Abstract
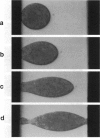
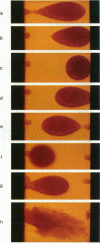
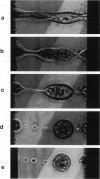
We report the use of high frequency alternating electric fields (AC) to induce deformation of sea urchin eggs, leading to budding of membrane vesicles or fission of cells. Several mini cell bodies can be prepared from a single egg by carefully manipulating the frequency and amplitude of the AC field and the ratio between the interelectrode spacing and the cell diameter, alpha. alpha values between 2.2 and 3.5 have been found to be optimal for inducing fission of sea urchin eggs. In a typical experiment, a sea urchin egg (diameter = 75 microns), suspended in a low ionic medium (conductance < 2 mS/m), was located under the microscope between two platinum wire electrodes, separated by a distance of approximately 200 microns. A medium strength AC field (< 100 V/cm at 2 MHz) was applied to attract the egg to one of the two electrodes via dielectrophoresis. This process took place in a few seconds. The voltage was then slowly increased to approximately 1000 V/cm over approximately 30 s. The cell elongated and separated into two fragments, the larger one containing the nucleus. When the field was turned off, the mother cell and the daughter vesicle retracted to form spherical mini cell bodies that appear to be stable as assessed by the absence of swelling for the duration of the experiment (approximately 15 min). This indicates that membranes of these mini cell bodies were not leaky to ions and small molecules. This procedure could be repeated a few times to make several mini cell bodies from a single egg. With practice, several minicell bodies could also be prepared in a single fission experiment by adjusting the field parameters and the a value. Cell fission is a result of the mechanical stress produced by the AC field. These procedures may be used to prepare mini membrane vesicles for voltage clamp experiments or to perform microsurgical manipulation of cells, embryos, or chromosomes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dimitrov D. S., Apostolova M. A., Sowers A. E. Attraction, deformation and contact of membranes induced by low frequency electric fields. Biochim Biophys Acta. 1990 Apr 30;1023(3):389–397. doi: 10.1016/0005-2736(90)90131-7. [DOI] [PubMed] [Google Scholar]
- Döbereiner H. G., Käs J., Noppl D., Sprenger I., Sackmann E. Budding and fission of vesicles. Biophys J. 1993 Oct;65(4):1396–1403. doi: 10.1016/S0006-3495(93)81203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt H., Sackmann E. On the measurement of shear elastic moduli and viscosities of erythrocyte plasma membranes by transient deformation in high frequency electric fields. Biophys J. 1988 Sep;54(3):495–508. doi: 10.1016/S0006-3495(88)82982-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A. Bending elastic modulus of red blood cell membrane derived from buckling instability in micropipet aspiration tests. Biophys J. 1983 Jul;43(1):27–30. doi: 10.1016/S0006-3495(83)84319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUEREDI A. A., OHAD I. EFFECTS OF HIGH-FREQUENCY ELECTRIC FIELDS ON THE LIVING CELL. I. BEHAVIOUR OF HUMAN ERYTHROCYTES IN HIGH-FREQUENCY ELECTRIC FIELDS AND ITS RELATION TO THEIR AGE. Biochim Biophys Acta. 1964 Jan 27;79:1–8. [PubMed] [Google Scholar]
- Foster K. R., Sauer F. A., Schwan H. P. Electrorotation and levitation of cells and colloidal particles. Biophys J. 1992 Jul;63(1):180–190. doi: 10.1016/S0006-3495(92)81588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass G. V., Chernomordik L. V., Margolis L. B. Local deformation of human red blood cells in high frequency electric field. Biochim Biophys Acta. 1991 Jul 10;1093(2-3):162–167. doi: 10.1016/0167-4889(91)90118-h. [DOI] [PubMed] [Google Scholar]
- Gass G. V., Chernomordik L. V. Reversible large-scale deformations in the membranes of electrically-treated cells: electroinduced bleb formation. Biochim Biophys Acta. 1990 Mar 30;1023(1):1–11. doi: 10.1016/0005-2736(90)90002-6. [DOI] [PubMed] [Google Scholar]
- Kaler K. V., Xie J. P., Jones T. B., Paul R. Dual-frequency dielectrophoretic levitation of Canola protoplasts. Biophys J. 1992 Jul;63(1):58–69. doi: 10.1016/S0006-3495(92)81586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. T. Hemolysis of human erythrocytes by transient electric field. Proc Natl Acad Sci U S A. 1977 May;74(5):1923–1927. doi: 10.1073/pnas.74.5.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov M. M., Lerche D., Meier W. RBC membrane instability for large pipette deformation. A theoretical approach. Biorheology. 1988;25(6):843–856. doi: 10.3233/bir-1988-25605. [DOI] [PubMed] [Google Scholar]
- Liu D. S., Astumian R. D., Tsong T. Y. Activation of Na+ and K+ pumping modes of (Na,K)-ATPase by an oscillating electric field. J Biol Chem. 1990 May 5;265(13):7260–7267. [PubMed] [Google Scholar]
- Mahaworasilpa T. L., Coster H. G., George E. P. Forces on biological cells due to applied alternating (AC) electric fields. I. Dielectrophoresis. Biochim Biophys Acta. 1994 Jul 13;1193(1):118–126. doi: 10.1016/0005-2736(94)90340-9. [DOI] [PubMed] [Google Scholar]
- Pliquett F. Das Verhalten von Oxytrichiden unter dem Einfluss des elektrischen Feldes. Z Biol. 1968 Feb;116(1):10–22. [PubMed] [Google Scholar]
- Popov S. V., Svitkina T. M., Margolis L. B., Tsong T. Y. Mechanism of cell protrusion formation in electrical field: the role of actin. Biochim Biophys Acta. 1991 Jul 22;1066(2):151–158. doi: 10.1016/0005-2736(91)90181-7. [DOI] [PubMed] [Google Scholar]
- Poznański J., Pawłowski P., Fikus M. Bioelectrorheological model of the cell. 3. Viscoelastic shear deformation of the membrane. Biophys J. 1992 Mar;61(3):612–620. doi: 10.1016/S0006-3495(92)81866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenger D. A., Kaler K. V., Hui S. W. Dipole interactions in electrofusion. Contributions of membrane potential and effective dipole interaction pressures. Biophys J. 1991 May;59(5):1074–1084. doi: 10.1016/S0006-3495(91)82322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekle E., Astumian R. D., Chock P. B. Electroporation by using bipolar oscillating electric field: an improved method for DNA transfection of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4230–4234. doi: 10.1073/pnas.88.10.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsong T. Y. Electroporation of cell membranes. Biophys J. 1991 Aug;60(2):297–306. doi: 10.1016/S0006-3495(91)82054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsong T. Y. Molecular recognition and processing of periodic signals in cells: study of activation of membrane ATPases by alternating electric fields. Biochim Biophys Acta. 1992 Mar 26;1113(1):53–70. doi: 10.1016/0304-4157(92)90034-8. [DOI] [PubMed] [Google Scholar]
- Weaver J. C. Molecular basis for cell membrane electroporation. Ann N Y Acad Sci. 1994 May 31;720:141–152. doi: 10.1111/j.1749-6632.1994.tb30442.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann U. Electric field-mediated fusion and related electrical phenomena. Biochim Biophys Acta. 1982 Nov 30;694(3):227–277. doi: 10.1016/0304-4157(82)90007-7. [DOI] [PubMed] [Google Scholar]