Abstract
Purple membranes adsorbed to mica were imaged in buffer solution using the atomic force microscope. The hexagonal diffraction patterns of topographs from the cytoplasmic and the extracellular surface showed a resolution of 0.7 and 1.2 nm, respectively. On the cytoplasmic surface, individual bacteriorhodopsin molecules consistently exhibited a distinct substructure. Depending on the pH value of the buffer solution, the height of the purple membranes decreased from 5.6 nm (pH 10.5) to 5.1 nm (pH 4). The results are discussed with respect to the structure determined by cryo-electron microscopy.
Full text
PDF


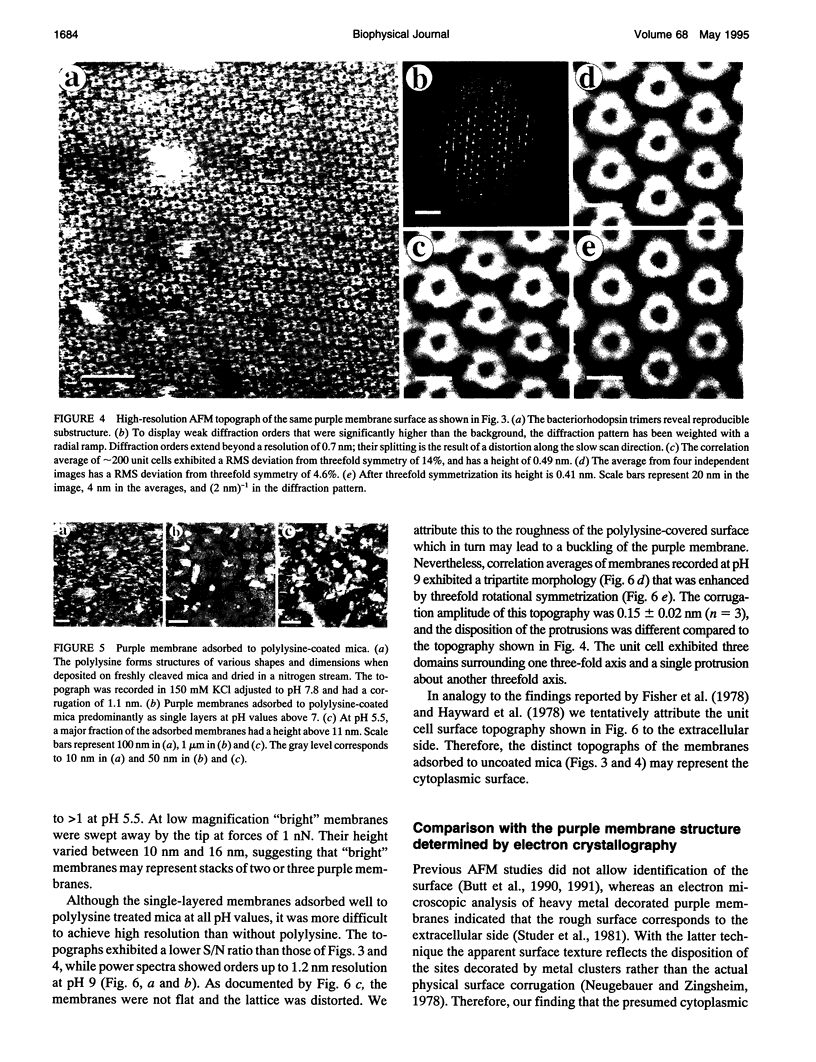
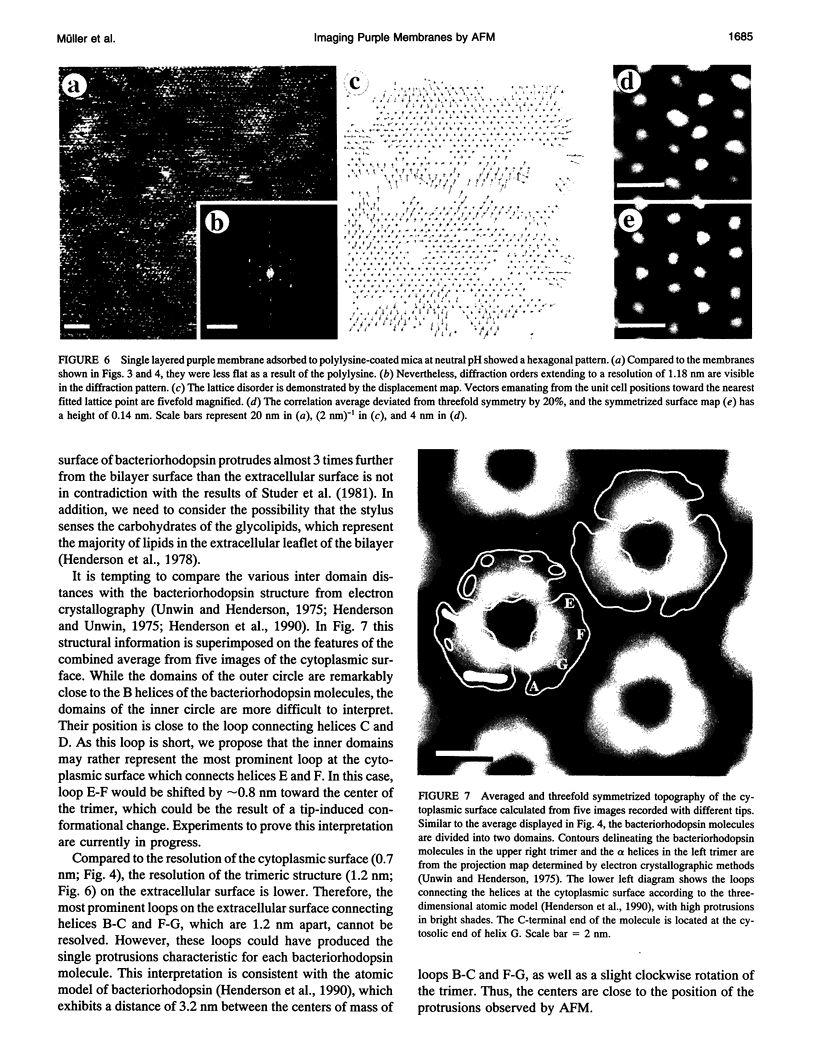

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J. M. The probable arrangement of the helices in G protein-coupled receptors. EMBO J. 1993 Apr;12(4):1693–1703. doi: 10.1002/j.1460-2075.1993.tb05814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986 Mar 3;56(9):930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E., Stoeckenius W. Structure of the purple membrane. Nat New Biol. 1971 Sep 29;233(39):152–155. doi: 10.1038/newbio233152a0. [DOI] [PubMed] [Google Scholar]
- Butt H. J., Downing K. H., Hansma P. K. Imaging the membrane protein bacteriorhodopsin with the atomic force microscope. Biophys J. 1990 Dec;58(6):1473–1480. doi: 10.1016/S0006-3495(90)82492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake B., Prater C. B., Weisenhorn A. L., Gould S. A., Albrecht T. R., Quate C. F., Cannell D. S., Hansma H. G., Hansma P. K. Imaging crystals, polymers, and processes in water with the atomic force microscope. Science. 1989 Mar 24;243(4898):1586–1589. doi: 10.1126/science.2928794. [DOI] [PubMed] [Google Scholar]
- Fisher K. A., Yanagimoto K., Stoeckenius W. Oriented adsorption of purple membrane to cationic surfaces. J Cell Biol. 1978 May;77(2):611–621. doi: 10.1083/jcb.77.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelka W. A., Henderson R., Heymann J. A., Oesterhelt D. Projection structure of halorhodopsin from Halobacterium halobium at 6 A resolution obtained by electron cryo-microscopy. J Mol Biol. 1993 Dec 5;234(3):837–846. doi: 10.1006/jmbi.1993.1629. [DOI] [PubMed] [Google Scholar]
- Hayward S. B., Grano D. A., Glaeser R. M., Fisher K. A. Molecular orientation of bacteriorhodopsin within the purple membrane of Halobacterium halobium. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4320–4324. doi: 10.1073/pnas.75.9.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Henderson R., Jubb J. S., Whytock S. Specific labelling of the protein and lipid on the extracellular surface of purple membrane. J Mol Biol. 1978 Aug 5;123(2):259–274. doi: 10.1016/0022-2836(78)90325-x. [DOI] [PubMed] [Google Scholar]
- Henderson R. The structure of the purple membrane from Halobacterium hallobium: analysis of the X-ray diffraction pattern. J Mol Biol. 1975 Apr 5;93(2):123–138. doi: 10.1016/0022-2836(75)90123-0. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Hoh J. H., Sosinsky G. E., Revel J. P., Hansma P. K. Structure of the extracellular surface of the gap junction by atomic force microscopy. Biophys J. 1993 Jul;65(1):149–163. doi: 10.1016/S0006-3495(93)81074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungblut H., Campbell S. A., Giersig M., Müller D. J., Lewerenz H. J. Scanning tunnelling microscopy observations of biomolecules on layered materials. Faraday Discuss. 1992;(94):183–197. doi: 10.1039/fd9929400183. [DOI] [PubMed] [Google Scholar]
- Karrasch S., Hegerl R., Hoh J. H., Baumeister W., Engel A. Atomic force microscopy produces faithful high-resolution images of protein surfaces in an aqueous environment. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):836–838. doi: 10.1073/pnas.91.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer D. C., Zingsheim H. P. The two faces of the purple membrane. Structural differences revealed by metal decoration. J Mol Biol. 1978 Aug 5;123(2):235–246. doi: 10.1016/0022-2836(78)90323-6. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Ross P. E., Helgerson S. L., Miercke L. J., Dratz E. A. Isoelectric focusing studies of bacteriorhodopsin. Biochim Biophys Acta. 1989 Apr 25;991(1):134–140. doi: 10.1016/0304-4165(89)90038-x. [DOI] [PubMed] [Google Scholar]
- Schabert F. A., Engel A. Reproducible acquisition of Escherichia coli porin surface topographs by atomic force microscopy. Biophys J. 1994 Dec;67(6):2394–2403. doi: 10.1016/S0006-3495(94)80726-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schertler G. F., Villa C., Henderson R. Projection structure of rhodopsin. Nature. 1993 Apr 22;362(6422):770–772. doi: 10.1038/362770a0. [DOI] [PubMed] [Google Scholar]
- Studer D., Moor H., Gross H. Single bacteriorhodopsin molecules revealed on both surfaces of freeze-dried and heavy metal-decorated purple membranes. J Cell Biol. 1981 Jul;90(1):153–159. doi: 10.1083/jcb.90.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin P. N., Henderson R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J Mol Biol. 1975 May 25;94(3):425–440. doi: 10.1016/0022-2836(75)90212-0. [DOI] [PubMed] [Google Scholar]
- Worcester D. L., Miller R. G., Bryant P. J. Atomic force microscopy of purple membranes. J Microsc. 1988 Dec;152(Pt 3):817–821. doi: 10.1111/j.1365-2818.1988.tb01454.x. [DOI] [PubMed] [Google Scholar]
- Yang J., Mou J., Shao Z. Molecular resolution atomic force microscopy of soluble proteins in solution. Biochim Biophys Acta. 1994 Mar 2;1199(2):105–114. doi: 10.1016/0304-4165(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Yang J., Mou J., Shao Z. Structure and stability of pertussis toxin studied by in situ atomic force microscopy. FEBS Lett. 1994 Jan 24;338(1):89–92. doi: 10.1016/0014-5793(94)80122-3. [DOI] [PubMed] [Google Scholar]
- Yang J., Tamm L. K., Tillack T. W., Shao Z. New approach for atomic force microscopy of membrane proteins. The imaging of cholera toxin. J Mol Biol. 1993 Jan 20;229(2):286–290. doi: 10.1006/jmbi.1993.1033. [DOI] [PubMed] [Google Scholar]








