Abstract
The efficacy of intramammary (Ima) immunization with a live attenuated (la) Staphylococcus aureus mutant to protect the mouse mammary gland from infection has previously been established. The present study was aimed at evaluating whether Ima immunization with la-S. aureus can induce cell-mediated immune responses to the pathogen within the mammary gland. Mice were immunized by Ima route with la-S. aureus, and regional lymph node mononuclear cells were obtained thereafter. A higher expression of the interleukin-2 receptor was found on B and T cells from immunized mice when they were compared with control mice. Immunization with la-S. aureus induced strong proliferative responses to S. aureus. Moreover, significantly increased levels of gamma interferon (IFN-γ) were produced by CD4+ T cells when lymphocytes from immunized mice, but not from control mice, were cultured in the presence of staphylococcal antigens. Moreover, a significant increase in the percentage of IFN-γ-producing CD4+ and CD8+ T cells was observed after S. aureus Ima challenge in immunized mice compared to challenged control mice. Our results demonstrated that Ima immunization with la-S. aureus induced primed lymphocyte populations capable of responding against staphylococcal antigens during in vitro stimulation, as well as during in vivo infection by S. aureus. CD4+ and CD8+ T cells appear to be the main lymphocyte subpopulations involved in this response. It is suggested that IFN-γ production induced by Ima immunization may play a pivotal role in the eradication of intracellular staphylococci.
Staphylococcus aureus is one of the main causative agents of bovine mastitis, the most economically important disease of dairy ruminants (16, 28). S. aureus intramammary (Ima) infections tend to become persistent and chronic, even in animals placed under antibiotic treatment (36). Several attempts have been made to prevent S. aureus mastitis through vaccination. The efficacy of current experimental S. aureus vaccines, however, has not yet been fully demonstrated in field trials (4, 8, 37), although certain protection was achieved in bovine herds with high prevalence of S. aureus mastitis (35). Indeed, whereas available vaccines can induce high levels of circulating specific anti-S. aureus antibodies (21), they failed to reduce the incidence of new Ima infection or eliminate chronic mastitis (20, 32, 37). These facts emphasize the need for a better understanding of the immune responses required to prevent S. aureus infection of the mammary gland.
One confirmed mechanism utilized by certain bacteria to evade host defenses is to become internalized within host cells. Although S. aureus has not traditionally been considered an intracellular pathogen of the magnitude associated with classical facultative intracellular pathogens such as Mycobacterium, Salmonella, and Listeria spp., its ability to enter and survive within phagocytic and nonphagocytic cells is now well established (17). Intracellular survival may contribute to persistence of S. aureus in certain pathological conditions, such as endocarditis, osteomyelitis, and bovine mastitis (17). Indeed, survival of S. aureus within professional phagocytes, such as neutrophils, may be a key step toward chronic infection (10). In vitro studies have shown that S. aureus may be internalized and survive within endothelial cells (38), mammary epithelial cells (2), pulmonary epithelial cells (14) and osteoblasts (12). In addition, the intracellular presence of living bacteria has recently been demonstrated within alveolar cells and macrophages isolated from milk of cows chronically infected with S. aureus (11). Internalization and survival of S. aureus may explain why the humoral immune response alone is inefficient and why antibiotic treatment fails to eliminate this pathogen, which is so well adapted to its environment (2, 10). In the light of these findings we hypothesize that the prevention of S. aureus mammary gland infection through vaccination may only be achieved if both humoral and cell-mediated local specific immune responses against the pathogen are raised.
Using a mouse model of infection, we have previously demonstrated the feasibility of inducing mammary gland specific humoral immunity against S. aureus by local but not systemic immunization with a live attenuated (la) strain (6, 9). The present study was aimed at evaluating whether Ima immunization with la-S. aureus can also induce cell-mediated immune responses to the pathogen within the mammary gland. Thus, we characterized the lymphocyte subpopulations involved and the profile of cytokines produced during in vitro and in vivo secondary responses to S. aureus in a mouse model of mastitis.
MATERIALS AND METHODS
Bacteria and antigens.
Wild-type S. aureus 8325-4 (phi-11) was provided by John Iandolo (Department of Pathology, College of Veterinary Medicine, Kansas State University, Manhattan). Bovine mastitis strain 319 provided by Elida Gentilini (Veterinary School, University of Buenos Aires, Buenos Aires, Argentina) was included in certain experiments. Live attenuated, temperature-sensitive derivatives were obtained from the wild-type strain by chemical mutagenesis with nitrosoguanidine (Sigma Chemical Co., St. Louis, Mo.) as described previously (29). The attenuated mutant used in this study (A523) replicates well at low temperatures (below 30°C) and only undergoes limited replication when transferred to the mammalian body temperature. Bacteria were cultured on tryptic soy agar (Difco Laboratories, Detroit, Mich.) at 28°C (la mutant) or 37°C (wild-type strain) for 14 h, and harvested with saline. Bacteria were washed by centrifugation at 500 × g for 10 min at 4°C and then suspended to the appropriate density in saline. Heat-killed S. aureus was prepared by heating a suspension of the wild-type S. aureus strain (wt-S. aureus) in saline at 60°C for 1 h. Total proteins from wt-S. aureus were obtained as described previously (9). Preparations of BCG and purified protein derivative (PPD) were obtained from Lucía Barrera (ANLIS; Carlos G. Malbrán National Institute of Microbiology, Buenos Aires, Argentina).
Mice, immunization schedule and challenge.
Eight- to ten-week-old Swiss mice were obtained from our vivarium at the Department of Microbiology, School of Medicine, and were maintained under standard conditions (34). Groups of six to eight female mice in their first pregnancy received an Ima inoculation of 50 μl containing 5 × 106 CFU of la-S. aureus 7 days before parturition as described elsewhere (9). Mice received a second inoculation 7 days later (at the day of parturition). In the in vivo experiments, mice were challenged by the Ima route with 5 × 105 CFU of wt-S. aureus as described previously (6, 9). In experiments designed to test γδ T-cell expansion, positive control cells were obtained from mice inoculated by the Ima route with 5 × 105 CFU of viable BCG (13).
Lymphocyte isolation and culture.
Lymphocytes from regional mammary lymph nodes were obtained from control and immunized mice by mechanical dissociation. Cells were cultured in RPMI 1640 medium (Life Technologies, Gaithersburg, Md.) supplemented with 2 mM glutamine (Life Technologies), 10% fetal calf serum (Life Technologies), and 5 × 10−2 mM 2-mercaptoethanol (Sigma) and then adjusted to pH 7.4 with 2 N NaOH. For the γδ T-cell culture, 25 mM HEPES (Life Technologies), 0.1 mM sodium pyruvate (Life Technologies), 1× minimal essential medium nonessential amino acids, and 0.5× minimal essential medium essential amino acids (Life Technologies) were added to the culture medium already supplemented as described above. Cells (2 × 105 in 200 μl) were cultured in 96-well, round-bottom plates (Corning Glass Works, Corning, N.Y.) in the presence of whole heat-killed S. aureus (5 × 108 CFU/ml) or total S. aureus proteins (10 μg/ml). Control cultures were made in medium alone or medium containing 2 μg of concanavalin A (Sigma)/ml. Cultures were maintained at 37°C in a water-saturated air atmosphere containing 5% CO2. For proliferation experiments, mice were immunized by the Ima route, and 10 days thereafter mononuclear cells from regional mammary gland lymph nodes were obtained as described above. Cells were cultured in medium or stimulated with either whole heat-killed wt-S. aureus or total S. aureus proteins over 4 days. Proliferation was assessed by addition of [3H]thymidine (1 μCi/well) during the last 16 h. Cells were harvested, and thymidine intake was measured by liquid scintillation counting.
Cytokine detection by enzyme-linked immunosorbent assay.
Culture supernatants were sampled after the first 48 h to evaluate the presence of gamma interferon (IFN-γ) and interleukin-4 (IL-4) by enzyme-linked immunosorbent assay. The IFN-γ concentration in culture supernatants was determined by using the mouse IFN-γ MiniKit (Endogen, Woburn, Mass.) according to a modification of the original protocol described by the manufacturer (7). The IL-4 concentration in culture supernatants was measured by using the Duoset kit for mouse IL-4 detection (Genzyme Diagnostics, Cambridge, Mass.) according to the manufacturer's directions. For both cytokines, a standard curve was made, and IFN-γ and IL-4 concentrations in each sample were determined by regression analysis. The sensitivity of both of the assays was 40 pg/ml.
Phenotypic cell analysis.
Phenotypes of freshly isolated cells from regional mammary gland lymph nodes or cells after culture in the presence of whole heat-killed S. aureus (5 × 108 CFU/ml) or PPD (50 μg/ml) were determined by flow cytometry. Cells (106) were washed in phosphate-buffered saline (PBS), suspended in PBS containing 10% normal mouse serum, incubated during 30 min at 37°C, and stained with labeled antibodies specific for the different murine surface antigens evaluated. The following monoclonal antibodies were used: fluorescein isothiocyanate (FITC)-labeled or Tri-Color (TC)-labeled anti-CD3, FITC-labeled or phycoerythrin (PE)-labeled anti-CD4, TC-labeled anti-CD8, PE-labeled anti-CD25 (Caltag, South San Francisco, Calif.), PE-labeled CD19, biotin-labeled anti-γδ T-cell receptor, PE-labeled Pan NK, biotin-labeled anti-CD25 (Pharmingen, San Diego, Calif.). When biotin-labeled antibodies were used, cells were incubated with TC-labeled streptavidin (Caltag). Cells were washed, and fluorescence was measured by using a Coulter EPICS XL cytometer (Miami, Fla.). Flow data were analyzed by using XL II software. Negative control samples were incubated with irrelevant, isotype-matched antibodies in parallel with all experimental samples.
Intracellular analysis of cytokine production.
Cytokine production by different lymphocyte subpopulations was evaluated at the single-cell level by intracellular staining by using the Citofix/Cytoperm Plus kit (Pharmingen) according to the manufacturer's instructions. Briefly, cultured cells or freshly isolated cells from mice challenged with wt-S. aureus were incubated consecutively with monensin, Cytofix/Cytoperm solution (Pharmingen), and FITC-conjugated anti-IFN-γ (Pharmingen). Cells were then washed with Perm/Wash solution (Pharmingen) and finally with PBS-2% fetal calf serum. Samples were analyzed by using the cytometer and the software described above. Negative control samples were incubated with irrelevant, isotype-matched antibodies in parallel with all experimental samples. The percentage of CD4+ and CD8+ T cells producing IFN-γ was determined. The change in mean fluorescence intensity (ΔMFI) was determined as the difference between the MFI measured when cells were incubated with specific antibodies and the MFI measured when cells were incubated with irrelevant isotype-matched antibodies.
Statistical analysis.
The Mann-Whitney test for nonparametrics was used to statistically compare nonpaired samples. The Wilcoxon signed-rank test for paired samples was used for cell comparisons before and after culture. Statistical analysis was performed with the GraphPad program (PRISM, version 2.2). P values lower than 0.05 were considered significant.
RESULTS
Effect of Ima immunization on specific priming of mammary gland lymphocytes.
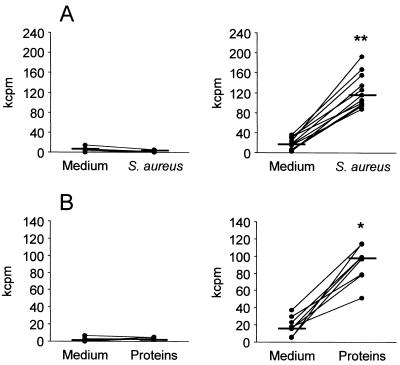
To evaluate the efficacy of local immunization with la-S. aureus on the induction of cell-mediated immune responses within the mammary gland, lymphocyte proliferation after in vitro specific stimulation was assessed. Lymphocytes from immunized mice exhibited a significant proliferative response in the presence of heat-killed S. aureus (Fig. 1A, right) (P < 0.001) or total S. aureus proteins (Fig. 1B, right) (P < 0.01). No proliferative response was observed in lymphocytes from control mice upon in vitro stimulation with either heat-killed bacteria or total S. aureus proteins (Fig. 1A and B, left panels). These results suggest that local immunization with la-S. aureus induces primed lymphocyte populations capable of responding to staphylococcal antigens in vitro.
FIG. 1.
In vitro proliferative responses of mammary gland lymph node cells to S. aureus. Cells from regional mammary gland lymph nodes of control (left panels) and immunized mice (right panels) were cultured in the presence or absence of whole heat-killed S. aureus (A) or S. aureus total proteins (B). Lines connect the counts per minute (cpm) measured in the presence or absence of antigen at the individual level. Horizontal short lines indicate the median cpm value obtained for each group. P values were obtained by using the Wilcoxon signed-rank test for paired samples by comparing the median cpm measured after stimulation with S. aureus or staphylococcal proteins versus the median cpm measured in medium (✽, P < 0.01; ✽✽, P < 0.001). One representative experiment of three is shown.
Cytokine production by lymphocytes stimulated with S. aureus in vitro.
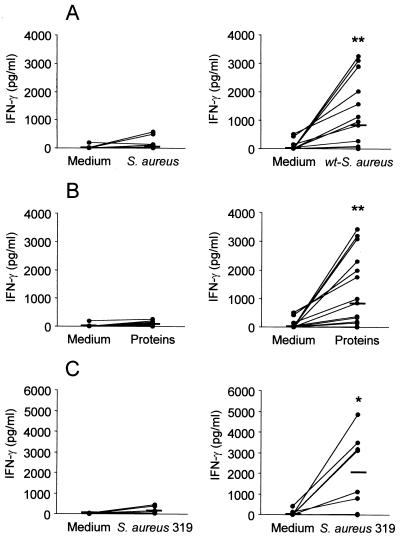
The levels of IFN-γ and IL-4 produced by lymphocytes from immunized mice in response to S. aureus were investigated. Mononuclear cells from mammary gland regional lymph nodes from control and immunized mice were stimulated with either heat-killed wt-S. aureus or total S. aureus proteins. A significant increase in the levels of IFN-γ produced by lymphocytes from immunized mice after stimulation with heat-killed S. aureus (Fig. 2A, right) or total S. aureus proteins (Fig. 2B, right) (P < 0.001) was observed when compared with control mice (Fig. 2A and B, left panels). Similar results were obtained when lymphocytes from immunized mice were stimulated with a heat-killed S. aureus strain of bovine mastitis origin (Fig. 2C). No IL-4 production was detected during the in vitro response to S. aureus (whole heat-killed bacteria or total proteins [data not shown]).
FIG. 2.
Effect of Ima immunization on cytokine production in response to S. aureus. Mononuclear cells from mammary gland lymph nodes of control mice (left panels) and immunized mice (right panels) were cultured in the presence of medium alone, heat-killed wt-S. aureus (A), total S. aureus proteins (B), or heat-killed S. aureus of bovine origin (strain 319) (C). Lines connect IFN-γ levels measured in the presence or absence of antigen at the individual level. Horizontal short lines indicate the median values obtained for each group. P values were determined by using the Wilcoxon signed-rank test for paired samples in order to compare median IFN-γ levels measured after stimulation with S. aureus or staphylococcal proteins versus the median IFN-γ levels measured in medium (✽, P < 0.01; ✽✽, P < 0.001). One representative experiment of three is shown.
Lymphocyte subpopulations responding during in vitro stimulation with S. aureus.
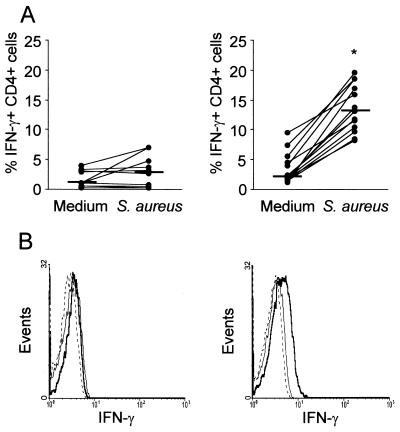
Similar percentages (relative proportion) of CD4+ and CD8+ T cells were found before and after S. aureus stimulation. A significant increase (P < 0.01) in the percentage of CD4+ T cells producing IFN-γ was detected when lymphocytes from immunized mice were cultured in the presence of whole heat-killed S. aureus (Fig. 3A and B, right panels). Moreover, S. aureus-stimulated CD4+ T cells from immunized mice carried a significantly higher number of IFN-γ molecules per cell compared to nonstimulated cells, as evidenced by analysis of the MFI (ΔMFI with medium alone, 1.03 [range, 0.5 to 1.47]; ΔMFI with S. aureus, 1.5 [range, 1.2 to 2.3]; P < 0.02). No differences were found in the number of IFN-γ molecules per cell when mononuclear cells from control mice were stimulated with S. aureus (ΔMFI with medium, 0.7 [range, 0.6 to 0.8]; ΔMFI with S. aureus, 0.8 [range, 0.4 to 0.9]; P > 0.5). No production of IFN-γ by CD8+ T cells was observed (data not shown). No preferential expansion of γδ T cells from control or immunized mice was observed (control medium [1.0%; range, 0.1 to 4.0%] versus control S. aureus [0.6%; range, 0.0 to 5.0%] [P > 0.5] and immunized medium [1.4%; range, 0.4 to 3.2%] versus immunized S. aureus [0.7%; range, 0.0 to 9.3%] [P > 0.5]). Moreover, no subsequent expansion of γδ T cells was detected when cells were cultured in the presence of S. aureus for 6 to 8 days. In additional control experiments a considerable expansion of γδ T cells from mice immunized by Ima route with BCG was observed after in vitro stimulation with PPD (medium, positive-stained cells, 7.0% [range, 6.8 to 12.0%]; PPD, positive-stained cells, 30.0% [range, 20.0 to 35.0%]). There was also no differential expansion of natural killer (NK) cells (either CD3+ or CD3−) after in vitro stimulation with S. aureus (control medium [1.5%; range, 0.7 to 2.0%] versus control S. aureus [3.4%; range, 1.0 to 5.9%] [P > 0.5] and immunized medium [3.0%; range, 0.0 to 4.3%] versus immunized S. aureus [6.9%; range, 6.0 to 6.7%] [P > 0.5]). The results of our experiments suggest that the CD4+ T cells constitute the main lymphocyte subpopulation involved during in vitro secondary responses to S. aureus induced by local immunization with the live attenuated mutant.
FIG. 3.
Intracellular IFN-γ expression by CD4+ T cells in response to S. aureus. Mononuclear cells from mammary gland lymph nodes of control mice (left panels) and immunized mice (right panels) were cultured in the presence of medium alone or heat-killed S. aureus for 72 h, and double-color flow cytometry for CD4 and IFN-γ was performed. Lymphoblasts were gated based on their forward-scatter-side-scatter profile and gated further based on CD4 expression. (A) Horizontal short lines depict the median values obtained for each group. P values were determined by using the Wilcoxon Signed Rank test for paired samples to compare the percentage of CD4+ T cells producing IFN-γ after stimulation with S. aureus versus medium alone (✽, P < 0.01). One representative experiment of three is shown. (B) Histograms show the intracellular IFN-γ production by CD4+ T cells from one representative control mouse (left) and one immunized mouse (right). Histogram curves: thick lines, cells stimulated with S. aureus; thin lines, cells cultured in medium; dotted lines, cells stained with irrelevant, isotype-matched antibodies.
Effect of Ima immunization on mammary lymphocyte responses during S. aureus infection.
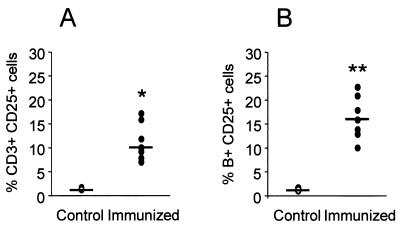
Mice were immunized by the Ima route and, by day 10 after the second inoculation, mice were challenged by the Ima route with wt-S. aureus. Regional mammary lymph nodes were obtained at days 0, 2, and 6 postchallenge, and lymphocyte subpopulations were characterized by flow cytometry. The total number of mononuclear cells in mammary lymph nodes from immunized mice was significantly higher than that in control mice (5.25 × 106 versus 1.99 × 106, respectively; P < 0.05) on the day of the challenge. At that time a significant increase in the relative percentage of B lymphocytes was also detected in mammary gland lymph nodes from immunized mice (16.0% [range, 7.4 to 24.0%]) compared to control mice (1.3% [range, 1.0 to 2.2%]) (P < 0.01). Moreover, all B cells and 10% of CD3+ T cells present in lymph nodes of immunized mice expressed the IL-2 receptor, which indicates that these cells were activated (Fig. 4). The CD4/CD8 ratio in control and immunized mice did not differ significantly (control, 6.1 [range, 5.3 to 7.4]; immunized, 7.3 [range, 5.8 to 8.4]). By the day of the challenge, there were no significant differences in the percentages of either γδ T cells or NK cells among the different groups investigated (data not shown). The total number of mononuclear cells increased significantly thereafter in both groups of challenged mice (immunized and nonimmunized) compared to nonchallenged control mice. Significant changes in the relative percentages of CD8+ T cells were observed after S. aureus-Ima challenge in both immunized and control mice between the day of the challenge (control, 10.1% [range, 6.2 to 17.0%]; immunized, 12.2% [range, 7.1 to 16.0%]) and day 6 after challenge (control, 29.3% [range, 8.1 to 36.2%]; immunized, 20.4% [range, 11.2 to 32.1%]) (P < 0.05). Concomitant changes in the relative percentage of CD4+ T cells were observed after S. aureus-Ima challenge (data not shown). These changes, however, were observed in control, nonchallenged mice during lactation. No significant changes in the relative percentages of γδ T or NK cells from mammary lymph nodes were observed in the host response to S. aureus mammary gland infection.
FIG. 4.
IL-2 receptor (CD25) expression in T (A) and B (B) lymphocytes from regional mammary gland lymph nodes after Ima immunization with la-S. aureus. Open circles represent cells from control mice, and solid circles represent cells from immunized mice. Horizontal short lines indicate the median percentage of positive-stained cells for each group. Significance: ✽, P < 0.05; ✽✽, P < 0.01 (Mann-Whitney test).
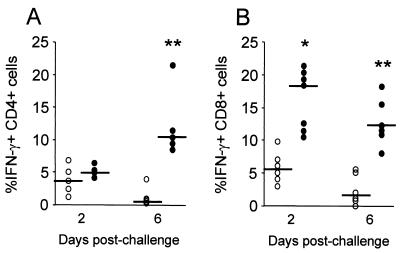
To investigate the effect of local immunization on the mammary T-cell responses involved in eradication of S. aureus during infection, IFN-γ production by regional CD4+ and CD8+ T cells was determined. Mononuclear cells from mammary lymph nodes of S. aureus-challenged, immunized and control mice were obtained by days 2 and 6 after challenge, and the production of IFN-γ by CD4+ and CD8+ T cells was analyzed at the single-cell level. Although the relative percentage of CD4+ and CD8+ T cells did not differ between S. aureus-challenged control and immunized mice, only cells from immunized mice were able to produce IFN-γ. Indeed, by day 6 after S. aureus Ima challenge, a significant increase in the percentage of IFN-γ-producing CD4+ and CD8+ T cells was observed among lymphocytes from immunized mice compared to those from control mice (Fig. 5; P < 0.01). Moreover, IFN-γ production by CD8+ T cells from immunized mice was detected as early as 2 days after S. aureus Ima challenge (Fig. 5B; P < 0.05). Since it occurred after in vitro stimulation, not only was a significant increase in the percentage of cells producing IFN-γ in response to S. aureus infection observed but also a significant increase in the ΔMFI measured in CD4+ and CD8+ T cells from immunized mice compared to control mice (data not shown). These results suggest that both CD4+ and CD8+ T cells are involved in responses elicited by S. aureus during experimental Ima infection.
FIG. 5.
Intracellular IFN-γ expression by CD4+ and CD8+ T cells during in vivo infection with S. aureus. Mononuclear cells from mammary gland lymph nodes of S. aureus-challenged control (○) and immunized (•) mice were obtained and triple-color flow cytometry for CD4, CD8, and IFN-γ was performed. Lymphoblasts were gated based on their forward-scatter-side-scatter profile and gated further based on CD4 (A) or CD8 (B) expression. Horizontal short lines show the median values obtained for each group. P values were determined by using the Mann-Whitney test to compare the percentage of CD4+ or CD8+ T cells producing IFN-γ in each group (✽, P < 0.05; ✽✽, P < 0.01). One representative experiment of three is shown.
DISCUSSION
In a broad sense, mastitis vaccines are expected to eliminate chronic Ima infection, prevent the establishment of new infections, and reduce the frequency and severity of clinical disease. One possible explanation for the limited success of previous field trials (8, 35) may lie in the approach utilized to construction of these vaccines. In fact, in most cases empirical criteria for vaccine administration have prevailed over previous systematic acquisition of basic knowledge on the type of immune responses that would be required in the mammary gland to prevent S. aureus Ima infections. It is surprising that, although an increased incidence of mastitis has been associated with impaired phagocyte and lymphocyte function in the mammary gland (15, 27, 32), the contribution of current and potential available S. aureus mastitis vaccines to the enhancement of adaptive cell-mediated immune responses within this gland has not yet been established. The rationale of our current research is that a deeper knowledge on mammary gland immune responses leading to the eradication of intracellular S. aureus is required for the rational design of a vaccine for protecting ruminant hosts from staphylococcal Ima infection.
Current evidence shows that S. aureus superantigens might cause a shift from a protective immune response to one that is not competent to clear intracellular pathogens (3). In this regard, evaluation of lacteal secretions from mammary glands of dairy cows infected with S. aureus revealed a subpopulation of activated CD8+ lymphocytes that are capable of altering or suppressing CD4+-lymphocyte proliferative responses (23). In addition, the low levels of IFN-γ mRNA found in milk samples from S. aureus-infected cows (25), concomitantly with the invasion of mammary epithelial cells and a nonstimulatory entry into macrophages (3), may enable S. aureus to survive in an immunocompetent host. Therefore, eradication of intracellular S. aureus may only be achieved if an appropriate Th1 response is triggered at the site of infection. This study provides evidence that, in a mouse model, Ima immunization with a live attenuated prototypic vaccine strain induces cell-mediated immunity in the mammary gland. Ima immunization induced activation of T lymphocytes in mammary gland lymph nodes, and these cells were able to proliferate and produce IFN-γ upon in vitro stimulation with S. aureus antigens. Production of IFN-γ, a key indicator of the cell-mediated immune response induced by immunization, was not strain dependent, since it was found after stimulation with not only strain 8325-4 but also a S. aureus strain obtained from a cow with mastitis. Activated B lymphocytes were also detected in mammary lymph nodes from immunized mice, a finding which agreed with our previous results (6, 9). The number of mononuclear cells in mammary lymph nodes from both immunized and control mice significantly increased after Ima challenge with wt-S. aureus, but only lymphocytes from immunized mice were able to produce IFN-γ in response to S. aureus infection. In the light of available information, the significance that these findings may have in protection of the ruminant udder from staphylococcal infection merits discussion from different viewpoints.
Previous evidence has pointed out the potential role of IFN-γ in the mammary gland environment to prevent S. aureus Ima infections. The in vitro treatment with IFN-γ can reverse the suppressive effects of mammary gland secretions and increase the functional capabilities of bovine mammary gland phagocytes against S. aureus (30). Moreover, Ima administration of recombinant IFN-γ was also effective in enhancing phagocytic and bactericidal activities of mammary gland neutrophils in vivo (5). Based upon these findings, administration of IFN-γ has been suggested as a possible, albeit very expensive, prophylactic strategy for the prevention of S. aureus mastitis (32). In the present study, we demonstrated the capability of CD4+ T cells from immunized mice to produce IFN-γ upon in vitro stimulation with S. aureus antigens. Moreover, CD4+ T cells from Ima-immunized mice were able to produce IFN-γ after experimental Ima challenge with wt-S. aureus. The relevance of these findings is that immunization by the Ima route with a live attenuated strain induced primed lymphocyte populations which, once confronted with the wild-type pathogen, were able to produce IFN-γ, a cytokine that may play a pivotal role in eradication of intracellular staphylococci throughout phagocytic cell activation. We suggest that the presence of both specific antibodies (9) and active phagocytic cells would prevent the establishment of chronic S. aureus infection in the mammary gland of locally vaccinated hosts.
The diminished cellular activity observed in the bovine mammary gland was attributed to a high percentage of suppressor CD8+ T lymphocytes present in this gland (23). One important finding of the present study is that not only CD4+ but also CD8+ T lymphocytes from Ima-immunized mice were able to produce IFN-γ during staphylococcal experimental infection. The significance of this finding can be analyzed from two viewpoints. On the one hand, IFN-γ produced by CD8+ T lymphocytes may contribute to cytokine-mediated macrophage activation, thus promoting S. aureus clearance by these cells. In this regard, it has been shown that control of M. tuberculosis infection also requires IFN-γ production by CD8+ T cells (33). On the other hand, although further studies are required, IFN-γ production by CD8+ T lymphocytes suggests that Ima immunization with a live attenuated strain can prime cytotoxic T lymphocytes, which may play an important role in eliminating infected mammary epithelial cells.
In addition to CD4+ and CD8+ T cells, T cells bearing the γδ T-cell receptor and NK cells may participate in the control of infection caused by certain intracellular bacteria (1, 18, 19, 22). A potential role for γδ T cells in the bovine immune responses against mastitis-causing bacteria has been hypothesized. This hypothesis was based upon the fact that the percentage of γδ T lymphocytes in the mammary parenchyma decreases significantly during the postpartum period, when susceptibility to Ima infection is increased (15, 26, 27, 32). In our mouse model, we did not observe differential expansion of γδ T cells during in vitro secondary stimulation with S. aureus. Moreover, in the present study, we did not observe differential expansion of γδ T cells during in vivo experimental Ima infection by S. aureus in either immunized or control mice. These results agree with those of Riollet et al., who demonstrated the lack of participation of γδ T cells during early host responses in cows previously immunized with alpha-toxin (24). Furthermore, S. aureus mammary gland infection did not induce significant changes in the percentage of milk γδ T cells in bovines (25). The results obtained in the present study and the experimental evidences available to the present suggest that, if γδ T cells play any role during immune mediated inflammation, it is likely to occur in the intraepithelial milieu. Regarding NK cells, a population of lymphoid cells with NK-like activity and antibacterial properties has been isolated from the bovine mammary gland (31). In our mouse model, there was no differential expansion of NK cells, either from immunized or control mice, after S. aureus stimulation, which suggests that these cells may not be involved in either in vivo or in vitro secondary responses to S. aureus. Further studies in bovines will be required to elucidate whether NK cells play a significant role in protection from S. aureus Ima infection.
In conclusion, the feasibility of using local immunization with a live attenuated strain to induce cell-mediated immunity to S. aureus in the mouse mammary gland was established. Although differences may exist between ruminant and murine mammary glands, our findings may contribute to the future rational design of a vaccine for preventing S. aureus mastitis, since in this study we have shown that Ima immunization can induce immune responses known to be involved in the eradication of intracellular pathogens. Further controlled trials in cows are required to extend the validity of our findings for dairy ruminant vaccination.
Acknowledgments
This study was supported in part by grants from UBACyT Argentina (TM-55 and ME-063), CONICET Argentina (PIP 0944/98), and FONCyT-SEPCyT (PICT-04746) and from the International Foundation for Science, Stockholm, Sweden.
We thank Mónica Saracco and Mariel Bibini for expert technical assistance in flow cytometry determinations.
Editor: V. J. DiRita
REFERENCES
- 1.Bancroft, G. J., R. D. Schreiber, and E. R. Unanue. 1991. Natural immunity: a T-cell-independent pathway of macrophage activation defined in the scid mouse. Immunol. Rev. 124:5-24. [DOI] [PubMed] [Google Scholar]
- 2.Bayles, K. W., C. A. Wesson, L. E. Liou, L. K. Fox, G. A. Bohach, and W. R. Trumble. 1998. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect. Immun. 66:336-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferens, W. A., and G. A. Bohach. 2000. Persistence of Staphylococcus aureus on mucosal membranes: superantigens and internalization by host cells. J. Lab. Clin. Med. 135:225-230. [DOI] [PubMed] [Google Scholar]
- 4.Foster, T. J. 1991. Potential for vaccination against infections caused by Staphylococcus aureus. Vaccine 9:221-227. [DOI] [PubMed] [Google Scholar]
- 5.Fox, L. K., H. D. Liggit, T. Yilma, and L. B. Corbeil. 1990. The effects of interferon intramammary administration on mammary phagocyte function. Zentbl. Veterinarmed. [B] 37:28-30. [DOI] [PubMed] [Google Scholar]
- 6.García, V. E., M. I. Gómez, M. F. Iglesias, N. Sanjuan, M. M. Gherardi, M. C. Cerquetti, and D. O. Sordelli. 1996. Intramammary immunization with live-attenuated Staphylococcus aureus: microbiological and immunological studies in a mouse mastitits model. FEMS Immunol. Med. Microbiol. 14:45-51. [DOI] [PubMed] [Google Scholar]
- 7.García, V. E., K. Uyemura, P. A. Sieling, M. T. Ochoa, C. T. Morita, H. Okamura, M. Kurimoto, T. H. Rea, and R. L. Modlin. 1999. IL-18 promotes type 1 cytokine production from NK cells and T cells in human intracellular infection. J. Immunol. 162:6114-6121. [PubMed] [Google Scholar]
- 8.Giraudo, J. A., A. Calzolari, H. Rampone, A. Rampone, A. T. Giraudo, C. Bogni, A. Larriestra, and R. Nagel. 1997. Field trials of a vaccine against bovine mastitis. 1. Evaluation in heifers. J. Dairy Sci. 80:845-853. [DOI] [PubMed] [Google Scholar]
- 9.Gómez, M. I., V. E. García, M. M. Gherardi, M. C. Cerquetti, and D. O. Sordelli. 1998. Intramammary immunization with live-attenuated Staphylococcus aureus protects mice from experimental mastitis. FEMS Immunol. Med. Microbiol. 20:21-27. [DOI] [PubMed] [Google Scholar]
- 10.Gresham, H. D., J. H. Lowrance, T. E. Caver, B. S. Wilson, A. L. Cheung, and F. P. Lindberg. 2000. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J. Immunol. 164:3713-3722. [DOI] [PubMed] [Google Scholar]
- 11.Hebert, A., K. Sayasith, S. Senechal, P. Dubreuil, and J. Lagace. 2000. Demonstration of intracellular Staphylococcus aureus in bovine mastitis alveolar cells and macrophages isolated from naturally infected cow milk. FEMS Microbiol. Lett. 193:57-62. [DOI] [PubMed] [Google Scholar]
- 12.Hudson, M., N. Ramp, A. Nicholson, A. Williams, and M. Nousisinen. 1995. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb. Pathog. 19:409-419. [DOI] [PubMed] [Google Scholar]
- 13.Inoue, T., Y. Yoshikai, G. Matsuzaki, and K. Nomoto. 1991. Early appearing γδ-bearing T cells during infection with Calmette-Guerin bacillus. J. Immunol. 146:2754-2762. [PubMed] [Google Scholar]
- 14.Kahl, B. C., M. Goulian, W. V. Wamel, M. Herrmann, S. M. Simon, G. Kaplan, G. Peters, and A. L. Cheung. 2000. Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infect. Immun. 68:5385-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura, K., J. P. Goff, M. E. Kehrli, Jr., and J. A. Harp. 1999. Phenotype analysis of peripheral blood mononuclear cells in periparturient dairy cows. J. Dairy Sci. 82:315-319. [DOI] [PubMed] [Google Scholar]
- 16.Lee, J. C., and G. B. Pier. 1997. Vaccine-based strategies for prevention of staphylococcal diseases, p. 631-654. In K. B. Crossley and G. L. Archer (ed.), The staphylococci in human disease. Churchill-Livingstone, New York, N.Y.
- 17.Lowy, F. D. 2000. Is Staphylococcus aureus an intracellular pathogen? Trends Microbiol. 8:341-343. [DOI] [PubMed] [Google Scholar]
- 18.Mombaerts, P., J. Arnoldi, F. Russ, S. Tonegawa, and S. H. E. Kaufmann. 1993. Different roles of α/β and γ/δ T cells in immunity against an intracellular bacterial pathogen. Nature 365:53-56. [DOI] [PubMed] [Google Scholar]
- 19.Muller, I., S. P. Cobbold, H. Waldmann, and S. H. E. Kaufmann. 1987. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect. Immun. 55:2037-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nordhaug, M. L., L. L. Nesse, N. L. Norcross, and R. Gudding. 1994. A field trial with an experimental vaccine against Staphylococcus aureus mastitis in cattle. 1. Clinical parameters. J. Dairy Sci. 77:1267-1275. [DOI] [PubMed] [Google Scholar]
- 21.Nordhaug, M. L., L. L. Nesse, N. L. Norcross, and R. Gudding. 1994. A field trial with an experimental vaccine against Staphylococcus aureus mastitis in cattle. 2. Antibody response. J. Dairy Sci. 77:1276-1284. [DOI] [PubMed] [Google Scholar]
- 22.Orme, I. M. 1987. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J. Immunol. 138:293-298. [PubMed] [Google Scholar]
- 23.Park, Y. H., L. K. Fox, M. J. Hamilton, and W. C. Davis. 1993. Suppression of proliferative response of BoCD4+ T lymphocytes by activated BoCD8+ T lymphocytes in the mammary gland of cows with Staphylococcus aureus mastitis. Vet. Immunol. Immunopathol. 36:137-151. [DOI] [PubMed] [Google Scholar]
- 24.Riollet, C., P. Rainard, and B. Poutrel. 2000. Kinetics of cells and cytokines during immune-mediated inflammation in the mammary gland of cows systemically immunized with Staphylococcus aureus α-toxin. Inflamm. Res. 49:486-496. [DOI] [PubMed] [Google Scholar]
- 25.Riollet, C., P. Rainard, and B. Poutrel. 2001. Cell subpopulations and cytokine expression in cow milk in response to chronic Staphylococcus aureus infection. J. Dairy Sci. 84:1077-1084. [DOI] [PubMed] [Google Scholar]
- 26.Shafer-Weaver, K. A., and L. M. Sordillo. 1996. Enhancing bactericidal activity of bovine lymphoid cells during the perparturient period. J. Dairy Sci. 79:1347-1352. [DOI] [PubMed] [Google Scholar]
- 27.Shafer-Weaver, K. A., G. M. Pighetti, and L. M. Sordillo. 1996. Diminished mammary gland lymphocyte functions parallel shifts in trafficking patterns during the postpartum period. Proc. Soc. Exp. Biol. Med. 212:271-279. [DOI] [PubMed] [Google Scholar]
- 28.Shoshani, E., G. Leitner, B. Hanochi, A. Saran, N. Y. Shpigel, and A. Berman. 2000. Mammary infection with Staphylococcus aureus in cows: progress from inoculation to chronic infection and its detection. J. Dairy Res. 67:155-169. [DOI] [PubMed] [Google Scholar]
- 29.Sordelli, D. O., M. F. Iglesias, M. C. Cerquetti, M. Catalano, and A. Morris-Hooke. 1993. Temperature-sensitive mutants of Staphylococcus aureus: isolation and preliminary characterization. Curr. Microbiol. 27:125-129. [DOI] [PubMed] [Google Scholar]
- 30.Sordillo, L. M., and L. A. Babiuk. 1991. Modulation of mammary neutrophil function during the periparturient period following in vivo exposure to recombinant interferon-gamma. Vet. Immunol. Immunopathol. 27:393-402. [DOI] [PubMed] [Google Scholar]
- 31.Sordillo, L. M., M. Y. Campos, and L. A. Babiuk. 1991. Antibacterial activity of bovine mammary gland lymphocytes following treatment with interleukin-2. J. Dairy. Sci. 74:3370-3375. [DOI] [PubMed] [Google Scholar]
- 32.Sordillo, L. M., K. Shafer-Weaver, and D. DeRosa. 1997. Immunobiology of the mammary gland. J. Dairy Sci. 80:1851-1865. [DOI] [PubMed] [Google Scholar]
- 33.Tascon, R. E., E. Stavropoulos, K. V. Lukacs, and M. J. Colston. 1998. Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect. Immun. 66:830-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.U.S. Department of Health and Human Services. 1985. Guide for the care and use of laboratory animals. NIH publication 86-23 Public Health Service, National Institutes of Health, Bethesda, Md.
- 35.Watson, D. L., M. L. McColl, and H. I. Davis. 1996. Field trial of a staphylococcal mastitis vaccine in dairy herds: clinical, subclinical and microbiological assessments. Aust. Vet. J. 74:447-450. [DOI] [PubMed] [Google Scholar]
- 36.Yancey, R. J., Jr., M. S. Sanchez, and C. W. Ford. 1991. Activity of antibiotics against Staphylococcus aureus within polymorphonuclear neutrophils. Eur. J. Clin. Microbiol. Infect. Dis. 10:107-113. [DOI] [PubMed] [Google Scholar]
- 37.Yancey, R. J., Jr. 1999. Vaccines and diagnostic methods for bovine mastitis: fact and fiction. Adv. Vet. Med. 41:257-273. [DOI] [PubMed] [Google Scholar]
- 38.Yao, L., V. Bengualid, F. D. Lowy, J. J. Gibbons, V. B. Hatcher, and J. W. Berman. 1995. Internalization of Staphylococcus aureus by endothelial cells induces cytokine gene expression. Infect. Immun. 63:1835-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]