Abstract
Bordetella pertussis, the etiological agent of whooping cough, produces a number of factors, such as toxins and adhesins, that are required for full expression of virulence. Filamentous hemagglutinin (FHA) is the major adhesin of B. pertussis. It is a protein of approximately 220 kDa, found both associated at the bacterial cell surface and secreted into the extracellular milieu. Despite its importance in B. pertussis pathogenesis and its inclusion in most acellular pertussis vaccines, little is known about the functional importance of individual domains in infection and in the induction of protective immunity. In this study, we analyzed the role of the approximately 80-kDa N-terminal domain of FHA, designated Fha44, in B. pertussis adherence, colonization, and immunogenicity. Although Fha44 contains the complete heparan sulfate-binding domain, it is not sufficient for adherence to epithelial cells or macrophages. It also cannot replace FHA during colonization of the mouse respiratory tract. Infection with a B. pertussis strain producing Fha44 instead of FHA does not induce anti-FHA antibodies, whereas such antibodies can readily be induced by intranasal administration of purified Fha44. In addition, mice immunized with purified Fha44 were protected against challenge with wild-type B. pertussis, indicating that Fha44 contains protective epitopes. Compared to FHA, Fha44 is much smaller and much more soluble and is therefore easier to purify and to store. These advantages may perhaps warrant considering Fha44 for inclusion in acellular pertussis vaccines.
Bordetella pertussis is the main etiologic agent of whooping cough, a highly contagious human respiratory disease responsible for more than 355,000 deaths annually worldwide, mostly unimmunized young children (6). Inactivated whole-cell vaccines (WCVs) have been used for more than 30 years and have successfully reduced the incidence of pertussis (11). However, some adverse side effects have been associated with the administration of WCVs (30), and these have led to development of new, acellular vaccines (ACVs). The safety and reduced reactogenicity of ACVs compared to WCVs have been well established by their extensive use in Japan (15) and by several clinical trials (7, 10). More than 15 ACVs are now commercially available (29). They are composed of one to five defined purified antigens.
Filamentous hemagglutinin (FHA) is included in most ACVs. Its protective role against respiratory B. pertussis challenge has been extensively studied and demonstrated with mice (4, 5, 19, 35, 36). In addition, the inclusion of FHA in ACVs has been shown to decrease the rates of infection in humans (39). FHA is first synthesized as a 370-kDa precursor and is then processed to yield a 220-kDa mature, nontoxic protein. FHA is considered to be one of the main adhesins produced by virulent B. pertussis (21), together with pertactin, tracheal colonization factor, and fimbriae. Three distinct binding sites have been defined and mapped in full-length FHA: an N-terminal glycosaminoglycan-binding site (13, 25), an arginine-glycine-aspartate (RGD) sequence (33), and a carbohydrate recognition domain (CRD) (31). FHA mediates adherence of B. pertussis to pulmonary and tracheal epithelial cells as well as to macrophages (1, 3, 14, 40). It has been postulated that an effective antibody response to FHA, which interferes with the attachment of the bacteria to the respiratory mucosal surface, could prevent infection (4). The C-terminal moiety of mature FHA has been demonstrated to be the immunodominant part of the protein (9, 20, 28, 42). It also contains most of the cell-binding sites. Much less is known about the function of the N-terminal moiety of the protein, except that it contains the secretion determinant and is thus implicated in displaying FHA at the surfaces of B. pertussis organisms (16).
In order to study the roles of the N-terminal moiety of FHA in adherence and immunogenicity, we used a truncated form of FHA, designated Fha44, corresponding to the first 772 amino acids of the mature protein (34). This 80-kDa fragment contains the glycosaminoglycan-binding site but lacks the CRD and RGD domains, as well as the immunodominant regions. We found that the N-terminal region of FHA is not sufficient for colonization in vivo and that, when produced by B. pertussis in an FHA-deficient background, it is not immunogenic during infection. However, when administered by the intranasal (i.n.) route as a purified protein, it induces a strong antibody response and protection against respiratory challenge.
MATERIALS AND METHODS
B. pertussis strains and growth conditions.
The B. pertussis strains used in this study are listed in Table 1. They are all derived from the Tohama I strain (25) and were grown on Bordet-Gengou (BG) agar (Difco, Detroit, Mich.) supplemented with 1% glycerol, 20% defibrinated sheep blood, and 100 μg of streptomycin (Sigma Chemical Co., St. Louis, Mo.)/ml at 37°C for 72 h. Liquid cultures of B. pertussis were performed as described previously (23) in Stainer-Scholte (SS) medium containing 1 g of heptakis(2,6-di-o-methyl) β-cyclodextrin (Sigma)/liter. For cell adherence assays, exponentially growing B. pertussis was inoculated at an optical density at 600 nm (OD600) of 0.15 in 2.5 ml of SS medium supplemented with 65 μCi of l-[35S]methionine plus l-[35S]cysteine (NEN, Boston, Mass.)/ml and grown for 24 h at 37°C. Bacteria were then washed three times in phosphate-buffered saline (PBS) and resuspended in RPMI 1640 medium (Gibco, Grand Island, N.Y.) at the desired density.
TABLE 1.
B. pertussis strains used in this study
B. pertussis mutant constructs.
B. pertussis BPSA90 and BPSA87 were obtained by allelic exchange as described by Stibitz (38) by using pAS34 to replace the fhaB genes of BPSM and BPRA, respectively, with the DNA encoding Fha44. pAS34 was obtained by cloning the 2200-bp SalI fragment of pAS32 into the unique SalI site of pJQmp200rpsL18 (32). pAS32 resulted from the cloning of the 2,850-bp EcoRI-XbaI fragment from pBG4STOP into EcoRI-NheI-digested pAS2. pAS2 is a pBR328 derivative (37) in which the EcoRI-BglII fragment of pBG1 (34) was cloned after digestion with EcoRI and BamHI. pBG4STOP is a derivative of pBG4 (34) in which the oligonucleotide 5′-GATCCTAGTCTAGACTAG-3′ has been inserted into the BamHI site to create an XbaI site containing a termination codon at the end of the first 2.8 kb of fhaB corresponding to the Fha44-coding sequence. Strains BPSA90 and BPSA87 were analyzed by Southern blotting to ensure that the expected allelic replacements had occurred and by immunoblotting of the culture supernatants, as described previously (34), to verify the absence of FHA and the production and secretion of Fha44. Fha44 was produced in both strains at levels at least as high as those of FHA in the parent strains.
Cells and growth conditions.
The human pulmonary epithelial cell line A549 (ATCC CCL-185) was cultured in RPMI medium containing sodium penicillin G (1,000 U/ml) and streptomycin (50 μg/ml), 2 mM l-glutamine, and 10% heat-inactivated fetal calf serum (all from Gibco) by using uncoated tissue culture flasks and 24-well plates (Costar). The murine alveolar macrophage cell line MH-S (ATCC CRL-2019) was propagated in uncoated tissue culture flasks and 24-well plates in the same RPMI-based medium described above, supplemented with 1.5 g of sodium bicarbonate/liter, 4.5 g of glucose/liter, 10 mM HEPES, 1 mM sodium pyruvate, and 50 μM 2-β-mercaptoethanol (all from Gibco). Cells were detached mechanically by scraping.
Cell adherence assay.
A total of 2 × 105 cells per well were cultured for 2 days in 24-well plates. Cells were washed once with RPMI medium before addition of 4 × 106 [35S]-labeled bacteria per well and incubation for 1 h 30 min at 37°C under 5% CO2. After three washes with RPMI medium to remove nonadherent bacteria, the mammalian cells were lysed with 0.5% sodium dodecyl sulfate. The radioactivity in the whole-cell lysates was quantified by liquid scintillation counting.
Antigens.
FHA and Fha44 were purified from B. pertussis BPRA and BPSA87, respectively, as described previously (24). In both strains the ptx genes are deleted to avoid any possible contamination with pertussis toxin (PT) during purification by heparin-Sepharose chromatography. The antigen concentration was estimated by the Bradford method. Purified PT, cholera toxin (CT), and the cholera toxin B subunit (CTB) were purchased from Sigma.
i.n. infection.
B. pertussis grown on BG agar was suspended in sterile PBS and adjusted to a concentration of approximately 2.5 × 108 CFU/ml for colonization and immunogenicity studies or 2.5 × 107 CFU/ml for protection studies. Infections were performed by the i.n. route, with 20 μl of the bacterial suspensions deposited in the nostrils of 9-week-old BALB/c mice (Iffa Credo, L'Arbresle, France) that had been anesthesized with a cocktail of physiological water containing 20% (vol/vol) Immalgen 1000 (Merial, Lyon, France), 10% atropine (Aguettant, Lyon, France), and 6% valium (Roche, Neuilly-sur-Seine, France), given intraperitoneally (150 μl per 20 g of body weight) (8). At the indicated time points, the lungs were aseptically removed and homogenized in PBS. Serial dilutions from individual lung homogenates were plated onto BG agar, and the numbers of CFU were determined after 3 to 4 days of incubation at 37°C. Four mice per time point and per group of mice were assessed. All animal studies were carried out according to the guidelines of the Institut Pasteur animal study board.
i.n. immunization.
A 50-μl volume of various concentrations of antigen in sterile PBS was administered by the i.n. route to BALB/c mice that had been anesthesized as described above.
Passive immunization.
Immune serum (0.2 ml) was intravenously (i.v.) injected into BALB/c mice 1 h before i.n. infection with 1.5 × 108 CFU of strain BPSM/ml. Lung homogenates were plated out on day 5 after challenge, as described above. Five to seven mice per group were assessed.
Antibody detection.
Mice under anesthesia were bled by retro-orbital plexus puncture, and the serum samples were stored at −20°C until further use. Levels of antibodies to PT and FHA were measured by enzyme-linked immunosorbent assays. Microtiter plates (Maxisorp; Nunc) were coated with 50 μl of 0.05 M carbonate buffer (pH 9.6; Sigma) containing 5 μg of purified antigen/ml. After blocking with PBS containing 0.1% Tween and 1% bovine serum albumin, 50 μl of serum was added in twofold serial dilutions. Plates were incubated for 2 h at 37°C, and goat anti-mouse total immunoglobulin G (IgG), IgG2a, IgG2b, and IgG1 conjugated with horseradish peroxidase (Amersham, Les Ulis, France) were added at dilutions of 1/4,000, 1/500, 1/500, and 1/1,000, respectively. After 1 h at 37°C, antibodies were detected by using 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS; Boehringer Mannheim, Mannheim, Germany) according to the manufacturer's specifications. Results are expressed as the highest dilution yielding an absorbency at 405 nm three times above the control values.
Statistical analysis.
The results were analyzed by an unpaired Student t test. Differences were considered significant at a P value of ≤0.05.
RESULTS
Lung colonization by B. pertussis strains producing Fha44.
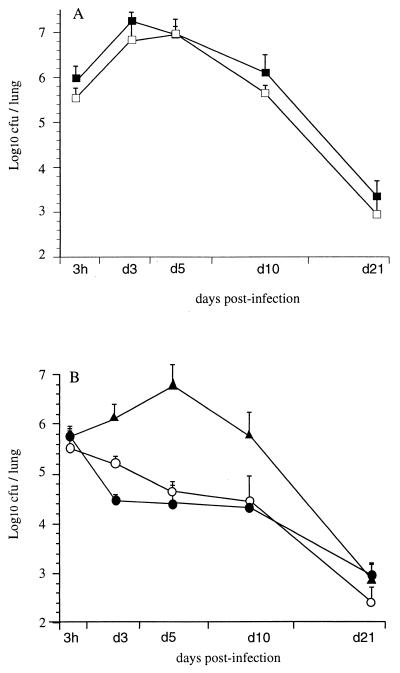
To determine whether the 80-kDa N-terminal portion of FHA, designated Fha44, plays an appreciable role in B. pertussis colonization of the lungs, mice were i.n. infected with wild-type B. pertussis BPSM or with its derivative BPSA90, which produces Fha44 instead of FHA. BPSA90 showed a colonization profile similar to that of BPSM, with a multiplication peak 3 to 5 days after inoculation followed by progressive clearance over the next 3 weeks (Fig. 1A). In contrast, BPSA87, producing Fha44 and lacking PT (Fha44 ΔPT), presented a colonization profile without a multiplication peak, similar to the profile observed for a mutant lacking both FHA and PT, whereas BPRA, a strain lacking only PT, behaved like the wild-type strain (Fig. 1B). These results indicate that the 80-kDa N-terminal moiety of FHA is not sufficient to play the functional role of FHA during lung colonization by B. pertussis.
FIG. 1.
Lung colonization by B. pertussis. OF1 mice were infected i.n. either with B. pertussis BPSM (solid squares) or BPSA90 (open squares) (A) or with BPRA (solid triangles), BPSA87 (solid circles), or BPDR (open circles) (B). At the indicated time points, mice were sacrificed, and viable bacteria present in the lungs were counted. Four mice were analyzed per time point for each group.
In vitro adherence of B. pertussis strains.
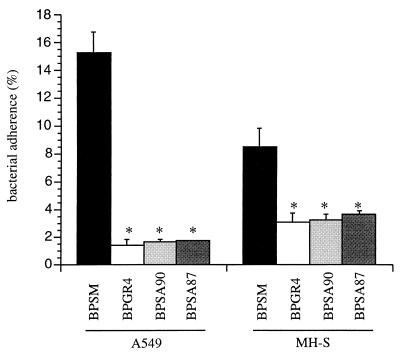
To investigate the functional role of Fha44 in direct host-cell binding of B. pertussis, we studied the adherence of BPSM and its derivatives BPGR4 (ΔFHA), BPSA90 (Fha44), and BPSA87 (Fha44 ΔPT) to A549 human pulmonary epithelial cells and to MH-S murine alveolar macrophages. Figure 2 shows that the Fha44-producing strains adhered as poorly as the ΔFHA strain to both types of cells, indicating that Fha44 does not allow the bacteria to adhere efficiently to macrophages or pulmonary epithelial cells.
FIG. 2.
Adherence of B. pertussis to cells of two different cell lines. Human pulmonary epithelial cells (A549) and murine alveolar macrophages (MH-S) were incubated with the indicated [35S]-labeled B. pertussis strains for 1 h 30 min at a multiplicity of infection of 20. After a wash, adherence was estimated by scintillation counting. Results are expressed as percentages of counts per minute present in the inoculum. Data are averages and standard deviations from quadruplicate experiments. ∗, P < 0.05 relative to BPSM values.
Absence of anti-FHA antibodies after i.n. infection.
To evaluate the immunogenicity of Fha44 in the context of infection with Fha44-producing B. pertussis, mice were i.n. infected with BPSA90 or BPSA87. Production of anti-FHA antibodies was determined in serum samples 8 weeks after infection. No specific anti-FHA IgG was detected in these serum samples. In contrast, anti-FHA antibodies could readily be detected after infection with the FHA-producing strain BPSM (Table 2), indicating that, in contrast to full-length FHA, the 80-kDa N-terminal moiety of FHA is not immunogenic when expressed by B. pertussis.
TABLE 2.
Anti-FHA and anti-PT IgG titers in sera of i.n. immunized micea
| Immunization | Anti-FHA IgG titer | Anti-PT IgG titer |
|---|---|---|
| B. pertussis | ||
| BPSM | 1.6 × 104 | NDb |
| BPSA87 | <10 | ND |
| BPSA90 | <10 | ND |
| Antigens | ||
| FHA | 3.6 × 105 | <10 |
| Fha44 | 3.1 × 104 | <10 |
| PT | <10 | 4.1 × 105 |
| FHA + PT | 1.7 × 106 | 7.3 × 105 |
| Fha44 + PT | 2.5 × 105 | 6.5 × 105 |
| PBS | <10 | <10 |
For B. pertussis-infected mice, serum samples were collected and pooled from eight mice per group 8 weeks after infection. For mice immunized with purified antigens, serum samples were collected and pooled from 10 mice per group 2 weeks after the last immunization.
ND, not determined.
Immunogenicity and protective activity of purified Fha44.
To determine whether purified Fha44 given i.n. is immunogenic, adult mice were immunized with two i.n. doses of 45 nmol of FHA or Fha44 (corresponding to 10 and 3.6 μg, respectively), given 1 month apart, and anti-FHA antibodies were measured 2 weeks after the last immunization. As shown in Table 2, i.n. immunization with purified Fha44 resulted in high titers of anti-FHA IgG in the serum, although i.n. immunization with purified FHA resulted in 10-fold higher serum anti-FHA IgG titers. These results show that Fha44 is immunogenic when given by the i.n. route.
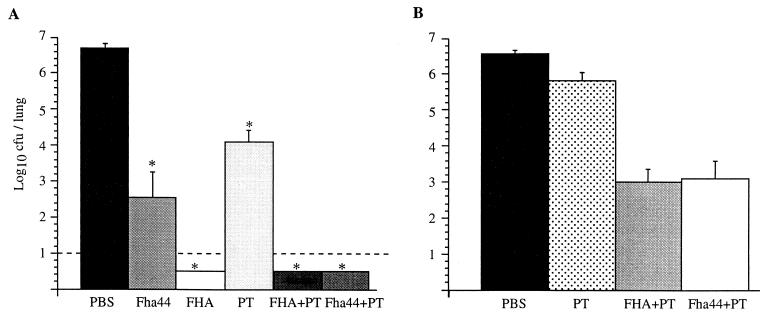
When the mice were challenged 2 weeks after the second i.n. immunization with Fha44, they were found to be significantly protected (Fig. 3A), indicating that the epitopes of Fha44 are protective epitopes. However, protection was significantly stronger when the mice were immunized with FHA rather than Fha44. This correlates well with the higher specific IgG titers in mice immunized with FHA compared to those in mice immunized with Fha44. Alternatively, it is possible that FHA contains protective epitopes that are not present in Fha44.
FIG. 3.
Protection against B. pertussis colonization. Mice were infected i.n. with B. pertussis BPSM after i.n. immunization with either purified Fha44, FHA, PT, FHA plus PT, or Fha44 plus PT, as indicated (A), or after i.v. administration of antisera from mice immunized with either PBS, PT, FHA plus PT, or Fha44 plus PT (B). Five days after infection, the mice were sacrificed, and the viable bacteria present in the lungs were counted. Values shown are means ± standard deviations for at least four mice per group. ∗, P < 0.05 relative to the PBS control. The dashed line in panel A represents the limit of detection of the number of CFU present in the lungs.
To test whether the difference in protection levels might be attributed to a quantitative difference in antibody titers or to a qualitative difference in important epitopes that would be present on FHA but lacking on Fha44, we added small amounts of PT to Fha44 as an adjuvant to increase the anti-FHA IgG titers. As shown in Table 2, the addition of 1 μg of PT to 45 nmol of Fha44 increased serum anti-FHA IgG titers approximately 10-fold and brought them up to the levels in mice immunized with FHA. Isotypic profile analysis indicated no significant difference in the relative proportions of anti-FHA IgG2a, IgG2b, and IgG1 induced in mice immunized with Fha44 or Fha44 plus PT (data not shown). Addition of 1 μg of PT to FHA also increased anti-FHA IgG titers about fivefold over titers obtained after immunization with FHA alone.
When the mice immunized with 45 nmol of Fha44 in the presence of 1 μg of PT were challenged, they were found to be protected as well as those that were immunized with 45 nmol of FHA in the presence or absence of 1 μg of PT (Fig. 3A). One microgram of PT alone resulted in only marginal protection. Since similar anti-PT IgG levels were obtained for mice immunized with PT and mice immunized with Fha44 plus PT, the anti-PT antibodies cannot account for the Fha44-mediated protection observed in Fig. 3. These results suggest that Fha44 contains epitopes that are immunogenic when given by the i.n. route and that are fully protective as long as the anti-Fha44 antibody titers are sufficiently high.
Protective effect of anti-Fha44 polyclonal antibodies.
To confirm that the protective effect observed in Fig. 3A was mediated by antibodies and was not due to a synergistic effect via PT-induced cell-mediated immunity, passive immunization was carried out by using anti-FHA antisera from mice i.n. immunized with either Fha44 plus PT or FHA plus PT. The anti-FHA antisera were adjusted to a titer of 2.5 × 105 and injected i.v. 1 h before i.n. challenge with virulent B. pertussis BPSM. Numbers of viable bacteria in the lungs were determined 5 days later. The results shown in Fig. 3B indicate that the anti-FHA antibodies protected the mice equally efficiently, regardless of whether the antiserum was obtained from mice immunized with FHA plus PT or with Fha44 plus PT. In contrast, when only anti-PT antibodies were administered at titers similar to those found in the anti-FHA antisera, only marginal protection was observed, confirming that Fha44-induced antibodies are able to fully protect mice from respiratory B. pertussis infection.
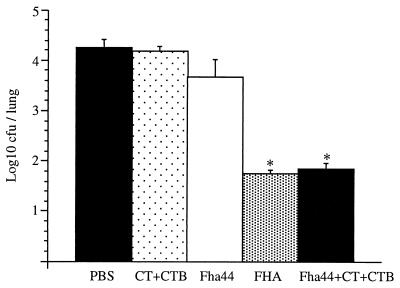
To rule out the possibility that the protection observed in mice immunized with Fha44 plus PT might be due to a synergistic effect of the anti-Fha44 and anti-PT antibodies, PT was replaced by a mixture of CT and CTB known to have powerful adjuvant activity. When mice were i.n. immunized with 45 nmol of Fha44 in the presence of 10 ng of CT plus 5 μg of CTB, anti-FHA IgG titers were increased about 10-fold over those in Fha44-immunized animals (data not shown), similar to what was observed when mice immunized with Fha44 plus PT were compared to Fha44-immunized mice. In addition, immunization with the Fha44-CT-CTB combination protected mice from B. pertussis respiratory challenge at the level obtained after immunization with FHA (Fig. 4), clearly demonstrating that high levels of anti-Fha44 antibodies are sufficient to fully protect mice from B. pertussis infection.
FIG. 4.
Protection against B. pertussis colonization by using CT plus CTB as an adjuvant. Mice were infected i.n. with B. pertussis BPSM after i.n. immunization with either purified CT plus CTB, Fha44, FHA, or Fha44 plus CT plus CTB, as indicated. Two days after infection, mice were sacrificed, and viable bacteria in the lungs were counted. Values shown are means ± standard deviations for at least four mice per group. ∗, P < 0.05 relative to the PBS control.
DISCUSSION
In this study, we investigated the biological properties of the 80-kDa N-terminal moiety of FHA, designated Fha44. As reported earlier, these first 772 amino acids contain the entire secretion determinant, and Fha44 is secreted at least as efficiently as full-length FHA (34). Immunoblot analyses on cell-associated protein fractions from Fha44-producing B. pertussis have indicated that Fha44 is also present at the surfaces of bacteria (34). Fha44 contains the heparin-binding site of FHA (13, 25), while the CRD, which is responsible for attachment to epithelial cilia (31), and the RGD sequence, which is involved in binding to the CR3 integrin of macrophages (33), are not present in Fha44. In vitro adherence studies using Fha44-producing B. pertussis strains that lack full-length FHA showed that the 80-kDa N-terminal region containing the heparin-binding site of FHA is not sufficient to promote adherence of the bacteria to pulmonary epithelial cells or macrophages. These results indicate that the adherence properties of FHA rely on the presence of binding regions other than its heparin-binding site.
To investigate the role of Fha44 in B. pertussis colonization of the respiratory tract, we took advantage of the recently discovered redundancy between FHA and PT during infection in the mouse model (1). Mutants lacking FHA alone (17-19) or lacking PT alone (12, 17, 18, 41) colonize the mouse respiratory tract almost as well as their isogenic parent strains producing both FHA and PT. However, when both FHA and PT are lacking, B. pertussis is severely impaired in its ability to colonize (1). Therefore, Fha44 was produced in a PT-deficient B. pertussis background. A strain that lacks PT and produces Fha44 instead of FHA colonized the mouse respiratory tract poorly, as has been observed for a strain deficient in both PT and FHA production (1). This finding indicates that Fha44 cannot replace full-length FHA in its ability to promote colonization, at least in the mouse model.
Curiously, when mice were i.n. infected with B. pertussis strains that produce Fha44 instead of FHA, no local or systemic anti-FHA antibodies were detected. However, anti-FHA antibodies could readily be induced after injection (34) or after i.n. administration (this study) of purified Fha44, indicating that Fha44 contains immunogenic epitopes and is not immunosilent. Since in vitro studies have shown that Fha44 is secreted at high levels and is surface associated (34), it should be accessible to the immune system of the host, unless secretion or surface display of Fha44 differs dramatically between in vitro- and in vivo-growing B. pertussis. Furthermore, infection of mice or children with wild-type B. pertussis producing full-length FHA results in the generation of anti-Fha44 antibodies (20), although the major antigenic determinants of FHA are located in its C-terminal moiety, which is absent in Fha44. This indicates that the Fha44 portion of FHA is immunoaccessible in the context of B. pertussis infection. It cannot be ruled out, however, that the conformation of the Fha44 domain may differ between strains that produce Fha44 alone and strains that produce full-length FHA, and that therefore Fha44 may be rapidly degraded by host proteases before it can induce immune responses. However, passive immunization with an anti-FHA immune serum significantly protected mice from challenge with a strain producing Fha44 instead of FHA (data not shown), suggesting that anti-FHA antibodies recognize Fha44 present at the bacterial surface during infection. Therefore, the reasons for the lack of immunogenicity of Fha44 during Fha44-producing B. pertussis infection remain unknown.
i.n. administration of purified Fha44 was able to induce protective immunity against respiratory challenge with virulent B. pertussis. Besides indicating that immunoprotective determinants are present on Fha44, these observations also confirm independently that the Fha44 moiety of FHA is accessible to the immune system of an infected host. The level of protection offered by Fha44 was lower than that induced by full-length FHA at an equimolar dose, suggesting either that FHA contains additional protective epitopes not present in Fha44 or that FHA is more immunogenic than Fha44, or both. However, when addition of small amounts of PT or of a mixture of CT and CTB augmented anti-FHA antibody levels in Fha44-immunized mice to the levels induced by FHA, Fha44-mediated protection was as high as that induced by FHA. These results suggest that anti-Fha44 antibodies may provide protection levels as strong as those induced by anti-FHA antibodies, as long as high enough titers are reached.
Although several studies have demonstrated that FHA is able to induce protective immunity in murine models (4, 5, 19, 35, 36), which is the basis for its inclusion in most of the current ACVs (29), attempts to protect by passive transfer of anti-FHA antibodies have not always been successful (26, 27). In most of these studies, the antibodies were administered intraperitoneally, whereas in this study, the i.v. route was used. In addition, most anti-FHA antibodies are directed against its C-terminal moiety (9, 20, 28, 42). The N-terminal moiety, essentially the Fha44 domain, contains only minor B-cell epitopes (20). It is conceivable that the protective epitopes of FHA are located not in the immunodominant domain but rather in the Fha44 region.
Fha44 has a number of advantages over FHA with regard to inclusion in ACVs. It is much more soluble than full-length FHA and can therefore be used at higher concentrations. This may also facilitate purification and storage of the antigen. In addition, Fha44 can be produced and secreted by heterologous gram-negative bacterial hosts that are easier to cultivate in large amounts than B. pertussis, provided that the FHA secretion apparatus is coexpressed (16). Full-length FHA, on the other hand, has so far been produced only by Bordetella organisms. Taken together, these advantages may warrant consideration of Fha44 for inclusion as a possible protective antigen in new ACVs against pertussis.
Editor: J. D. Clements
REFERENCES
- 1.Alonso, S., K. Pethe, N. Mielcarek, D. Raze, and C. Locht. 2001. Role of ADP-ribosyltransferase activity of pertussis toxin in toxin-adhesin redundancy with filamentous hemagglutinin during Bordetella pertussis infection. Infect. Immun. 69:6038-6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoine, R., and C. Locht. 1990. Roles of the disulfide bond and the carboxy-terminal region of the S1 subunit in the assembly and biosynthesis of pertussis toxin. Infect. Immun. 58:1518-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassinet, L., P. Gueirard, B. Maitre, B. Housset, P. Gounon, and N. Guiso. 2000. Role of adhesins and toxins in invasion of human tracheal epithelial cells by Bordetella pertussis. Infect. Immun. 68:1934-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahill, E. S., D. T. O'Hagan, L. Illum, A. Barnard, K. H. G. Mills, and K. Redhead. 1995. Immune responses and protection against Bordetella pertussis infection after intranasal immunization of mice with filamentous haemagglutinin in solution or incorporated in biodegradable microparticles. Vaccine 13:455-462. [DOI] [PubMed] [Google Scholar]
- 5.Cahill, E. S., D. T. O'Hagan, L. Illum, and K. Redhead. 1993. Mice are protected against Bordetella pertussis infection by intra-nasal immunization with filamentous haemagglutinin. FEMS Microbiol. Lett. 107:211-216. [DOI] [PubMed] [Google Scholar]
- 6.Cherry, J. D. 1996. Historical review of pertussis and the classical vaccine. J. Infect. Dis. 174(Suppl. 3):S259-S263. [DOI] [PubMed] [Google Scholar]
- 7.Decker, M. D., K. M. Edwards, M. C. Steinhoff, M. B. Rennels, M. E. Pichichero, J. A. Englund, E. L. Anderson, M. A. Deloria, and G. F. Reed. 1995. Comparison of 13 acellular pertussis vaccines: adverse reactions. Pediatrics 96:557-566. [PubMed] [Google Scholar]
- 8.Dei-Cas, E., M. Brun-Pascaud, V. Bille-Hansen, A. Allaert, and E. M. Aliouat. 1998. Animal models of pneumocystosis. FEMS Immunol. Med. Microbiol. 22:163-168. [DOI] [PubMed] [Google Scholar]
- 9.Delisse-Gathoye, A.-M., C. Locht, F. Jacob, M. Raashchou-Nielsen, I. Heron, J.-L. Ruelle, M. DeWilde, and T. Cabezon. 1990. Cloning, partial sequence, expression, and antigenic analysis of the filamentous hemagglutinin gene of Bordetella pertussis. Infect. Immun. 58:2895-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards, K. M., B. M. Meade, M. D. Decker, G. F. Reed, M. B. Rennels, M. C. Steinhoff, E. L. Anderson, J. A. Englund, M. E. Pichichero, and M. A. Deloria. 1995. Comparison of 13 acellular pertussis vaccines: overview and serologic response. Pediatrics 96:548-557. [PubMed] [Google Scholar]
- 11.Fine, P. E. M., and J. A. Clarkson. 1987. Reflections on the efficacy of pertussis vaccines. Rev. Infect. Dis. 9:866-883. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin, M. S., and A. A. Weiss. 1990. Adenylate cyclase toxin is critical for colonization and pertussis toxin is critical for lethal infection by Bordetella pertussis in infant mice. Infect. Immun. 58:3445-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hannah, J. H., F. D. Menozzi, G. Renauld, C. Locht, and M. J. Brennan. 1994. Sulfated glycoconjugate receptors for the Bordetella pertussis adhesin filamentous hemagglutinin (FHA) and mapping of the heparin-binding domain on FHA. Infect. Immun. 62:5010-5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazenbos, W. L. W., B. Van den Berg, J. W. Van't Wout, F. R. Mooi, and R. Van Furth. 1994. Virulence factors determine attachment and ingestion of nonopsonized Bordetella pertussis by human monocytes. Infect. Immun. 62:4818-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishitobi, A. 1983. Report of adverse reactions associated with new DTP vaccine available in Japan. J. Child Health 42:348-353. [Google Scholar]
- 16.Jacob-Dubuisson, F., C. Locht, and R. Antoine. 2001. Two-partner secretion in Gram-negative bacteria: a thrifty, specific pathway for large virulence proteins. Mol. Microbiol. 40:306-313. [DOI] [PubMed] [Google Scholar]
- 17.Khelef, N., C. M. Bachelet, B. B. Vargaftig, and N. Guiso. 1994. Characterization of murine lung inflammation after infection with parental Bordetella pertussis and mutants deficient in adhesins or toxins. Infect. Immun. 62:2893-2900. (Erratum, 62:5707.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khelef, N., H. Sakamoto, and N. Guiso. 1992. Both adenylate cyclase and hemolytic activities are required by Bordetella pertussis to initiate infection. Microb. Pathog. 12:227-235. [DOI] [PubMed] [Google Scholar]
- 19.Kimura, A., K. T. Mountzouros, D. A. Relman, S. Falkow, and J. L. Cowell. 1990. Bordetella pertussis filamentous hemagglutinin: evaluation as a protective antigen and colonization factor in a mouse respiratory infection model. Infect. Immun. 58:7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leininger, E., S. Bowen, G. Renauld-Mongenie, J. H. Rouse, F. D. Menozzi, C. Locht, I. Heron, and M. J. Brennan. 1997. Immunodominant domains present on the Bordetella pertussis vaccine component filamentous hemagglutinin. J. Infect. Dis. 175:1423-1431. [DOI] [PubMed] [Google Scholar]
- 21.Locht, C., P. Bertin, F. D. Menozzi, and G. Renauld. 1993. The filamentous haemagglutinin, a multifaceted adhesin produced by virulent Bordetella spp. Mol. Microbiol. 9:653-660. [DOI] [PubMed] [Google Scholar]
- 22.Locht, C., M.-C. Geoffroy, and G. Renauld. 1992. Common accessory genes for the Bordetella pertussis filamentous haemagglutinin and fimbriae share sequence similarities with the papC and papD gene families. EMBO J. 11:3175-3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menozzi, F. D., C. Gantiez, and C. Locht. 1991. Identification and purification of transferrin- and lactoferrin-binding proteins of Bordetella pertussis and Bordetella bronchiseptica. Infect. Immun. 59:3982-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menozzi, F. D., C. Gantiez, and C. Locht. 1991. Interaction of the Bordetella pertussis filamentous haemagglutinin with heparin. FEMS Microbiol. Lett. 62:59-64. [DOI] [PubMed] [Google Scholar]
- 25.Menozzi, F. D., R. Mutombo, G. Renauld, C. Gantiez, J. H. Hannah, E. Leininger, M. J. Brennan, and C. Locht. 1994. Heparin-inhibitable lectin activity of the filamentous hemagglutinin adhesin of Bordetella pertussis. Infect. Immun. 62:769-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munoz, J. J., H. Arai, and R. L. Cole. 1981. Mouse-protecting and histamine-sensitizing activities of pertussigen and fimbrial hemagglutinin from Bordetella pertussis. Infect. Immun. 32:243-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oda, M., J. L. Cowell, D. G. Burstyn, and C. R. Manclark. 1985. Protective activities of the filamentous hemagglutinin and the lymphocytosis-promoting factor of Bordetella pertussis in mice. J. Infect. Dis. 150:823-833. [DOI] [PubMed] [Google Scholar]
- 28.Piatti, G. 1999. Identification of immunodominant epitopes in the filamentous haemagglutinin of Bordetella pertussis. FEMS Immunol. Med. Microbiol. 23:235-241. [DOI] [PubMed] [Google Scholar]
- 29.Plotkin, S. A., and M. Cadoz. 1997. The acellular pertussis vaccine trials: an interpretation. Pediatr. Infect. Dis. J. 16:508-517. [DOI] [PubMed] [Google Scholar]
- 30.Pollock, T. M., J. Y. Mortimer, E. Miller, and G. Smith. 1984. Symptoms after primary immunization with DTP and DT vaccine. Lancet ii:146-149. [DOI] [PubMed] [Google Scholar]
- 31.Prasad, S. M., Y. Yin, E. Rodzinski, E.-I. Tuomanen, and H. R. Masure. 1993. Identification of a carbohydrate recognition domain in filamentous hemagglutinin from Bordetella pertussis. Infect. Immun. 61:2780-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in Gram-negative bacteria. Gene 127:15-21. [DOI] [PubMed] [Google Scholar]
- 33.Relman, D., E. I. Tuomanen, S. Falkow, D. T. Golenbock, K. Saukkonen, and S. D. Wright. 1990. Recognition of a bacterial adhesin by an integrin: macrophage CR3 (αMβ2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell 61:1375-1382. [DOI] [PubMed] [Google Scholar]
- 34.Renauld-Mongenie, G., J. Cornette, N. Mielcarek, F. D. Menozzi, and C. Locht. 1996. Distinct roles of the N-terminal and C-terminal precursor domains in the biogenesis of the Bordetella pertussis filamentous hemagglutinin. J. Bacteriol. 178:1053-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shahin, R., M. Leef, J. Eldridge, M. Hudson, and R. Gilley. 1995. Adjuvanticity and protective immunity elicited by Bordetella pertussis antigens encapsulated in poly(dl-lactide-co-glycolide) microspheres. Infect. Immun. 63:1195-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shahin, R. D., D. F. Amsbaugh, and M. F. Leef. 1992. Mucosal immunization with filamentous hemagglutinin protects against Bordetella pertussis respiratory infection. Infect. Immun. 60:1482-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soberon, X., L. Covarrubias, and E. Bolivar. 1980. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene 9:287-305. [DOI] [PubMed] [Google Scholar]
- 38.Stibitz, S. 1994. Use of conditionally counterselectable suicide vectors for allelic exchange. Methods Enzymol. 235:458-465. [DOI] [PubMed] [Google Scholar]
- 39.Storsaeter, J., H. Hallander, C. P. Farrington, P. Olin, R. Mollby, and E. Miller. 1990. Secondary analysis of the efficacy of two acellular vaccines evaluated in a Swedish phase III trial. Vaccine 8:457-461. [DOI] [PubMed] [Google Scholar]
- 40.Van den Berg, B. M., H. Beekhuizen, R. J. L. Willems, F. R. Mooi, and R. van Furth. 1999. Role of Bordetella pertussis virulence factors in adherence to epithelial cell lines derived from the human respiratory tract. Infect. Immun. 67:1056-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss, A. A., E. L. Hewlett, G. A. Myers, and S. Falkow. 1984. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J. Infect. Dis. 150:219-222. [DOI] [PubMed] [Google Scholar]
- 42.Wilson, D. R., A. Siebers, and B. B. Finlay. 1998. Antigenic analysis of Bordetella pertussis filamentous hemagglutinin with phage display libraries and rabbit anti-filamentous hemagglutinin polyclonal antibodies. Infect. Immun. 66:4884-4894. [DOI] [PMC free article] [PubMed] [Google Scholar]