Abstract
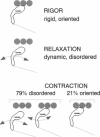
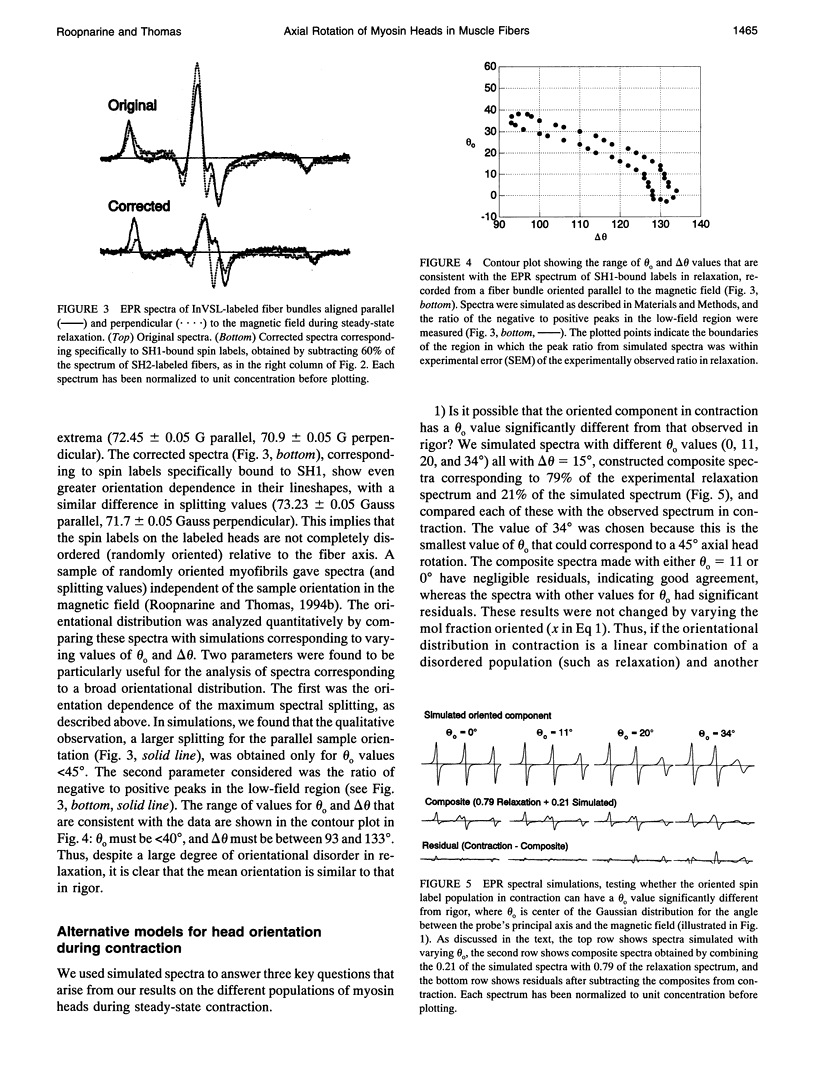
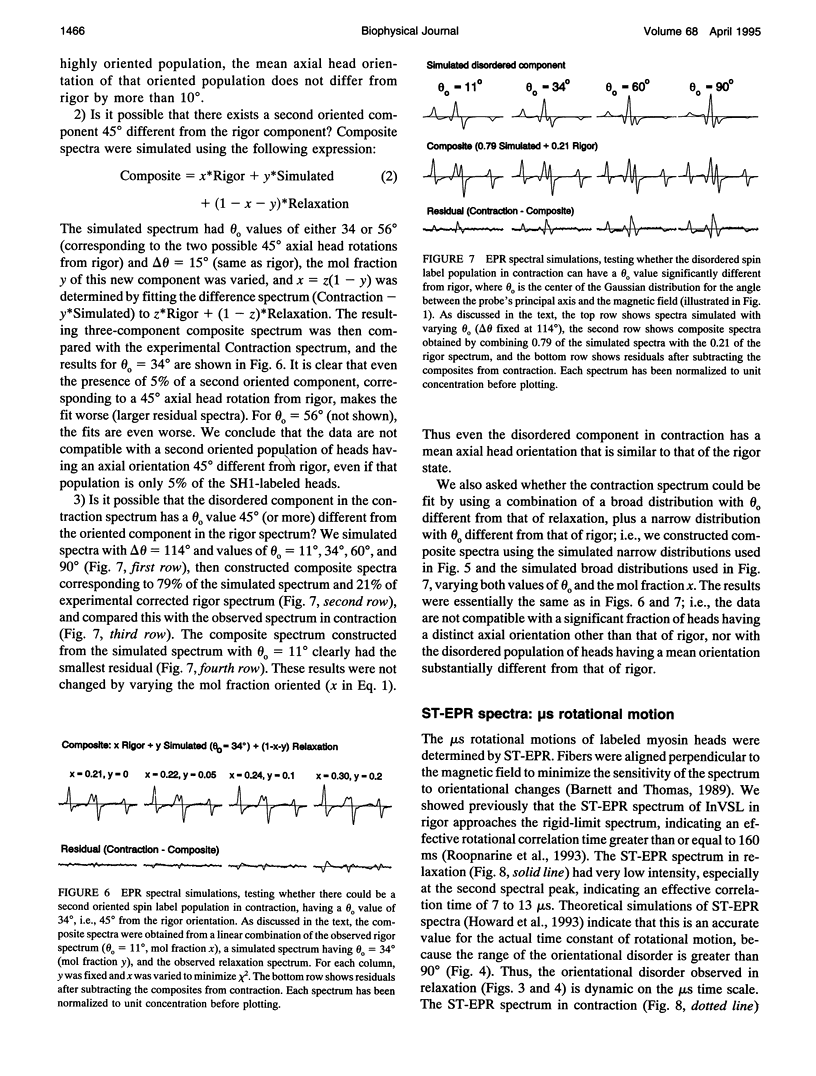
We have used electron paramagnetic resonance (EPR) spectroscopy to study the orientation and rotational motions of spin-labeled myosin heads during steady-state relaxation and contraction of skinned rabbit psoas muscle fibers. Using an indane-dione spin label, we obtained EPR spectra corresponding specifically to probes attached to Cys 707 (SH1) on the catalytic domain of myosin heads. The probe is rigidly immobilized, so that it reports the global rotation of the myosin head, and the probe's principal axis is aligned almost parallel with the fiber axis in rigor, making it directly sensitive to axial rotation of the head. Numerical simulations of EPR spectra showed that the labeled heads are highly oriented in rigor, but in relaxation they have at least 90 degrees (Gaussian full width) of axial disorder, centered at an angle approximately equal to that in rigor. Spectra obtained in isometric contraction are fit quite well by assuming that 79 +/- 2% of the myosin heads are disordered as in relaxation, whereas the remaining 21 +/- 2% have the same orientation as in rigor. Computer-simulated spectra confirm that there is no significant population (> 5%) of heads having a distinct orientation substantially different (> 10 degrees) from that in rigor, and even the large disordered population of heads has a mean orientation that is similar to that in rigor. Because this spin label reports axial head rotations directly, these results suggest strongly that the catalytic domain of myosin does not undergo a transition between two distinct axial orientations during force generation. Saturation transfer EPR shows that the rotational disorder is dynamic on the microsecond time scale in both relaxation and contraction. These results are consistent with models of contraction involving 1) a transition from a dynamically disordered preforce state to an ordered (rigorlike) force-generating state and/or 2) domain movements within the myosin head that do not change the axial orientation of the SH1-containing catalytic domain relative to actin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett V. A., Fajer P., Polnaszek C. F., Thomas D. D. High-Resolution Detection of muscle Crossbridge Orientation by Electron Paramagnetic Resonance. Biophys J. 1986 Jan;49(1):144–147. doi: 10.1016/S0006-3495(86)83628-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett V. A., Thomas D. D. Microsecond rotational motion of spin-labeled myosin heads during isometric muscle contraction. Saturation transfer electron paramagnetic resonance. Biophys J. 1989 Sep;56(3):517–523. doi: 10.1016/S0006-3495(89)82698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett V. A., Thomas D. D. Resolution of conformational states of spin-labeled myosin during steady-state ATP hydrolysis. Biochemistry. 1987 Jan 13;26(1):314–323. doi: 10.1021/bi00375a044. [DOI] [PubMed] [Google Scholar]
- Berger C. L., Svensson E. C., Thomas D. D. Photolysis of a photolabile precursor of ATP (caged ATP) induces microsecond rotational motions of myosin heads bound to actin. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8753–8757. doi: 10.1073/pnas.86.22.8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C. L., Thomas D. D. Rotational dynamics of actin-bound intermediates in the myosin ATPase cycle. Biochemistry. 1991 Nov 19;30(46):11036–11045. doi: 10.1021/bi00110a005. [DOI] [PubMed] [Google Scholar]
- Berger C. L., Thomas D. D. Rotational dynamics of actin-bound intermediates of the myosin adenosine triphosphatase cycle in myofibrils. Biophys J. 1994 Jul;67(1):250–261. doi: 10.1016/S0006-3495(94)80476-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C. L., Thomas D. D. Rotational dynamics of actin-bound myosin heads in active myofibrils. Biochemistry. 1993 Apr 13;32(14):3812–3821. doi: 10.1021/bi00065a038. [DOI] [PubMed] [Google Scholar]
- Cooke R., Crowder M. S., Thomas D. D. Orientation of spin labels attached to cross-bridges in contracting muscle fibres. Nature. 1982 Dec 23;300(5894):776–778. doi: 10.1038/300776a0. [DOI] [PubMed] [Google Scholar]
- Cooke R. The mechanism of muscle contraction. CRC Crit Rev Biochem. 1986;21(1):53–118. doi: 10.3109/10409238609113609. [DOI] [PubMed] [Google Scholar]
- Fajer P. G. Determination of spin-label orientation within the myosin head. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):937–941. doi: 10.1073/pnas.91.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajer P. G., Fajer E. A., Brunsvold N. J., Thomas D. D. Effects of AMPPNP on the orientation and rotational dynamics of spin-labeled muscle cross-bridges. Biophys J. 1988 Apr;53(4):513–524. doi: 10.1016/S0006-3495(88)83131-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajer P. G., Fajer E. A., Matta J. J., Thomas D. D. Effect of ADP on the orientation of spin-labeled myosin heads in muscle fibers: a high-resolution study with deuterated spin labels. Biochemistry. 1990 Jun 19;29(24):5865–5871. doi: 10.1021/bi00476a031. [DOI] [PubMed] [Google Scholar]
- Fajer P. G., Fajer E. A., Schoenberg M., Thomas D. D. Orientational disorder and motion of weakly attached cross-bridges. Biophys J. 1991 Sep;60(3):642–649. doi: 10.1016/S0006-3495(91)82093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajer P. G., Fajer E. A., Thomas D. D. Myosin heads have a broad orientational distribution during isometric muscle contraction: time-resolved EPR studies using caged ATP. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5538–5542. doi: 10.1073/pnas.87.14.5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funatsu T., Kono E., Tsukita S. Time-resolved electron microscopic analysis of the behavior of myosin heads on actin filaments after photolysis of caged ATP. J Cell Biol. 1993 Jun;121(5):1053–1064. doi: 10.1083/jcb.121.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highsmith S., Eden D. Ligand-induced myosin subfragment 1 global conformational change. Biochemistry. 1990 May 1;29(17):4087–4093. doi: 10.1021/bi00469a010. [DOI] [PubMed] [Google Scholar]
- Highsmith S., Eden D. Myosin-ATP chemomechanics. Biochemistry. 1993 Mar 16;32(10):2455–2458. doi: 10.1021/bi00061a001. [DOI] [PubMed] [Google Scholar]
- Hirose K., Lenart T. D., Murray J. M., Franzini-Armstrong C., Goldman Y. E. Flash and smash: rapid freezing of muscle fibers activated by photolysis of caged ATP. Biophys J. 1993 Jul;65(1):397–408. doi: 10.1016/S0006-3495(93)81061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard E. C., Lindahl K. M., Polnaszek C. F., Thomas D. D. Simulation of saturation transfer electron paramagnetic resonance spectra for rotational motion with restricted angular amplitude. Biophys J. 1993 Mar;64(3):581–593. doi: 10.1016/S0006-3495(93)81417-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Kress M. Crossbridge behaviour during muscle contraction. J Muscle Res Cell Motil. 1985 Apr;6(2):153–161. doi: 10.1007/BF00713057. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Ostap E. M., White H. D., Thomas D. D. Transient detection of spin-labeled myosin subfragment 1 conformational states during ATP hydrolysis. Biochemistry. 1993 Jul 6;32(26):6712–6720. doi: 10.1021/bi00077a026. [DOI] [PubMed] [Google Scholar]
- Pate E., Cooke R. Energetics of the actomyosin bond in the filament array of muscle fibers. Biophys J. 1988 Apr;53(4):561–573. doi: 10.1016/S0006-3495(88)83136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Bhandari D., Maupin P., Wachsstock D., Weeds A. G., Zot H. G. Direct visualization by electron microscopy of the weakly bound intermediates in the actomyosin adenosine triphosphatase cycle. Biophys J. 1993 Feb;64(2):454–471. doi: 10.1016/S0006-3495(93)81387-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Roopnarine O., Hideg K., Thomas D. D. Saturation transfer electron parametric resonance of an indane-dione spin-label. Calibration with hemoglobin and application to myosin rotational dynamics. Biophys J. 1993 Jun;64(6):1896–1907. doi: 10.1016/S0006-3495(93)81561-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopnarine O., Thomas D. D. A spin label that binds to myosin heads in muscle fibers with its principal axis parallel to the fiber axis. Biophys J. 1994 Oct;67(4):1634–1645. doi: 10.1016/S0006-3495(94)80636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel J. C., Gergely J. Electron spin resonance of myosin spin labeled at the S1 thiol groups during hydrolysis of adenosine triphosphate. Arch Biochem Biophys. 1973 Oct;158(2):853–863. doi: 10.1016/0003-9861(73)90581-x. [DOI] [PubMed] [Google Scholar]
- Stein R. A., Ludescher R. D., Dahlberg P. S., Fajer P. G., Bennett R. L., Thomas D. D. Time-resolved rotational dynamics of phosphorescent-labeled myosin heads in contracting muscle fibers. Biochemistry. 1990 Oct 30;29(43):10023–10031. doi: 10.1021/bi00495a003. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Cooke R. Orientation of spin-labeled myosin heads in glycerinated muscle fibers. Biophys J. 1980 Dec;32(3):891–906. doi: 10.1016/S0006-3495(80)85024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D. Spectroscopic probes of muscle cross-bridge rotation. Annu Rev Physiol. 1987;49:691–709. doi: 10.1146/annurev.ph.49.030187.003355. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K., Tokunaga M., Kohno I., Sugimoto Y., Hamanaka T., Takezawa Y., Wakabayashi T., Amemiya Y. Small-angle synchrotron x-ray scattering reveals distinct shape changes of the myosin head during hydrolysis of ATP. Science. 1992 Oct 16;258(5081):443–447. doi: 10.1126/science.1411537. [DOI] [PubMed] [Google Scholar]
- Walker M., White H., Belknap B., Trinick J. Electron cryomicroscopy of acto-myosin-S1 during steady-state ATP hydrolysis. Biophys J. 1994 May;66(5):1563–1572. doi: 10.1016/S0006-3495(94)80948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells C., Bagshaw C. R. The characterization of vanadate-trapped nucleotide complexes with spin-labelled myosins. J Muscle Res Cell Motil. 1984 Feb;5(1):97–112. doi: 10.1007/BF00713154. [DOI] [PubMed] [Google Scholar]
- Wilson M. G., Mendelson R. A. A comparison of order and orientation of crossbridges in rigor and relaxed muscle fibres using fluorescence polarization. J Muscle Res Cell Motil. 1983 Dec;4(6):671–693. doi: 10.1007/BF00712160. [DOI] [PubMed] [Google Scholar]