Abstract
Automated electron tomography is shown to be a suitable means to visualize the shape of phospholipid vesicles embedded in vitrified ice. With a slow-scan charge-coupled device camera as a recording device, the cumulative electron dose needed to record a data set of 60 projections at a magnification of 20,000X can be kept as low as 15 e-/A2 (or 1500 electrons/nm2). The membrane of the three-dimensionally reconstructed vesicles is clearly visible in two-dimensional sections through the three-dimensionally reconstructed volume. Some edges indicating a polygonal shape of the vesicles, frozen from the gel phase, are also clearly recognized. Because of the presently limited tilt angle range (+/- 60 degrees), the upper and lower "caps" of the vesicles (representing about 35% of the surface of the ellipsoidal particles) remain invisible in the three-dimensional reconstruction.
Full text
PDF



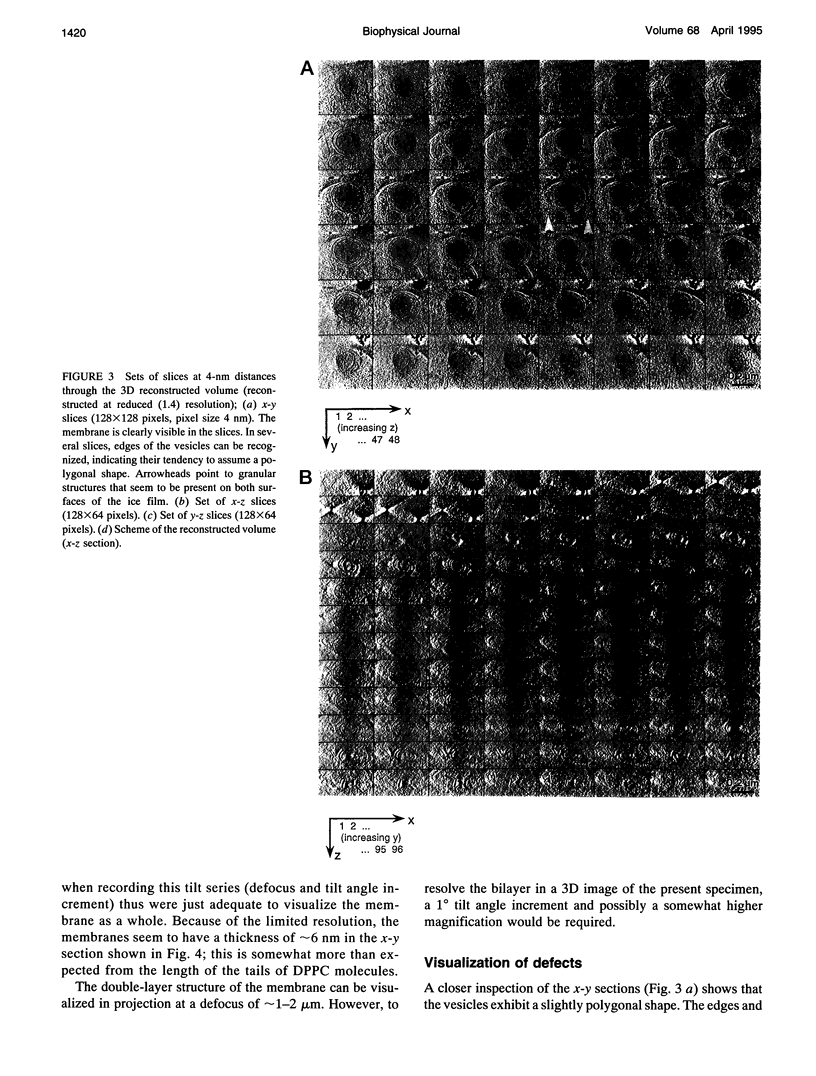
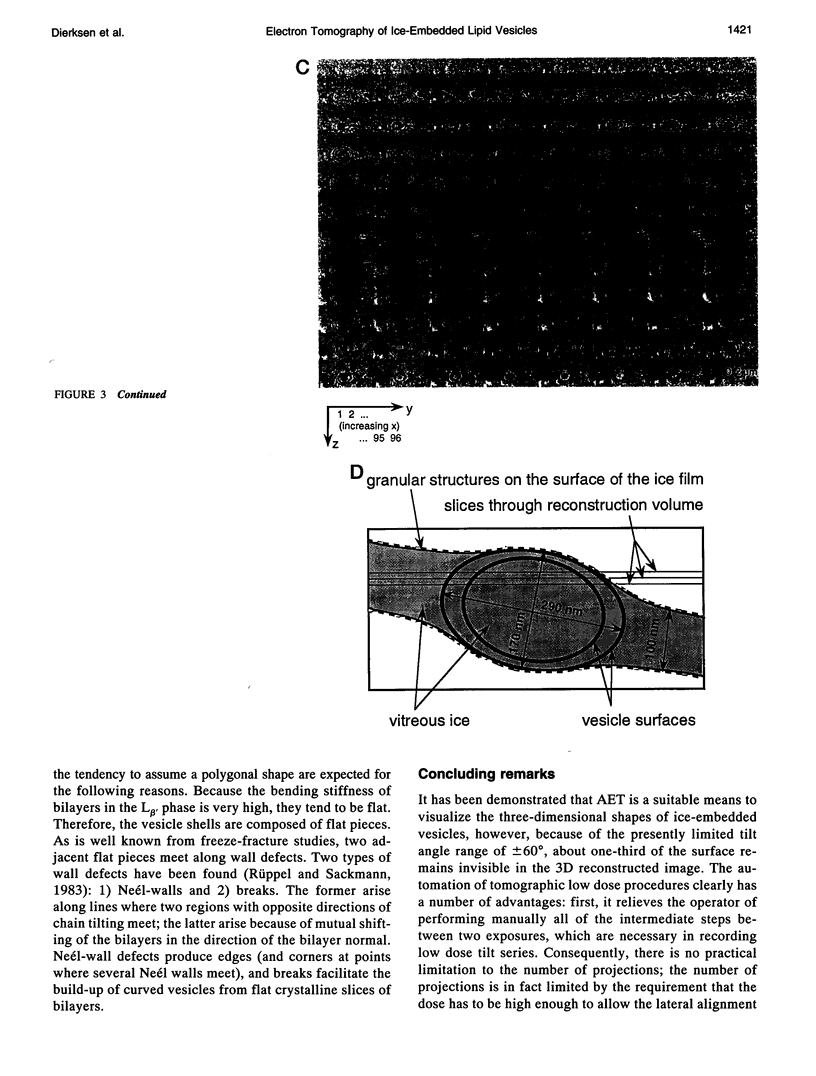

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellare J. R., Davis H. T., Scriven L. E., Talmon Y. Controlled environment vitrification system: an improved sample preparation technique. J Electron Microsc Tech. 1988 Sep;10(1):87–111. doi: 10.1002/jemt.1060100111. [DOI] [PubMed] [Google Scholar]
- Frederik P. M., Stuart M. C., Bomans P. H., Busing W. M. Phospholipid, nature's own slide and cover slip for cryo-electron microscopy. J Microsc. 1989 Jan;153(Pt 1):81–92. doi: 10.1111/j.1365-2818.1989.tb01469.x. [DOI] [PubMed] [Google Scholar]
- Hegerl R., Altbauer A. The "EM" program system. Ultramicroscopy. 1982;9(1-2):109–116. doi: 10.1016/0304-3991(82)90233-9. [DOI] [PubMed] [Google Scholar]
- Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch C. 1973 Nov-Dec;28(11):693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- Käs J., Sackmann E. Shape transitions and shape stability of giant phospholipid vesicles in pure water induced by area-to-volume changes. Biophys J. 1991 Oct;60(4):825–844. doi: 10.1016/S0006-3495(91)82117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipowsky R. The conformation of membranes. Nature. 1991 Feb 7;349(6309):475–481. doi: 10.1038/349475a0. [DOI] [PubMed] [Google Scholar]
- Siegel D. P., Green W. J., Talmon Y. The mechanism of lamellar-to-inverted hexagonal phase transitions: a study using temperature-jump cryo-electron microscopy. Biophys J. 1994 Feb;66(2 Pt 1):402–414. doi: 10.1016/s0006-3495(94)80790-8. [DOI] [PMC free article] [PubMed] [Google Scholar]