the blood of malarial patients. J. Lab. Clin. Med. 17:1124. [Google Scholar]
- 9.Guo, P. X., S. Goebel, S. Davis, M. E. Perkus, B. Languet, P. Desmettre, G. Allen, and E. Paoletti. 1989. Expression in recombinant vaccinia virus of the equine herpesvirus 1 gene encoding glycoprotein gp13 and protection of immunized animals. J. Virol. 63:4189-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gwadz, R. W., A. H. Cochrane, V. Nussenzweig, and R. S. Nussenzweig. 1979. Preliminary studies on vaccination of rhesus monkeys with irradiated sporozoites of Plasmodium knowlesi and characterization of surface antigens of these parasites. Bull. W. H. O. 57(Suppl. 1):165-173. [PMC free article] [PubMed] [Google Scholar]
- 11.Haddad, D., J. Ramprakash, M. Sedegah, Y. Charoenvit, R. Baumgartner, S. Kumar, S. L. Hoffman, and W. R. Weiss. 2000. Plasmid vaccine expressing granulocyte-macrophage colony-stimulating factor attracts infiltrates including immature dendritic cells into injected muscles. J. Immunol. 165:3772-3781. [DOI] [PubMed] [Google Scholar]
- 12.Hartikka, J., M. Sawdey, F. Cornefert Jensen, M. Margalith, K. Barnhart, M. Nolasco, H. L. Vahlsing, J. Meek, M. Marquet, P. Hobart, J. Norman, and M. Manthorpe. 1996. An improved plasmid DNA expression vector for direct injection into skeletal muscle. Hum. Gene Ther. 7:1205-1217. [DOI] [PubMed] [Google Scholar]
- 12a.Institute of Laboratory Animals Resources. 1996. Guide for the care and use of laboratory animals. National Research Council, National Academy Press, Washington, D.C.
- 13.Kumar, A., W. Weiss, J. A. Tine, S. L. Hoffman, and W. O. Rogers. 2001. ELISPOT assay for detection of peptide specific interferon-secreting cells in rhesus macaques. J. Immunol. Methods 247:49-60. [DOI] [PubMed] [Google Scholar]
- 14.Lanar, D. E., J. A. Tine, C. de Taisne, M. C. Seguin, W. I. Cox, J. P. Winslow, L. A. Ware, E. B. Kauffman, D. Gordon, W. R. Ballou, E. Paoletti, and J. C. Sadoff. 1996. Attenuated vaccinia virus-circumsporozoite protein recombinants confer protection against rodent malaria. Infect. Immun. 64:1666-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nussenzweig, R. S., J. Vanderberg, H. Most, and C. Orton. 1967. Protective immunity produced by the injection of X-irradiated sporozoites of Plasmodium berghei. Nature 216:160-162. [DOI] [PubMed] [Google Scholar]
- 15a.Paoletti, E., J. Taylor, B. Meignier, C. Menc, and J. Tartaglia. 1995. Highly attenuated poxvirus vectors: NYVAC, ALVAC, and TROVAC. Dev. Biol. Stand. 84:159-163. [PubMed] [Google Scholar]
- 16.Perkus, M. E., K. Limbach, and E. Paoletti. 1989. Cloning and expression of foreign genes in vaccinia virus, using a host range selection system. J. Virol. 63:3829-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson, H. L., D. C. Montefiori, R. P. Johnson, K. H. Manson, M. L. Kalish, J. D. Lifson, T. A. Rizvi, S. Lu, S.-L. Hu, G. P. Mazzara, D. L. Panicali, J. G. Herndon, R. Glickman, M. A. Candido, S. L. Lydy, M. S. Wyand, and H. M. McClure. 1999. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat. Med. 5:526-534. [DOI] [PubMed] [Google Scholar]
- 18.Rogers, W. O., J. K. Baird, A. Kumar, J. A. Tine, W. R. Weiss, J. C. Aguiar, K. Gowda, R. W. Gwadz, S. Kumar, M. Gold, and S. L. Hoffman. 2001. Multistage multiantigen heterologous prime boost vaccine for Plasmodium knowlesi malaria provides partial protection in rhesus macaques. Infect. Immun. 69:5565-5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers, W. O., and S. L. Hoffman. 1999. Malaria vaccines, p. 439-493. In M. Wahlgren and P. Perlmann (ed.), Malaria: molecular and clinical aspects. Harwood Academic Publishers, London, United Kingdom.
- 20.Roy, M. J., M. S. Wu, L. J. Barr, J. T. Fuller, L. G. Tussey, S. Speller, J. Culp, J. K. Burkholder, W. F. Swain, R. M. Dixon, G. Widera, R. Vessey, A. King, G. Ogg, A. Gallimore, J. R. Haynes, and F. D. Heydenburg. 2000. Induction of antigen-specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine 19:764-778. [DOI] [PubMed] [Google Scholar]
- 21.Sabchareon, A., T. Burnouf, D. Ouattara, P. Attanah, H. Bouharoun-Tayooun, P. Chantavanich, C. Foucault, T. Chongsuphajaisiddhi, and P. Druilhe. 1991. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am. J. Trop. Med. Hyg. 45:297-308. [DOI] [PubMed] [Google Scholar]
- 22.Sallusto, F., M. Cella, C. Danieli, and A. Lanzavecchia. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. Hill. 1998. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4:397-402. [DOI] [PubMed] [Google Scholar]
- 24.Sedegah, M., T. R. Jones, M. Kaur, R. Hedstrom, P. Hobart, J. A. Tine, and S. L. Hoffman. 1998. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of a malaria DNA vaccine. Proc. Natl. Acad. Sci. USA 95:7648-7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sedegah, M., W. Weiss, J. B. Sacci, Jr., Y. Charoenvit, R. Hedstrom, K. Gowda, V. F. Majam, J. Tine, S. Kumar, P. Hobart, and S. L. Hoffman. 2000. Improving protective immunity induced by DNA-based immunization: priming with antigen and GM-CSF-encoding plasmid DNA and boosting with antigen-expressing recombinant poxvirus. J. Immunol. 164:5905-5912. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan, N. J., A. Sanchez, P. E. Rollin, Z. Y. Yang, and G. J. Nabel. 2000. Development of a preventive vaccine for Ebola virus infection in primates. Nature 408:605-609. [DOI] [PubMed] [Google Scholar]
- 27.Wang, B., D. L. Doolan, T. P. Le, R. C. Hedstrom, K. M. Coonan, Y. Charoenvit, T. R. Jones, P. Hobart, M. Margalith, J. Ng, W. R. Weiss, M. Sedegah, C. de Taisne, J. A. Norman, and S. L. Hoffman. 1998. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 282:476-480. [DOI] [PubMed] [Google Scholar]
- 28.Weiss, W. R., K. J. Ishii, R. C. Hedstrom, M. Sedegah, M. Ichino, K. Barnhart, D. M. Klinman, and S. L. Hoffman. 1998. A plasmid encoding murine granulocyte-macrophage colony-stimulating factor increases protection conferred by a malaria DNA vaccine. J. Immunol. 161:2325-2332. [PubMed] [Google Scholar]
- 29.World Health Organization. 1994. World malaria situation in 1992. Wkly. Epidemiol. Rec. 69:309-314. [PubMed] [Google Scholar]