Abstract
Mannheimia (Pasteurella) haemolytica A1 produces several virulence factors that play an important role in the pathogenesis of bovine pneumonic pasteurellosis. Foremost among these is a leukotoxin (LKT) that specifically kills ruminant leukocytes. Recent evidence suggests that M. haemolytica LKT binding to bovine leukocytes is mediated by the β2-integrin CD11a/CD18 (lymphocyte function-associated antigen 1 [LFA-1]), which subsequently induces activation and cytolysis of these cells. Inflammatory cytokines, which are released during viral and bacterial infection, are reported to increase LFA-1 expression and conformational activation. We investigated the effects of the inflammatory cytokines interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ) on the interaction of M. haemolytica LKT with bovine peripheral blood neutrophils (PMNs). In this study we demonstrated, by flow cytometry, that bovine PMNs increased their binding to an anti-bovine LFA-1 monoclonal antibody (BAT75A) following in vitro incubation with IL-1β, TNF-α, or IFN-γ. Incubation with cytokines also increased CD18 expression, as assessed by real-time PCR and by Western blotting. Increased LFA-1 expression by PMNs exposed to cytokines was associated with increased LKT binding and cytotoxicity. The latter represented, at least in part, enhanced PMN apoptosis, as assessed by propidium iodine staining and caspase-3 activation. The results of this study suggest that inflammatory cytokines may play an important role in enhancing the biological response of bovine PMNs to M. haemolytica LKT.
Mannheimia (Pasteurella) haemolytica A1 is the primary bacterial agent of bovine pneumonic pasteurellosis (shipping fever), which is characterized by acute lobar fibronecrotizing pneumonia with extensive peripheral blood neutrophil (PMN) infiltration in small airways and alveoli (4, 39, 47). Several virulence factors of M. haemolytica play an important role in the pathogenesis of pasteurellosis (7, 13). Foremost among these is a leukotoxin (LKT), whose effects are specific for ruminant leukocytes and platelets (2, 6, 9, 44). The M. haemolytica LKT is member of the repeats-in-toxin (RTX) family of gram-negative bacterial pore-forming exotoxins (46). Members of the RTX family have similar mechanisms of toxin production, secretion, and target cell intoxication (8, 45). Previously, it has been reported that other members of the RTX family bind to β2-integrins on target cells (23). More recently, it has been demonstrated that M. haemolytica LKT binds to lymphocyte function-associated antigen 1 (LFA-1), a β2-integrin (CD 11a/CD18) on bovine leukocytes (1, 17, 25, 27). LKT binding to bovine leukocytes induces formation of pore-like structures in the plasma membrane, resulting in both activation of leukocytes and death by necrosis and apoptosis (14, 18, 24, 29, 34, 40, 43, 45, 53).
For reasons that are not well understood, active viral infections can greatly enhance the susceptibility of cattle to M. haemolytica pneumonia (11, 28, 42, 48, 49). One mechanism that might be involved is the release of inflammatory cytokines during viral infection (33, 34). Inflammatory cytokines secreted by respiratory tract cells, such as interleukin 1 (IL-1β), tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ), can stimulate leukocyte migration and functional activation of β2-integrins on lung leukocytes (10, 35, 38). Once M. haemolytica infection is established in the lung, the continued release of these inflammatory cytokines could be sustained by M. haemolytica virulence factors (i.e., LKT and lipopolysaccharide [LPS]) (15, 21, 22, 30, 50, 51, 52).
PMNs are thought to contribute to the lung pathology observed in pneumonic pasteurellosis (4). PMN depletion reduces the severity of lung damage in experimentally infected cattle (4, 39). We hypothesized that inflammatory cytokines released during viral infection might increase surface expression or conformational activation of LFA-1 on bovine PMNs, thus amplifying their interaction with M. haemolytica LKT. In this study, we demonstrated increased expression of LFA-1 on bovine PMNs, as detected by flow cytometry, following incubation of PMNs with IL-1β, TNF-α, or IFN-γ. This in turn was reflected in increased LKT binding to, and cytotoxicity for, bovine PMNs. These observations suggest that the ability of inflammatory cytokines to increase surface expression or conformational activation of LFA-1 on bovine PMNs increases their interaction with M. haemolytica LKT. The outcome of the response might increase the severity of bovine pasteurellosis.
MATERIALS AND METHODS
PMN preparation.
Peripheral blood was collected from healthy Holstein donor cows by using Vacutainer tubes (Becton-Dickinson, Rutherford, N.J.) containing 0.38% (vol/vol) (final concentration) sodium citrate as an anticoagulant. The blood was centrifuged (250 × g for 20 min), and the platelet-rich plasma was removed. PMNs were harvested from the remaining blood by rapid hypotonic lysis and centrifugation through a Percoll gradient (Pharmacia, Uppsala, Sweden), as described previously (5). The cell suspensions were greater than 95% PMNs, as determined by microscopic evaluation of Diff-Quick-stained cytocentrifuge smears, and greater than 95% viable, as estimated by trypan blue dye exclusion.
Inflammatory cytokine treatment.
Recombinant bovine IL-1β (generously provided by D. Schuster, American Cyanamid Company, Princeton, N.J.), recombinant human TNF-α (Promega, Madison, Wis.), and recombinant bovine IFN-γ (Genetech, San Francisco, Calif.) were used in this study. Bovine PMNs (1 × 106 cells) were incubated with 50-ng portions of the cytokines at 37°C for 15 or 60 min. After this incubation, the cells were washed with Hanks balanced salt solution (HBSS) and incubated with LKT or monoclonal antibodies (MAbs), as described below.
LKT production and partial purification.
Strain A1 of M. haemolytica, isolated from a pneumonic bovine lung (generously provided by R. E. Briggs, National Animal Disease Center, USDA Agricultural Research Service, Ames, Iowa) was used in this study. All LKT preparations were produced and partially purified as described previously (5). Briefly, M. haemolytica A1 was inoculated onto blood agar (Remel, Lenexa, Kans.) and incubated overnight at 37°C. The bacteria were washed from the agar surface with 10 ml of brain heart infusion broth containing 0.5% yeast extract (Difco, Detroit, Mich.) and incubated at 37°C for 1 h while rotating (8 rpm) in 15-ml polypropylene tubes. A 10-ml aliquot of the suspension was used to inoculate 200 ml of brain heart infusion broth with 0.5% yeast extract in a 500-ml flask, which was incubated for 2 h at 37°C with shaking (120 rpm). The bacteria were collected by centrifugation (1,600 × g for 15 min), resuspended in 200 ml of RPMI 1640 supplemented with l-glutamine (4.0 mM), and incubated on a shaker apparatus (at 120 rpm) for 4 h at 37°C. The bacteria were pelleted by centrifugation (1,600 × g for 20 min), and the crude LKT-containing supernatant was collected and passed through a 0.45-μm-pore-size bottletop filter (Nalgene, Rochester, N.Y.) to remove any residual bacteria. Aliquots of crude LKT were concentrated with an Amicon ultrafiltration unit equipped with a 62-mm-diameter XM-50 membrane. The volume was then reduced to 10 to 20 ml over a 1- to 2-h period by applying a transmembrane pressure of 60 lb/in2 with nitrogen gas. The partially purified LKT preparation that remained was then collected and stored as 5-ml aliquots at −70°C. One unit of LKT activity was defined as the dilution causing 50% killing of bovine peripheral blood leukocytes when preparations were incubated at 37°C for 1 h, as determined by trypan blue exclusion. Partially purified LKT was stored at −70°C until it was used in an experiment.
Biotinylation of LKT.
Biotinylated LKT was prepared as described previously (5). Briefly, a mixture of NHS-LC-biotin (Pierce Chemical, Rockford, Ill.) and partially purified LKT (molar ratio, 80:1) was incubated in an ice bath for 20 min. The mixture was then concentrated to 1 ml in a prechilled Amicon Centricon tube (50-kDa cutoff). The reaction was stopped by addition of crystalline bovine serum albumin (30 mg), and the mixture was incubated at 4°C for 30 min. Unbound biotin was eliminated by buffer exchange with a Sephadex G-25 column (1 by 25 cm) by using phosphate-buffered saline (pH 7.2) as the elution buffer. The LKT eluted in the void volume in 7 to 8 min, as monitored by absorbance at 280 nm. LKT activity was evaluated by incubating biotinylated LKT with bovine peripheral leukocytes for 30 min at 37°C, and then the cytotoxicity was assessed by using 2,3-bis(2-methoxy-4-nitro-5-sulfenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) (Sigma, St. Louis, Mo.) as previously described (25).
Flow cytometry detection of LFA-1.
Bovine PMNs (1 × 106 cells) were incubated with an anti-LFA-1 MAb (BAT 75A) at 4°C for 45 min. The cells were then washed and incubated at 4°C for 30 min with a fluorescein isothiocyanate (FITC)-labeled anti-mouse immunoglobulin G (IgG) second antibody (50 μg/ml; Sigma) for indirect staining. The cells were washed with HBSS, resuspended in 300 μl of HBSS, and fixed with paraformaldehyde (4%, vol/vol). Cells were analyzed by flow cytometry by using a Coulter Profile II flow cytometer (10,000 cells were scored for green fluorescence on a log scale).
Real-time PCR for quantitation of CD18 cDNA.
Total RNA was isolated by the guanidine isothiocyanate method by using Trizol (Life Technologies, Gibco, Grand Island, N.Y.), and RNA was transcribed into cDNA by using the SuperScript first-strand synthesis system for real-time reverse transcription (RT)-PCR (Invitrogen, Carlsbad, Calif.). mRNA sequences for bovine glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and CD18 were obtained from GenBank (accession numbers U85042 and M81233, respectively). Primers were designed by using the Primer Express software (Applied Biosystems, Wamington, United Kingdom) and were synthesized by the University of Wisconsin Biotechnology Center. The GAPDH primer sequences were ATTCCACCCACGGCAAGTT (forward) and CGCTCCTGGAAGATGGTGAT (reverse). The CD18 primer sequences were AGCGACCTCAGGGAGTACCAT (forward) and GTCGTGGTGGCACTCTTGAAA (reverse). Real-time PCR for bovine CD18 and GAPDH were performed in triplicate wells. The PCR mixtures (25 μl) contained 0.5 μl of each primer, 9 μl of water, 12.5 μl of a commercial SYBR Green PCR master mixture (Applied Biosystems), and 2.5 μl of cDNA. The samples were placed in 96-well plates (Bio-Rad, Hercules, Calif.) that were sealed with optical sealing tape (Bio-Rad). PCR amplifications were performed by using the iCycler iQ Multi-Color real-time PCR detection system (Bio-Rad). The thermal cycling conditions were 3 min at 95°C and then 45 cycles of 15 s at 95°C, followed 45 s at 60°C. Two methods are used to quantify gene transcription by real-time PCR: (i) absolute quantitation with a standard curve and (ii) the comparative cycle threshold (Ct) method. We chose the latter due to its ease and speed of setup and analysis. For relative quantitation by the comparative Ct method, values are expressed relative to the value for a reference sample, called the calibrator (unstimulated PMNs). First, the Ct for the target amplicon and the Ct for the internal control (GAPDH) were determined for each sample. Differences in the Ct for the target (CD18) and the Ct for the internal control (ΔCt) were calculated to normalize for the differences in the amounts of total nucleic acid added to the reaction mixtures and the efficiency of the RT step. The ΔCt for each experimental sample was subtracted from the ΔCt for the calibrator. This difference was called the ΔΔCt value. Finally, the arithmetic calibrator (2−ΔΔ Ct) was used to calculate the amount of target normalized to the amount of an internal control and relative to the amount of the calibrator. Thus, all the values for experimental samples were expressed as fold differences between the sample mRNA and the calibrator mRNA.
Western immunoblotting.
Bovine PMNs (1 × 106 cells) were incubated with 50-ng portions of the cytokines at 37°C for 60 min. After this, total cell lysates were prepared by using the MPER cell lysis buffer (Pierce) and the manufacturer's protocol. Total protein content was estimated by bicinchoninic acid analysis (Pierce). Equal samples were loaded onto Tris-HCl-4 to 20% polyacrylamide gradient gels (Bio-Rad), electrophoresed, and transferred to nitrocellulose membranes. The membranes were blocked, washed, and probed with anti-LFA-1 MAb BAT75A (VMRD, Pullman, Wash.) or HI111 (Pharmingen, San Diego, Calif.) at 37°C for 1 h. The blots were washed and probed with a horseradish peroxidase-labeled anti-mouse IgG second antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.) at 37°C for 1 h. Immunoreactive proteins were visualized by using a SuperSignal West Pico chemiluminescence kit and X-ray film (Pierce). Relative band intensities were determined by using ImageQuaNT software (Molecular Dynamics, Sunnyvale, Calif.).
Detection of LKT binding.
Flow cytometric analysis of LKT binding to intact PMNs was performed as described previously (5). Briefly, bovine PMNs (1 × 106 cells) were incubated with biotinylated LKT (1 U) for 10 min at 4°C. The cells were washed and resuspended in HBSS. Extra-avidin-FITC (Sigma) was added, and the cells were incubated for 30 min at 4°C. The cells were washed with HBSS, resuspended in 300 μl of HBSS, and fixed with paraformaldehyde (4%, vol/vol). The cells were analyzed by flow cytometry by using a Coulter Profile II flow cytometer (10,000 cells were scored for green fluorescence on a log scale).
Cytotoxicity assay.
XTT (Sigma) was used to evaluate LKT cytotoxicity for bovine PMNs as described previously (24). Briefly, control and cytokine-exposed bovine PMNs (1 × 106 cells) were incubated with partially purified LKT (1 U) for 15 or 60 min at 37°C on a rotating platform. The cells were plated into triplicate wells in a 96-well microplate and incubated at 37°C for 1 h. XTT (1 mg/ml) was added (50 μl/well), and the plate was incubated at 37°C until optimal color development occurred. Absorbance was determined with a microplate reader (EL-312; Bio-Tek, Winooski, Vt.). The percentage of cytotoxicity was calculated as follows: [1 − (optical density of toxin-incubated cells/optical density of toxin-free cells)] × 100.
Apoptosis assays.
Apoptosis of bovine PMNs was assessed as described previously (32). Briefly, control and cytokine-stimulated bovine PMNs (1 × 106 cells) were incubated with partially purified LKT (0.1 U) for 6 h at 37°C on a rotating platform. Then the cells were centrifuged for 5 min at 1,200 rpm and washed once in cold 1× phosphate-buffered saline containing 2% bovine serum albumin. For propidium iodide (PI) staining, the cells were fixed in 1 ml of ice-cold ethanol (80%, vol/vol) for 30 min at −20°C, centrifuged for 5 min at 1,200 rpm, and resuspended in phosphate-citric acid buffer (0.19 M Na2HPO4, 4 mM citric acid; pH 7.8) for 5 min at room temperature. The cell suspensions were then centrifuged and suspended in 0.5 ml of PI staining solution (33 μg of PI per ml, 1 mg of RNase A per ml, 0.2% Triton X-100) and analyzed with a Becton Dickinson FACScan flow cytometer. A caspase-3 assay was performed by using the CaspACE assay system (Promega). Briefly, cells were resuspended in 50 μl of cell lysis buffer. A 5-μl aliquot was removed, and the total protein concentration was estimated by the bicinchoninic acid protein assay (Pierce). Appropriate dilutions of lysates (125 μg of total protein in 100 μl [total volume]) were then placed in individual wells of a flat-bottom 96-well plate, and caspase-3 substrate was added to a final concentration of 200 μM. The plates were covered, incubated at 37°C for 3 h, and then read with an EL-312 microplate reader (Bio-Tek) at A405.
Statistical analysis.
The flow cytometry data were analyzed with the WInMDI (version 2.8) and ModFit LT (version 2.0) software programs. Other data were analyzed for statistical significance by using a one-way analysis of variance, followed by the Student-Newman-Keuls multiple-comparison test, using the Instat software program (GraphPad, San Diego, Calif.). Linear regression analysis was performed by using the same software package. Statistical significance for the analysis of variance and multiple-comparison tests was defined as a P value of <0.05.
RESULTS
Inflammatory cytokines enhance the binding of anti-LFA-1 MAb to bovine PMNs.
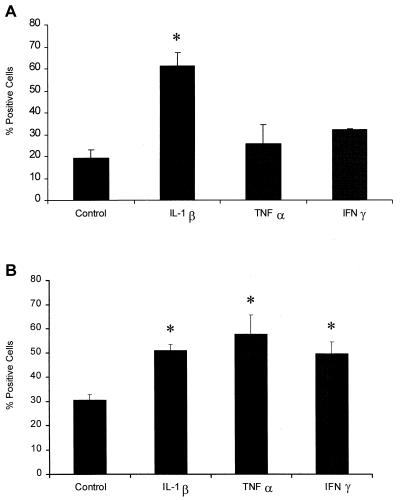
We first examined the expression of LFA-1 on cytokine-exposed bovine PMNs as assessed by flow cytometry, using MAb BAT75A, which recognizes bovine LFA-1 (CD11a/CD18). We observed a significant increase (P < 0.05) in anti-LFA-1 MAb binding to bovine PMNs that had been incubated with IL-1β for 15 min, at which time there was no difference between PMNs incubated with TNF-α or IFN-γ and unstimulated cells (Fig. 1A). However, after incubation for 1 h with IL-1β, TNF-α, or IFN-γ, we observed a significant increase (P < 0.05) in anti-LFA-1 MAb binding to bovine PMNs (Fig. 1B). Incubation with larger amounts of cytokines (up to 500 ng/ml) for up to 1 h or preincubation with 50 ng of cytokine per ml for longer periods of time (up to 24 h) did not further increase anti-LFA-1 MAb binding to bovine PMNs (data not shown).
FIG. 1.
Incubation with inflammatory cytokines increases the staining of bovine PMNs with the anti-LFA-1 MAb BAT75A. Freshly isolated bovine PMNs (1 × 106 cells/ml) were incubated with recombinant bovine IL-1β (50 ng), recombinant human TNF-α (50 ng), recombinant bovine IFN-γ (50 ng), or medium (control) for 15 min (A) or 60 min (B) at 37°C. The cells were then washed and incubated (40 min at 4°C) with anti-LFA-1 MAb BAT75A (final concentration, 50 μg/ml). The cells were washed, incubated with an FITC-labeled second antibody, and analyzed by flow cytometry (10,000 cells were scored for green fluorescence). The data are the means ± standard errors of the means for four independent experiments. An asterisk indicates that the data for stimulated cells was statistically significantly different from the data for control cells (P < 0. 05).
Analysis of CD18 mRNA by real-time RT-PCR.
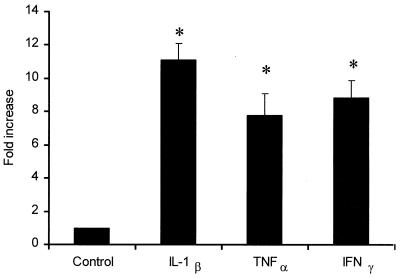
Expression of CD18 mRNA in bovine PMNs was studied by real-time PCR. Quantification was performed by using the comparative Ct method, and values were expressed as fold differences relative to the value for a calibrator cDNA. We observed 11-, 7-, and 8-fold increases in the expression levels of CD18 mRNA when the PMNs were exposed for 1 h with IL-1β, TNF-α, and IFN-γ, respectively, compared with the expression levels for control PMNs (Fig. 2) (P < 0.01).
FIG. 2.
Incubation with inflammatory cytokines increases the expression of CD18 mRNA in bovine PMNs. Freshly isolated bovine PMNs (1 × 106 cells/ml) were incubated with recombinant bovine IL-1β (50 ng), recombinant human TNF-α (50 ng), recombinant bovine IFN-γ (50 ng), or medium (control) for 60 min at 37°C. RNA was extracted and transcribed to cDNA, and then CD18 cDNA expression was assessed by real-time PCR. The results were expressed as fold increases relative to the data obtained with unstimulated (control) PMNs. The data are the means ± standard errors of the means for three independent experiments. An asterisk indicates that the data for cytokine-stimulated cells was statistically significantly different from the data for unstimulated cells (P < 0.01).
Analysis of Western immunoblots.
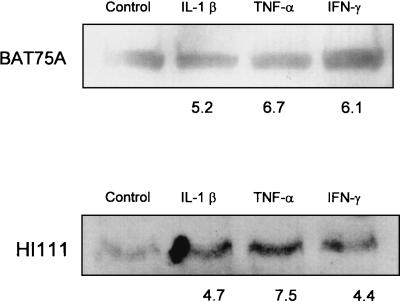
We also examined the expression of LFA-1 on cytokine-exposed bovine PMNs by Western immunoblotting by using MAb BAT75A, which recognizes bovine LFA-1 (CD11a/CD18), and MAb HI111, which recognizes human LFA-1 and cross-reacts with bovine LFA-1 (1). We observed 5.2-, 6.7-, and 6.1-fold increases in band intensity using BAT75A and 4.7-, 7.5-, and 4.4-fold increases in band intensity using HI111 for PMNs incubated for 1 h with IL-1β, TNF-α, and IFN-γ, respectively (Fig. 3).
FIG. 3.
Western blot analysis of LFA-1 expression by bovine PMNs incubated with inflammatory cytokines. Freshly isolated bovine PMNs (1 × 106 cells/ml) were incubated with recombinant bovine IL-1β (50 ng), recombinant human TNF-α (50 ng), recombinant bovine IFN-γ (50 ng), or medium (control) for 60 min at 37°C. Total cell lysates were prepared, and equal amounts of total protein were loaded onto Tris-HCl-4 to 20% polyacrylamide gradient gels, electrophoresed, and transferred to nitrocellulose membranes. The membranes were blocked, washed, and probed with anti-LFA-1 MAb BAT75A or HI111 at 37°C for 1 h. The blots were washed and probed with horseradish peroxidase-conjugated anti-mouse IgG at 37°C for 1 h. Immunoreactive proteins were visualized with a SuperSignal West Pico chemiluminescence kit, and relative band intensities were determined by using ImageQuaNT software. The numbers below the bands are the mean fold increases in LFA-1 expression by bovine PMNs incubated with the cytokines compared with the expression by unstimulated PMNs (three separate experiments).
Incubation of bovine PMNs with inflammatory cytokines enhances binding of partially purified LKT.
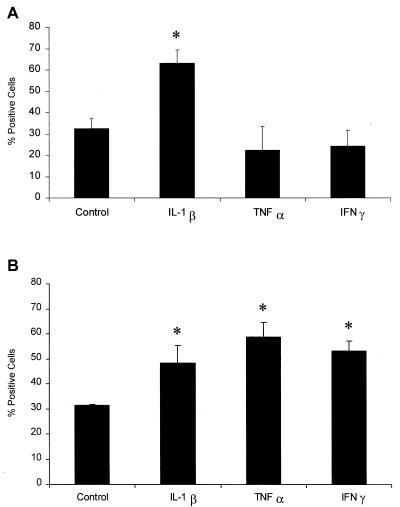
We next examined the binding of biotinylated LKT to cytokine-exposed PMNs. We observed a significant increase in LKT binding to PMNs incubated with IL-1β for 15 min, whereas the other cytokines had no effect after 15 min of incubation (Fig. 4A). This corresponded with the data in Fig. 1, since only IL-1β increased binding of the anti-LFA-1 MAb within 15 min. When PMNs were preincubated with IL-1β, TNF-α, or IFN-γ for 1 h, we observed increased LKT binding compared with the binding with unstimulated cells (Fig. 4B).
FIG. 4.
Incubation of bovine PMNs with inflammatory cytokines enhances LKT binding. Freshly isolated bovine PMNs (1 × 106 cells/ml) were incubated with recombinant bovine IL-1β (50 ng), recombinant human TNF-α (50 ng), recombinant bovine IFN-γ (50 ng), or medium (control) for 15 min (A) or 60 min (B) at 37°C. The cells were then incubated for 10 min in an ice bath with biotinylated LKT (1 U) and washed. Extra-avidin-FITC was added and incubated for 20 min on ice. The stained cells were washed, fixed with paraformaldehyde, and analyzed by flow cytometry (10,000 cells were scored for green fluorescence). The data are the means ± standard errors of the means for four independent experiments. An asterisk indicates that the data for stimulated cells was statistically significantly different from the data for control cells (P < 0.05).
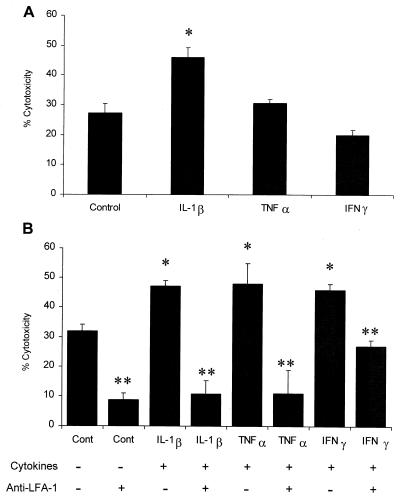
Incubation of bovine PMNs with inflammatory cytokines enhances the cytotoxicity of partially purified LKT for bovine PMNs.
We next examined the effects of preincubation of PMNs with cytokines on LKT-mediated cytotoxicity. We observed an increase in LKT cytotoxicity for PMNs incubated with IL-1β for 15 min compared with the LKT cytotoxicity for nonstimulated cells. Neither TNF-α nor IFN-γ had any effect on LKT-mediated cytotocixity after incubation for 15 min (Fig. 5A). We observed increased LKT-mediated cytotoxicity for PMNs incubated with IL-1β, TNF-α, or IFN-γ for 1 h. LKT-mediated cytotoxicity was greatly reduced when the cells were incubated with an anti-LFA-1 MAb (BAT75A) before addition of the LKT (Fig. 5B).
FIG. 5.
Incubation of bovine PMNs with inflammatory cytokines enhances LKT cytotoxicity. Freshly isolated bovine PMNs (1 × 106 cells/ml) were incubated with recombinant bovine IL-1β (50 ng), recombinant human TNF-α (50 ng), recombinant bovine IFN-γ (50 ng), or medium (control) for 15 min (A) or 1 h (B) at 37°C. In the latter experiments (B) some of the cells were incubated with an anti-LFA-1 MAb (BAT75A) (final concentration, 50 μg/ml) for 40 min before addition of LKT. Control and treated PMNs were then plated in 96-well plates and incubated with partially purified M. haemolytica LKT (1 U) for 1 h at 37°C. Cell viability was assessed by XTT reduction. The data are the means ± standard errors of the means for four independent experiments. One asterisk indicates that the value for cytokine-stimulated cells was statistically significantly greater than the value for control cells (P < 0.05). Two asterisks indicate that the value for anti-LFA-treated cytokine-stimulated PMNs was significantly less than the value for untreated cytokine-stimulated PMNs.
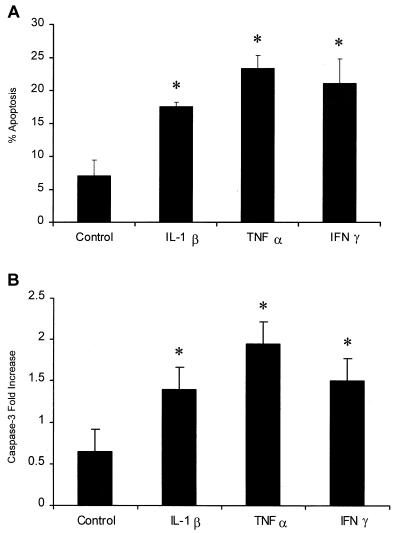
Incubation of bovine PMNs with inflammatory cytokines enhances apoptosis mediated by partially purified LKT.
To asses the effect of cytokine stimulation on LKT-mediated apoptosis, cytokine-treated and control PMNs were incubated with LKT (0.1 U) for 6 h, and then apoptosis was assessed by PI staining and caspase activation. We observed 2.5-, 3.3-, and 3-fold increases in PI staining for PMNs preincubated for 1 h with IL-1β, TNF-α, and IFN-γ, respectively, before exposure to LKT compared with the PI staining observed for control PMNs exposed to LKT. Likewise, we observed 2-, 3-, and 2.3-fold increases in caspase-3 activation when the PMNs were preincubated for 1 h with IL-1β, TNF-α, and IFN-γ, respectively, before exposure to LKT compared with the caspase-3 activation observed with control PMNs exposed to LKT (Fig. 6).
FIG. 6.
Incubation of bovine PMNs with inflammatory cytokines enhances LKT-mediated apoptosis. Freshly isolated bovine PMNs (1 × 106 cells/ml) were incubated with recombinant bovine IL-1β (50 ng), recombinant human TNF-α (50 ng), recombinant bovine IFN-γ (50 ng), or medium (control) for 1 h at 37°C. Partially purified M. haemolytica LKT (0.1 U) was then added to the PMNs and incubated for 6 h at 37°C. Apoptosis was assessed by PI staining by flow cytometry (A) and by caspase-3 activation by a colorimetric assay (B). The data are the means ± standard errors of the means for three independent experiments. An asterisk indicates that the data for cytokine-stimulated cells was statistically significantly different from the data for control cells (P < 0.05).
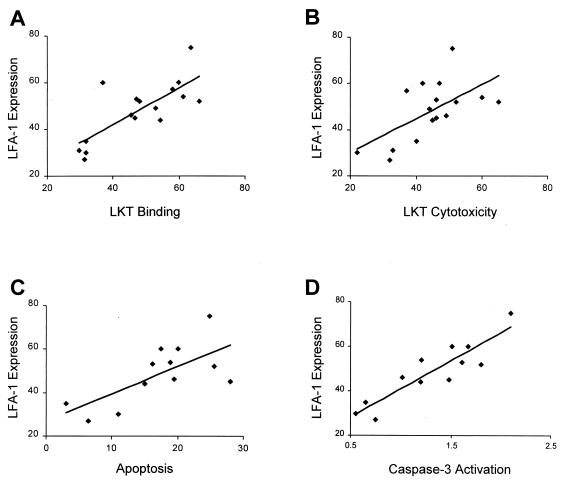
Correlation between LKT biological activities and anti-LFA-1 MAb binding to bovine PMNs.
To determine if there is a correlation between LKT biological activity and PMN expression of LFA-1, we performed linear regression analysis of the percentage of LFA-1-stained PMNs versus LKT binding, cytotoxicity, and apoptosis (PI staining and caspase-3 activation). As illustrated in Fig. 7, we observed statistically significant correlations between LFA-1 MAb-positive PMNs and LKT binding (P = 0.0006), cytotoxicity (P = 0.012), PI staining (P = 0.013), and caspase-3 activation (P < 0.0001).
FIG. 7.
Correlation between LKT biological activities and LFA-1 staining of bovine PMNs as determined by linear regression. (A) LKT binding versus LFA-1 staining (r = 0.76; y = 10.7 + 0.78x; P = 0.0006). (B) LKT cytotoxicity versus LFA-1 staining (r = 0.60; y = 15.5 + 0.73x; P = 0.0126). (C) LKT-mediated apoptosis assessed by PI staining versus LFA-1 staining (r = 0.68; y = 26.9 + 1.2x; P = 0.0138). (D) LKT-mediated caspase-3 activation versus LFA-1 staining (r = 0.89; y = 15.5 + 25.3x; P < 0.0001).
DISCUSSION
It is increasingly recognized that inflammatory cytokines contribute to pulmonary tissue damage in many diseases (20, 37). Studies with animal models of human adult respiratory distress syndrome have helped define the contributions of inflammatory cytokines to the pathogenesis of acute lung injury (16, 36). These cytokines promote the production and release of additional proteins, eicosanoids, and free radicals that perpetuate the inflammatory cascade, resulting in severe lung injury (3). There is compelling evidence that inflammatory cytokines play a central role in orchestrating the inflammatory cascade that leads to lung injury in bovine pasteurellosis (15, 19, 21, 22, 30, 31, 35, 50, 51, 52).
We recently reported that exposure of bovine PMNs to the inflammatory cytokine IL-1β resulted in increased binding of two different anti-LFA-1 MAbs, LKT binding, and cell death (25). In the present study, we extended these observations by comparing PMNs exposed to IL-1β, TNF-α, and IFN-γ. Using an MAb (BAT75A) that recognizes LFA-1 on bovine cells, we observed that all three cytokines increased the percentage of cells that stained positive for LFA-1. IL-1β increased LFA-1 staining most rapidly (within 15 min). Likewise, LKT binding and cytotoxicity also increased within 15 min. These results suggest that IL-1β may play an important role in the early response to M. haemolytica LKT. Although we cannot exclude the possibility that exposure to different cytokine concentrations might further alter the PMN response to LKT, we did not observe differences in the responses of PMNs that were incubated with the cytokines at concentrations of 25 to 500 ng/ml. The species of origin of the cytokines might also be a consideration, since the IL-1β and IFN-γ that we used were recombinant bovine cytokines and the TNF-α used was a recombinant human cytokine. However, we have shown previously that recombinant human TNF-α stimulates bovine PMNs in vitro (38). Thus, we believe that our data likely reflect the general response of bovine PMNs to these cytokines. Concomitant with increased LFA-1 staining of cytokine-exposed bovine PMNs, the cells exhibited an increased ability to bind M. haemolytica LKT and increased susceptibility to the cytotoxic effects of M. haemolytica LKT.
The results of the present study provide additional evidence that increased expression or conformational activation of LFA-1 on bovine PMNs is important for the biological effects of LKT. First, we observed a direct relationship between LFA-1 staining of bovine PMNs and LKT binding (Fig. 7A). Second, the cytotoxic effect of LKT was diminished when the cells were incubated with an anti-LFA-1 MAb (BAT 75A) before the LKT was added (Fig. 5). Our study corroborates the results reported by other investigators, who observed reductions in LKT binding and cytotoxicity following addition of a β2-integrin MAb (1, 27, 45) and noted that PMNs from animals deficient in β2-integrins exhibited diminished LKT binding and cytotoxicity (17). However, we cannot exclude the possibility that there are other receptors for LKT, since addition of the anti-LFA1 MAb did not totally block LKT cytotoxicity. This was particularly true when the PMNs were incubated with IFN-γ before exposure to LKT (Fig. 5B).
It has been reported previously that bovine leukocytes exposed to M. haemolytica LKT undergo apoptosis in vitro (41, 43). Recently, we reported that lungs from cattle experimentally infected with M. haemolytica exhibited increased numbers of TUNEL-positive (presumably apoptotic) cells (24). LKT-mediated apoptosis of cells responsible for defense of the lungs might increase the severity of bovine pasteurellosis. In the present study we observed that incubation with the inflammatory cytokines IL-1β, TNF-α, and IFN-γ increased LKT-mediated apoptosis of bovine PMNs, as assessed by PI staining and caspase-3 activation (Fig. 6). If similar events occur in the inflammatory milieu generated by viral infection of the lung, this could reduce host defense against pasteurellosis.
There are several limitations to our study. First, we used a partially purified LKT preparation. However, we have shown previously that the binding and cytotoxicity of partially purified LKT can be blocked by the anti-LKT MAb MM601 (5). The data suggest that the LKT, not simply contaminating LPS or other metabolic products, is required for the biological response. In the present study, we observed that heat (100°C for 15 min) destroyed the response to LKT, and pretreatment with polymyxin B did not reduce the response to LKT (data not shown). We have previously demonstrated that LKT from a noncytolytic M. haemolytica mutant exhibits little or no binding to bovine PMNs (25). These controls suggested that LPS contamination by itself does not account for the results obtained in our study. A second limitation is that we used only one MAb (BAT75A) to evaluate LFA-1 expression on bovine PMNs by flow cytometry. This antibody has been used by a number of investigators to detect LFA-1 expression on bovine cells, and workers in our laboratory and other workers have shown that it reduces LKT binding to bovine leukocytes and LKT cytotoxicity for these cells (17, 25, 45). Using MAbs BAT75A and HI111 in Western immunoblotting provided additional evidence that bovine PMNs have increased LFA-1 expression after cytokine exposure. However, these antibodies do not provide evidence of the state of LFA-1 activation, since they recognize LFA-1 on the surfaces of stimulated and unstimulated PMNs. This is an important consideration, since lymphocytes can modulate LFA-1 binding to its ligands without altering the numbers of LFA-1 molecules on the cell surface. LFA-1 molecules can change from an inactive state to an active state in two ways: (i) by altering the affinity of the individual integrin molecule by conformational changes and (ii) by increasing the avidity because of clustering of many LFA-1 molecules in the membrane (12). Thus, our data might reflect increased LFA-1 expression on the cell surface or an increase in the affinity or avidity of LFA-1 for both the BAT75A MAb and LKT. Finally, in our real-time PCR experiments we observed increased CD18 mRNA expression that corroborated our flow cytometry and Western immunoblot data. However, because CD18 is common to all β2-integrins, we cannot exclude the possibility that we were detecting expression of other β2-integrins (CD11b, CD11c, or CD11d) besides LFA-1.
In summary, the results of this study provide evidence that stimulation of bovine PMNs with the inflammatory cytokines IL-1β, TNF-α, and IFN-γ can enhance the biological response of these cells to M. haemolytica LKT by increasing cell surface expression or conformational activation of LFA-1. We recently reported that cattle experimentally infected with bovine herpesvirus 1 had LFA-1 up-regulated on their peripheral blood leukocytes, which in turn resulted in increased LKT binding and killing of these cells (26). If similar cytokine activation occurs during viral infection in vivo, the data might explain, in part, the virus-bacterium synergism observed in bovine pasteurellosis.
Acknowledgments
We thank Chester Thomas for his assistance with the statistical analysis. We thank Steve Giles for assistance with preparation of the illustrations. We thank Paul Lunn for his assistance with the real-time PCR.
This work was supported by funds from the Wisconsin Agricultural Experiment Station (grants142 4313 and 142 4543), the University of Wisconsin-Madison Industrial and Economic Development Research Program (grant 118 1034), the USDA National Research Initiative (grant 2000-02304), and the NIEHS Center for Developmental and Molecular Toxicology (grant P30 ES 0990). F. Leite was supported by CAPES-Ministério Da Educação, Brazil.
Editor: R. N. Moore
REFERENCES
- 1.Ambagala, T. C., A. P. N. Ambagala, and S. Srikumaran. 1999. The leukotoxin of Pasteurella haemolytica binds to β2 integrin on bovine leukocytes. FEMS Microbiol. Lett. 179:161-167. [DOI] [PubMed] [Google Scholar]
- 2.Baluyut, C. S., R. R. Simonson, W. J. Bemrick, and S. K. Maheswaran. 1981. Interaction of Pasteurella haemolytica with bovine neutrophils: identification and partial characterization of a cytotoxin. Am. J. Vet. Res. 42:1920-1926. [PubMed] [Google Scholar]
- 3.Billiau, A., and F. Vandekerckhove. 1991. Cytokines and their interaction with other inflammatory mediators in the pathogenesis of sepsis and septic shock. Eur. J. Clin. Investig. 21:559-573. [DOI] [PubMed] [Google Scholar]
- 4.Breider, M. A., R. D. Walker, F. M. Hopkins, T. W. Shultz, and T. L. Bowersock. 1988. Pulmonary lesions induced by Pasteurella haemolytica in neutrophil sufficient and neutrophil deficient calves. Can. J. Vet. Res. 52:205-209. [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, F. J., F. Leite, and C. J. Czuprynski. 1997. Binding of Pasteurella haemolytica leukotoxin to bovine leukocytes. Infect. Immun. 65:3719-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinkenbeard, K. D., C. R. Clarke, C. M. Hague, P. Clinkenbeard, S. Srikumaran, and R. J. Morton. 1994. Pasteurella haemolytica leukotoxin-induced synthesis of eicosanoids by bovine neutrophils in vitro. J. Leukoc. Biol. 56:644-649. [DOI] [PubMed] [Google Scholar]
- 7.Confer, A. W., R. J. Panciera, K. D. Clinkenbeard, and D. A. Mosier. 1990. Molecular aspects of virulence of Pasteurella haemolytica. Can. J. Vet. Res. 54:S48-S52. [PubMed] [Google Scholar]
- 8.Coote, J. G. 1992. Structural and functional relationships among the RTX toxin determinants of Gram-negative bacteria. FEMS Microbiol. Rev. 88:137-162. [DOI] [PubMed] [Google Scholar]
- 9.Czuprynski, C. J., E. J. Noel, O. Ortiz-Carranza, and S. Srikumaran. 1991. Activation of bovine neutrophils by partially purified Pasteurella haemolytica leukotoxin. Infect. Immun. 59:3126-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dallegri, F., and L. Otellonello. 1997. Tissue injury in neutrophilic inflammation. Inflamm. Res. 46:382-391. [DOI] [PubMed] [Google Scholar]
- 11.Frank, G. H. 1989. Pasteurellosis in cattle, p. 197-222. In C. Adlam and J. M. Rutter (ed.), Pasteurella and pasteurellosis. Academic Press, New York, N.Y.
- 12.Gahmberg, C. G. 1997. Leukocyte adhesion: CD11/CD18 integrins and intercellular adhesion molecules. Curr. Opin. Cell Biol. 9:643-650. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez, C. T., and S. K. Maheswaran. 1993. The role of induced virulence factors produced by Pasteurella haemolytica in the pathogenesis of bovine pulmonary pasteurellosis. Review and hypothesis. Br. Vet. J. 149:183-193. [DOI] [PubMed] [Google Scholar]
- 14.Henricks, P. A. J., G. J. Binkhorst, A. A. Drijver, and F. P. Nijkamp. 1992. Pasteurella haemolytica leukotoxin enhances production of leukotreine B4 and 5-hydroxyeicosatettraenoic acid by bovine polymorphonuclear leukocytes. Infect. Immun. 60:3238-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsuan, S. L., M. S. Kannan, S. Jeyaseelan, Y. S. Prakash, C. Malazdrewich, M. S. Abrahamsen, G. C. Sieck, and S. K. Maheswaran. 1999. Pasteurella haemolytica leukotoxin and endotoxin induced cytokine gene expression in bovine alveolar macrophages requires NF-kβ activation and calcium elevation. Microb. Pathog. 26:263-273. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs, R. F., D. R. Tabor, A. W. Burns, and G. D. Campbell. 1989. Elevated interleukin-1 release by human alveolar macrophages during the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 140:1686-1692. [DOI] [PubMed] [Google Scholar]
- 17.Jeyaseelan, S., S. L. Hsuan, M. S. Kannaa, B. K. Walcheck, J. F. Wang, M. E. Kehrli, Jr., E. T. Lally, G. C., Sleck, and S. Maheswaran. 2000. Lymphocyte function-associated antigen 1 is a receptor for Pasteurella haemolytica leukotoxin in bovine leukocytes. Infect. Immun. 68:72-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeyaseelan, S., M. S. Kannan, S. L. Hsuan, A. K. Singh, T. F. Walseth, and S. K. Maheswaran. 2001. Pasteurella (Mannheimia) haemolytica leukotoxin-induced cytolysis of bovine leukocytes: role of arachidonic acid and its regulation. Microb. Pathog. 30:59-69. [DOI] [PubMed] [Google Scholar]
- 19.Jeyaseelan, S., M. S. Kannan, R. E. Briggs, P. Thumbikat, and S. K. Maheswaran. 2001. Mannheimia haemolytica leukotoxin activates a nonreceptor tyrosine kinase signaling cascade in bovine leukocytes, which induces biological effects. Infect. Immun. 69:6131-6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly, J. 1990. Cytokines of the lung. Am. Rev. Respir. Dis. 141:765-788. [DOI] [PubMed] [Google Scholar]
- 21.Lafleur, R. L., M. S. Abrahamsen, and S. K. Maheswaran. 1998. The biphasic mRNA expression pattern of bovine interleukin-8 in Pasteurella haemolytica lipopolysaccharide-stimulated alveolar macrophages is primarily due to tumor necrosis factor alpha. Infect. Immun. 66:4087-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lafleur, R. L., C. Malazdrewich, S. Jeyaseelan, E. Bleifield, M. S. Abrahamsen, and S. K. Maheswaran. 2001. Lipopolysaccharide enhances cytolysis and inflammatory cytokine induction in bovine alveolar macrophages exposed to Pasteurella (Mannheimia) haemolytica leukotoxin. Microb. Pathog. 30:347-357. [DOI] [PubMed] [Google Scholar]
- 23.Lally, E. T., I. R. Kieba, A. Sato, C. L. Green, J. Rosenbloom, J. Korostoff, J. F. Wang, B. J. Shenker, S. Ortlepp, M. K. Robinson, and P. C. Billings. 1997. RTX toxins recognize a β2 integrin on the surface of human target cells. J. Biol. Chem. 272:30463-30469. [DOI] [PubMed] [Google Scholar]
- 24.Leite, F., C. Malazdrewich, H. S. Yoo, S. K. Maheswaran, and C. J. Czuprynski. 1999. Use of TUNEL staining to detect apoptotic cells in lungs of cattle experimentally infected with Pasteurella haemolytica. Microb. Pathog. 27:179-185. [DOI] [PubMed] [Google Scholar]
- 25.Leite, F., J. F. Brown, M. J. Sylte, R. E. Briggs, and C. J. Czuprynski. 2000. Recombinant bovine interleukin-1β amplifies the effect of partially purified Pasteurella haemolytica leukotoxin on bovine neutrophils in a β2-integrin-dependent manner. Infect. Immun. 68:5581-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leite, F., M. J. Sylte, S. O'Brien, R. Schultz, S. Peek, K. van Reeth, and C. J. Czuprynski. 2001. Effect of experimental infection of cattle with bovine herpesvirus-1 (BHV-1) on the ex vivo interaction of bovine leukocytes with Mannheimia (Pasteurella) haemolytica leukotoxin. Vet. Immunol. Immunopathol. 84:97-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, J., K. D. Clinkenbeard, and J. W. Ritchey. 1999. Bovine CD18 identified as a species specific receptor for Pasteurella haemolytica leukotoxin. Vet. Microbiol. 2:91-97. [DOI] [PubMed] [Google Scholar]
- 28.Liu, L., H. D. Lehmkuhul, and M. L. Kaeberle. 1999. Synergistic effects of bovine respiratory syncytial virus and non-cytopathic bovine viral diarrhea virus infection on selected bovine alveolar macrophage functions. Can. J. Vet. Res. 63:41-48. [PMC free article] [PubMed] [Google Scholar]
- 29.Maheswaran, S. K., D. J. Weiss, M. S. Kannan, E. L. Townsend, K. R. Reddy, L. O. Whiteley, and S. Srikumaran. 1992. Effects of Pasteurella haemolytica A1 leukotoxin on bovine neutrophils: degranulation and generation of oxygen-derived free radicals. Vet. Immunol. Immunopathol. 33:51-68. [DOI] [PubMed] [Google Scholar]
- 30.Malazdrewich, C., T. R. Ames, M. S. Abrahamsen, and S. K. Maheswaran. 2001. Pulmonary expression of tumor necrosis factor alpha, interleukin-1 beta, and interleukin-8 in the acute phase of bovine pneumonic pasteurellosis. Vet. Pathol. 38:297-310. [DOI] [PubMed] [Google Scholar]
- 31.Morsey, M. A., A. G. Van-Kessel, Y. Popowych, D. Gordon, M. Campos, and L. A. Babiuk. 1999. Cytokine profiles following interaction between bovine alveolar macrophages and Pasteurella haemolytica. Microb. Pathog. 26:325-331. [DOI] [PubMed] [Google Scholar]
- 32.Nicoletti, J., G. Migliorati, M. C. Pagliacci, F. Grignani, and C. Riccardi. 1991. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 139:271-279. [DOI] [PubMed] [Google Scholar]
- 33.Ohmann, H. B., and L. A. Babiuk. 1985. Viral-bacterial pneumonia in calves: effect of bovine herpesvirus-1 on immunologic functions. J. Infect. Dis. 151:937-947. [DOI] [PubMed] [Google Scholar]
- 34.Ohmann, H. B., L. A. Babiuk, and R. Harlan. 1991. Cytokine synergy with viral cytopathic effects and bacterial products during the pathogenesis of respiratory tract infection. Clin. Immunol. Immunopathol. 60:152-170. [DOI] [PubMed] [Google Scholar]
- 35.Pace, L. W., J. M. Kreeger, K. L. Bailey, S. E. Turnquist, and W. H. Fales. 1993. Serum levels of tumor necrosis-α in calves experimentally infected with Pasteurella haemolytica A1. Vet. Immunol. Immunopathol. 35:353-364. [DOI] [PubMed] [Google Scholar]
- 36.Parson, P. E., F. A. Moore, E. E. Moore, D. N. Ikle, P. M. Henson, and G. S. Worthen. 1992. Studies on the role of tumor necrosis factor in adult respiratory distress syndrome. Am. Rev. Respir. Dis. 146:694-700. [DOI] [PubMed] [Google Scholar]
- 37.Said, S. I., and H. D. Foda. 1989. Pharmacological modulation of lung injury. Am. Rev. Respir. Dis. 13:1553-1564. [DOI] [PubMed] [Google Scholar]
- 38.Sample, A. K., and C. J. Czuprynski. 1991. Priming and stimulation of bovine neutrophils by recombinant interleukin-1 α and tumor necrosis factor-α. J. Leukoc. Biol. 49:107-115. [DOI] [PubMed] [Google Scholar]
- 39.Slocombe, R. F., J. Malark, R. T. Ingersoll, F. J. Derksen, and N. E. Robinson. 1985. Importance of neutrophils in the pathogenesis of acute pneumonic pasteurellosis in calves. Am. J. Vet. Res. 46:2253-2258. [PubMed] [Google Scholar]
- 40.Stevens, P. K., and C. J. Czuprynski. 1995. Dissociation of cytolysis and monokine release by bovine mononuclear phagocytes incubated with Pasteurella haemolytica partially-purified leukotoxin and lipopolysaccharide. Can. J. Vet. Res. 59:110-117. [PMC free article] [PubMed] [Google Scholar]
- 41.Stevens, P. K., and C. J. Czuprynski. 1996. Pasteurella haemolytica leukotoxin induces bovine leukocytes to undergo morphologic changes consistent with apoptosis in vitro. Infect. Immun. 64:2687-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Storz, J., X. Lin, C. W. Purdy, V. N. Chouljenko, K. G. Kousoulas, F. M. Enright, W. C. Gilmore, R. E. Briggs, and R. W. Loan. 2000. Coronavirus and Pasteurella infections in bovine shipping fever pneumonia and Evan's criteria for causation. J. Clin. Microbiol. 38:3291-3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun, Y., K. D. Clinkenbeard, L. A. Cudd, C. R. Clarke, and P. A. Clinkenbeard. 1999. Correlation of Pasteurella haemolytica leukotoxin binding with susceptibility to intoxication of lymphoid cells from various species. Infect. Immun. 67:6264-6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tatum, F. M., R. E. Briggs, S. S. Sreevatsan, E. S. Zehr, H. S. Ling, L. O. Whiteley, T. R. Ames, and S. K. Maheswaran. 1998. Construction of an isogenic leukotoxin deletion mutant of Pasteurella haemolytica serotype 1: characterization and virulence. Microb. Pathog. 24:37-46. [DOI] [PubMed] [Google Scholar]
- 45.Wang, J. F., I. R. Kieba, J. Korostoff, T. L. Guo, N. Yamaguchi, H. Rozmiarek, P. C. Billings, B. J. Shenker, and E. T. Lally. 1998. Molecular and biochemical mechanisms of Pasteurella haemolytica leukotoxin-induced cell death. Microb. Pathog. 25:317-331. [DOI] [PubMed] [Google Scholar]
- 46.Welch, R. A. 1991. Pore-forming cytolysins of gram-negative bacteria. Mol. Microbiol. 5:521-528. [DOI] [PubMed] [Google Scholar]
- 47.Whiteley, L. O., S. K. Maheswaran, D. J. Weiss, and T. R. Ames. 1991. The morphologic and hematologic alteration caused by intratracheal inoculation of live and ultraviolet light-killed Pasteurella haemolytica A1 in calves. Vet. Pathol. 28:275-285. [DOI] [PubMed] [Google Scholar]
- 48.Yates, W. D. G. 1982. A review of infectious bovine rhinotracheitis, shipping fever pneumonia and viral-bacterial synergism in respiratory disease in cattle. J. Comp. Med. 46:225-263. [PMC free article] [PubMed] [Google Scholar]
- 49.Yates, W. D. G., K. W. F. Jericho, and C. E. Doige. 1982. Effects of bacterial dose on pneumonia induced by aerosol exposure of calves to bovine herpesvirus-1 and Pasteurella haemolytica. Am. J. Vet. Res. 44:238-243. [PubMed] [Google Scholar]
- 50.Yoo, H. S., B. S. Rajagopal, S. K. Maheswaran, and T. R. Ames. 1995. Purified Pasteurella haemolytica leukotoxin induces expression of inflammatory cytokines from bovine alveolar macrophages. Microb. Pathog. 18:237-252. [DOI] [PubMed] [Google Scholar]
- 51.Yoo, H. S., S. K. Maheswaran, S. Srinand, T. R. Ames, and M. Suresh. 1995. Increased tumor necrosis factor-α and interleukin-β1 expression in the lungs of calves with experimental pneumonic pasteurellosis. Vet. Immunol. Immunopathol. 49:15-28. [DOI] [PubMed] [Google Scholar]
- 52.Yoo, H. S., S. K. Maheswaran, G. Lin, E. L. Townsend, and T. R. Ames. 1995. Induction of inflammatory cytokines in bovine alveolar macrophages following stimulation with Pasteurella haemolytica lipopolysaccharide. Infect. Immun. 63:381-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yude, S., K. D. Clinkenbeard, C. L. Ownby, L. Cudd, C. R. Clarke, and S. K. Highlander. 2000. Ultrastructural characterization of apoptosis in bovine lymphocytes exposed to Pasteurella haemolytica leukotoxin. Am. J. Vet. Res. 61:51-56. [DOI] [PubMed] [Google Scholar]