Abstract
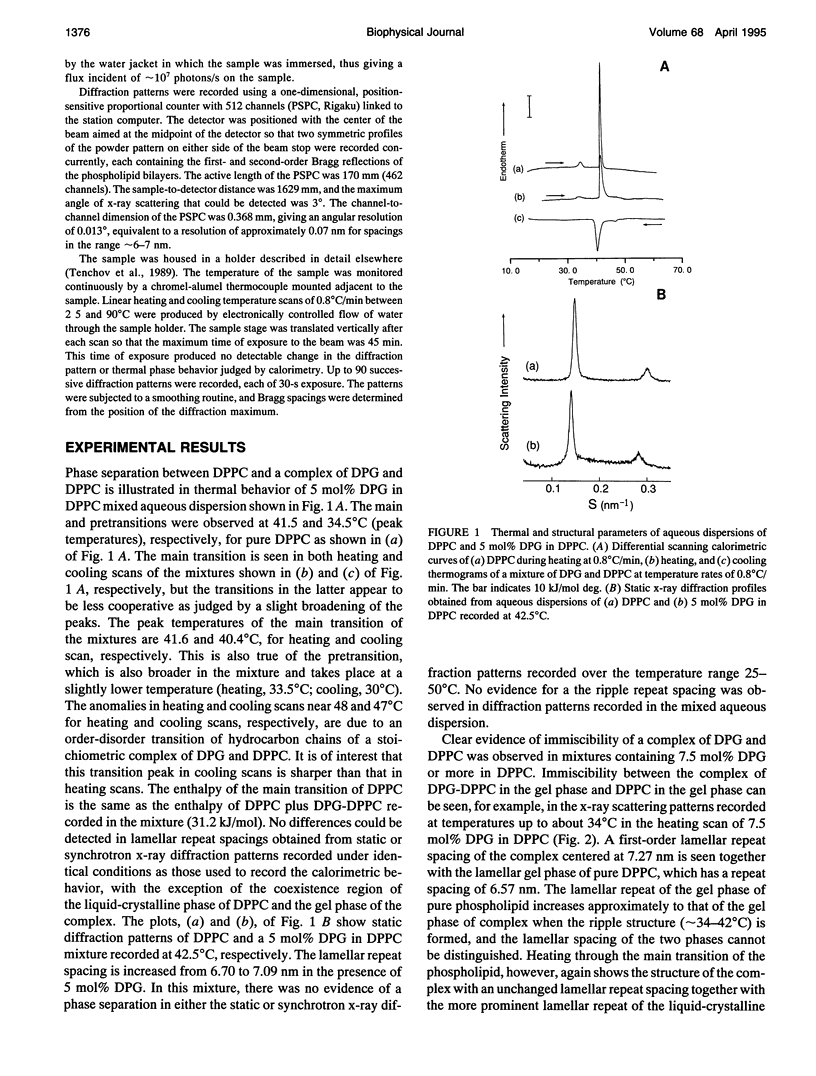
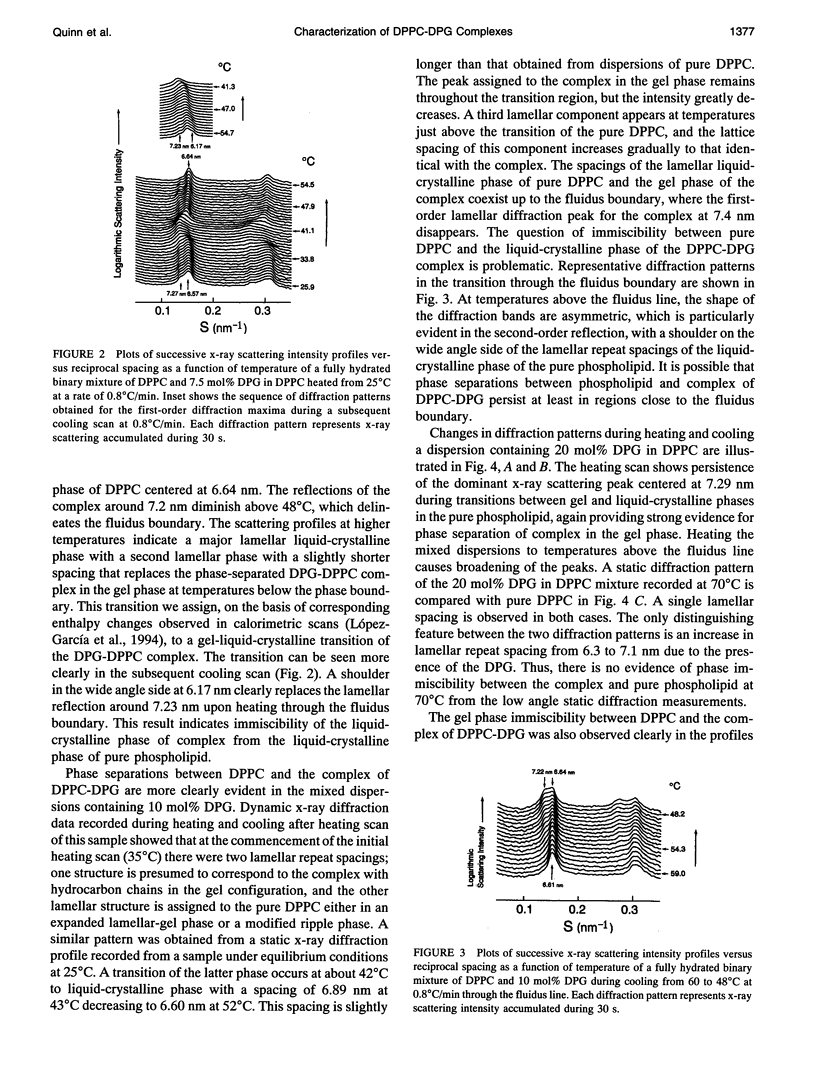
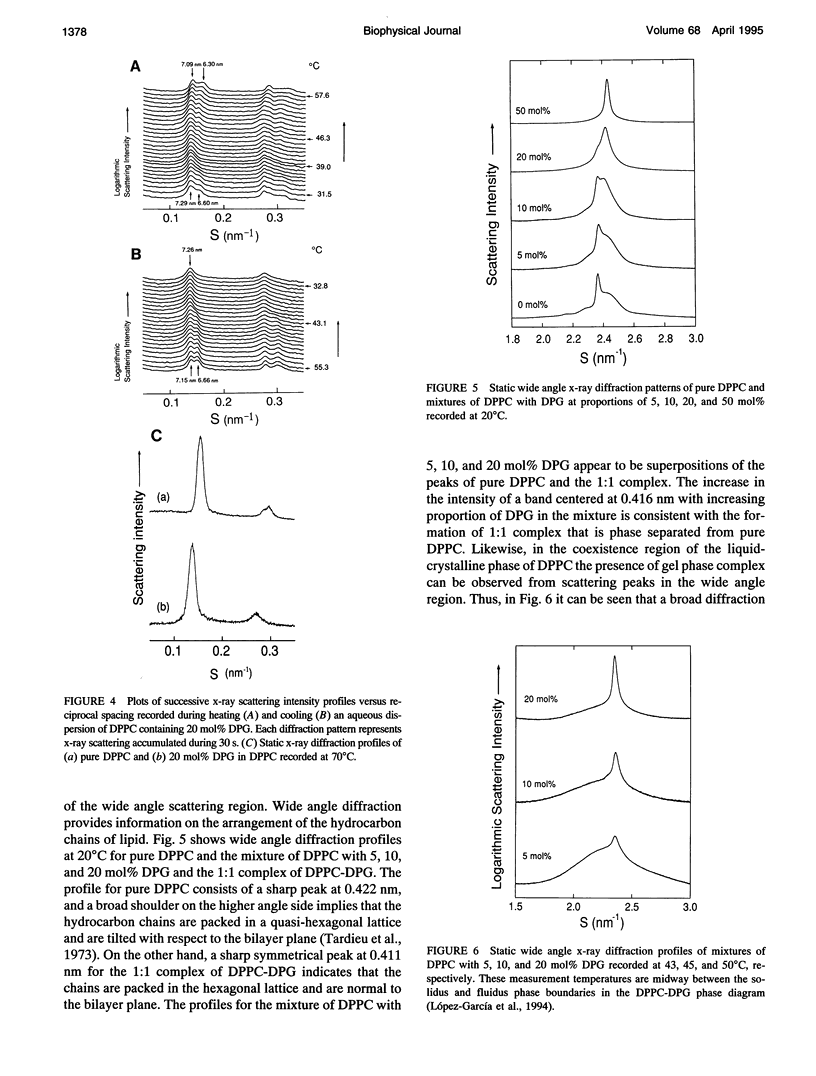
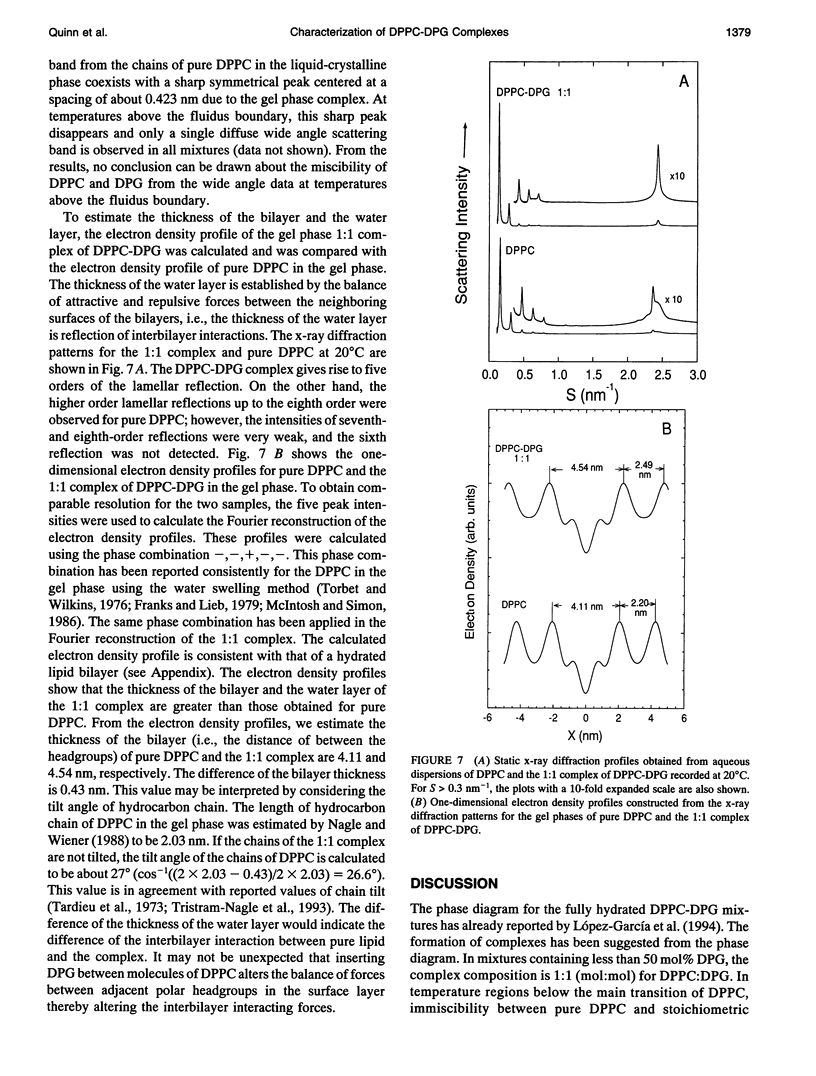
The phase diagram of fully hydrated binary mixtures of dipalmitoylphosphatidylcholine (DPPC) with 1,2-dipalmitoylglycerol (DPG) published recently by López-García et al. identifies regions where stoichiometric complexes of 1:1 and 1:2 DPPC:DPG, respectively, are formed. In this study, the structural parameters of the 1:1 complex in the presence of pure DPPC was characterized by synchrotron low angle and static x-ray diffraction methods. Structural changes upon transitions through phase boundaries were correlated with enthalpy changes observed by differential scanning calorimetry in mixtures of DPPC with 5, 7.5, 10, and 20 mol% DPG dispersed in excess water. Phase separation of a complex in gel phase could be detected by calorimetry in the mixture containing 5 mol% DPG but was not detectable by synchrotron low angle x-ray diffraction. Static x-ray measurements show evidence of phase separation, particularly in the reflections indexing chain packing. In the mixture containing 7.5 mol% DPG, two distinct lamellar repeat spacings could be seen in the temperature range from 25 to 34 degrees C. The lamellar spacing of about 6.6 nm was assigned to pure gel phase DPPC because the change in the spacing corresponds with thermal transition of the pure phospholipid, and a longer repeat spacing of about 7.2 nm was assigned to domains of the 1:1 complex of DPPC-DPG. In the temperature range from 34 to 420C, i.e., in the region of coexistence of the ripple phase of DPPC and the gel phase of the complex, a single, rather broad lamellar reflection appears because of superposition of two reflections of DPPC and the complex; the lamellar spacing of DPPC in the ripple phase is similar to that of the gel phase of complex. In the coexistence region of the liquid-crystalline phase of DPPC and the gel phase of complex (-42-480C), the lamellar reflections of the both phases are present. The fluidus boundary lies between the coexistence region and the fluid region.In the fluid region (-48-550C), the gel state of complex persists up to the fluidus boundary, whereupon the liquid-crystalline state of complex replaces the gel state of the complex. This indicates that the complex is also immiscible with DPPC even above the fluidus boundary at least in the temperature range close to the phase boundary. For mixtures comprising 10 and 20 mol%DPG in DPPC, complex formation is clearly detectable in both the gel region and the coexistence region by x-ray diffraction.Synchrotron x-ray measurements indicate phase separation between pure DPPC and liquid-crystalline complex just above thefluidus boundary. Static, wide angle x-ray measurements also suggest phase separations of the 1:1 complex not only from the gel phase but also the liquid-crystalline phase of pure DPPC. Two distinct diffraction peaks were detected for the mixture of DPPC with 5, 10, and 20 mol% DPG. One is due to the chain spacing of the complex, and the other is due to that of the pure DPPC. In the coexistence region of the liquid-crystalline phase of DPPC and the gel phase of complex, two kinds of diffraction peaks of the hydrocarbon chain of the gel phase complex and the broad scattering profile for the chain melting of DPPC were observed in the wide angle region. Electron density reconstructed from the lamellar reflections indicates that the thicknesses of both the bilayer and the water layer of the gel phase complex are greater than those of the respective thicknesses of gel phase DPPC.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. M. Protein kinase C activation by diacylglycerol second messengers. Cell. 1986 Jun 6;45(5):631–632. doi: 10.1016/0092-8674(86)90774-9. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Tsujita T., Brockman H. L. Enzymatic and physical characterization of diacylglycerol-phosphatidylcholine interactions in bilayers and monolayers. Biochemistry. 1989 Jan 10;28(1):32–40. doi: 10.1021/bi00427a006. [DOI] [PubMed] [Google Scholar]
- Das S., Rand R. P. Diacylglycerol causes major structural transitions in phospholipid bilayer membranes. Biochem Biophys Res Commun. 1984 Oct 30;124(2):491–496. doi: 10.1016/0006-291x(84)91580-8. [DOI] [PubMed] [Google Scholar]
- Das S., Rand R. P. Modification by diacylglycerol of the structure and interaction of various phospholipid bilayer membranes. Biochemistry. 1986 May 20;25(10):2882–2889. doi: 10.1021/bi00358a022. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Hemington N. L., Irvine R. F. Diacylglycerol potentiates phospholipase attack upon phospholipid bilayers: possible connection with cell stimulation. Biochem Biophys Res Commun. 1983 Nov 30;117(1):196–201. doi: 10.1016/0006-291x(83)91560-7. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Irvine R. F., Bray J., Quinn P. J. Long-chain unsaturated diacylglycerols cause a perturbation in the structure of phospholipid bilayers rendering them susceptible to phospholipase attack. Biochem Biophys Res Commun. 1984 Dec 14;125(2):836–842. doi: 10.1016/0006-291x(84)90615-6. [DOI] [PubMed] [Google Scholar]
- Dennis E. A., Rhee S. G., Billah M. M., Hannun Y. A. Role of phospholipase in generating lipid second messengers in signal transduction. FASEB J. 1991 Apr;5(7):2068–2077. doi: 10.1096/fasebj.5.7.1901288. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. The structure of lipid bilayers and the effects of general anaesthetics. An x-ray and neutron diffraction study. J Mol Biol. 1979 Oct 9;133(4):469–500. doi: 10.1016/0022-2836(79)90403-0. [DOI] [PubMed] [Google Scholar]
- Heimburg T., Würz U., Marsh D. Binary phase diagram of hydrated dimyristoylglycerol-dimyristoylphosphatidylcholine mixtures. Biophys J. 1992 Nov;63(5):1369–1378. doi: 10.1016/S0006-3495(92)81714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong-wei S., McConnell H. Phase separations in phospholipd membranes. Biochemistry. 1975 Feb 25;14(4):847–854. doi: 10.1021/bi00675a032. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Nishizuka Y. The role of protein kinase C in transmembrane signalling. Annu Rev Cell Biol. 1986;2:149–178. doi: 10.1146/annurev.cb.02.110186.001053. [DOI] [PubMed] [Google Scholar]
- Koynova R. D., Tenchov B. G., Quinn P. J., Laggner P. Structure and phase behavior of hydrated mixtures of L-dipalmitoylphosphatidylcholine and palmitic acid. Correlations between structural rearrangements, specific volume changes and endothermic events. Chem Phys Lipids. 1988 Oct;48(3-4):205–214. doi: 10.1016/0009-3084(88)90091-6. [DOI] [PubMed] [Google Scholar]
- López-García F., Villalaín J., Gómez-Fernández J. C., Quinn P. J. The phase behavior of mixed aqueous dispersions of dipalmitoyl derivatives of phosphatidylcholine and diacylglycerol. Biophys J. 1994 Jun;66(6):1991–2004. doi: 10.1016/S0006-3495(94)80992-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Wiener M. C. Structure of fully hydrated bilayer dispersions. Biochim Biophys Acta. 1988 Jul 7;942(1):1–10. doi: 10.1016/0005-2736(88)90268-4. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Ortiz A., Villalaín J., Gómez-Fernández J. C. Interaction of diacylglycerols with phosphatidylcholine vesicles as studied by differential scanning calorimetry and fluorescence probe depolarization. Biochemistry. 1988 Dec 13;27(25):9030–9036. doi: 10.1021/bi00425a022. [DOI] [PubMed] [Google Scholar]
- Siegel D. P., Banschbach J., Alford D., Ellens H., Lis L. J., Quinn P. J., Yeagle P. L., Bentz J. Physiological levels of diacylglycerols in phospholipid membranes induce membrane fusion and stabilize inverted phases. Biochemistry. 1989 May 2;28(9):3703–3709. doi: 10.1021/bi00435a012. [DOI] [PubMed] [Google Scholar]
- Smaby J. M., Brockman H. L. Miscibility, chain packing, and hydration of 1-palmitoyl-2-oleoyl phosphatidylcholine and other lipids in surface phases. Biophys J. 1985 Nov;48(5):701–707. doi: 10.1016/S0006-3495(85)83828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Matuoka S., Kato S., Ohki K., Hatta I. Electrostatic interaction of poly(L-lysine) with dipalmitoylphosphatidic acid studied by X-ray diffraction. Biochim Biophys Acta. 1991 Nov 4;1069(2):229–234. doi: 10.1016/0005-2736(91)90129-v. [DOI] [PubMed] [Google Scholar]
- Tardieu A., Luzzati V., Reman F. C. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water phases. J Mol Biol. 1973 Apr 25;75(4):711–733. doi: 10.1016/0022-2836(73)90303-3. [DOI] [PubMed] [Google Scholar]
- Tenchov B. G., Yao H., Hatta I. Time-resolved x-ray diffraction and calorimetric studies at low scan rates: I. Fully hydrated dipalmitoylphosphatidylcholine (DPPC) and DPPC/water/ethanol phases. Biophys J. 1989 Oct;56(4):757–768. doi: 10.1016/S0006-3495(89)82723-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torbet J., Wilkins M. H. X-ray diffraction studies of lecithin bilayers. J Theor Biol. 1976 Oct 21;62(2):447–458. doi: 10.1016/0022-5193(76)90129-6. [DOI] [PubMed] [Google Scholar]
- Tristram-Nagle S., Zhang R., Suter R. M., Worthington C. R., Sun W. J., Nagle J. F. Measurement of chain tilt angle in fully hydrated bilayers of gel phase lecithins. Biophys J. 1993 Apr;64(4):1097–1109. doi: 10.1016/S0006-3495(93)81475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener M. C., Suter R. M., Nagle J. F. Structure of the fully hydrated gel phase of dipalmitoylphosphatidylcholine. Biophys J. 1989 Feb;55(2):315–325. doi: 10.1016/S0006-3495(89)82807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington C. R. The interpretation of low-angle X-ray data from planar and concentric multilayered structures. The use of one-dimensional electron density strip models. Biophys J. 1969 Feb;9(2):222–234. doi: 10.1016/S0006-3495(69)86381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


