Abstract
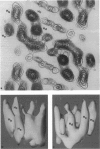
The visual pigment rhodopsin is a member of the G protein-coupled receptor family. Electron cryo-microscopy was used to determine the three-dimensional structure of bovine rhodopsin from tilted two-dimensional crystals embedded in vitrified water. The effective resolution in a map obtained from the 23 best crystals was about 9.5 A horizontally and approximately 47 A normal to the plane of the membrane. Four clearly resolved tracks of density in the map correspond to four alpha-helices oriented nearly perpendicular to the plane of the membrane. One of these helices appears to be more tilted than anticipated from the projection structure published previously. The remaining three helices are presumably more highly tilted, given that they form a continuous "arc-shaped" feature and could not be resolved to the same extent. The overall density distribution in the low resolution map shows an arrangement of the helices in which the "arc-shaped" feature is extended by a fourth, less tilted helix. The band of these four tilted helices is flanked by a straight helix on the outer side and a pair of straight helices on its inner side.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agard D. A. A least-squares method for determining structure factors in three-dimensional tilted-view reconstructions. J Mol Biol. 1983 Jul 15;167(4):849–852. doi: 10.1016/s0022-2836(83)80114-4. [DOI] [PubMed] [Google Scholar]
- Baldwin J. M., Henderson R., Beckman E., Zemlin F. Images of purple membrane at 2.8 A resolution obtained by cryo-electron microscopy. J Mol Biol. 1988 Aug 5;202(3):585–591. doi: 10.1016/0022-2836(88)90288-4. [DOI] [PubMed] [Google Scholar]
- Baldwin J. M. The probable arrangement of the helices in G protein-coupled receptors. EMBO J. 1993 Apr;12(4):1693–1703. doi: 10.1002/j.1460-2075.1993.tb05814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabre M. Diamagnetic anisotropy and orientation of alpha helix in frog rhodopsin and meta II intermediate. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5471–5474. doi: 10.1073/pnas.75.11.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Grip W. J. Purification of bovine rhodopsin over concanavalin A--sepharose. Methods Enzymol. 1982;81:197–207. doi: 10.1016/s0076-6879(82)81032-x. [DOI] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Hargrave P. A., McDowell J. H., Curtis D. R., Wang J. K., Juszczak E., Fong S. L., Rao J. K., Argos P. The structure of bovine rhodopsin. Biophys Struct Mech. 1983;9(4):235–244. doi: 10.1007/BF00535659. [DOI] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Henderson R. Image contrast in high-resolution electron microscopy of biological macromolecules: TMV in ice. Ultramicroscopy. 1992 Oct;46(1-4):1–18. doi: 10.1016/0304-3991(92)90003-3. [DOI] [PubMed] [Google Scholar]
- Kühlbrandt W., Wang D. N., Fujiyoshi Y. Atomic model of plant light-harvesting complex by electron crystallography. Nature. 1994 Feb 17;367(6464):614–621. doi: 10.1038/367614a0. [DOI] [PubMed] [Google Scholar]
- Lamba O. P., Borchman D., O'Brien P. J. Fourier transform infrared study of the rod outer segment disk and plasma membranes of vertebrate retina. Biochemistry. 1994 Feb 22;33(7):1704–1712. doi: 10.1021/bi00173a012. [DOI] [PubMed] [Google Scholar]
- Michel-Villaz M., Saibil H. R., Chabre M. Orientation of rhodopsin alpha-helices in in retinal rod outer segment membranes studied by infrared linear dichroism. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4405–4408. doi: 10.1073/pnas.76.9.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saibil H., Chabre M., Worcester D. Neutron diffraction studies of retinal rod outer segment membranes. Nature. 1976 Jul 22;262(5566):266–270. doi: 10.1038/262266a0. [DOI] [PubMed] [Google Scholar]
- Sakmar T. P., Franke R. R., Khorana H. G. Glutamic acid-113 serves as the retinylidene Schiff base counterion in bovine rhodopsin. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8309–8313. doi: 10.1073/pnas.86.21.8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schertler G. F., Villa C., Henderson R. Projection structure of rhodopsin. Nature. 1993 Apr 22;362(6422):770–772. doi: 10.1038/362770a0. [DOI] [PubMed] [Google Scholar]
- Shichi H., Shelton E. Assessment of physiological integrity of sonicated retinal rod membranes. J Supramol Struct. 1974;2(1):7–16. doi: 10.1002/jss.400020103. [DOI] [PubMed] [Google Scholar]
- Toyoshima C., Sasabe H., Stokes D. L. Three-dimensional cryo-electron microscopy of the calcium ion pump in the sarcoplasmic reticulum membrane. Nature. 1993 Apr 1;362(6419):467–471. doi: 10.1038/362469a0. [DOI] [PubMed] [Google Scholar]
- Unwin N. Acetylcholine receptor channel imaged in the open state. Nature. 1995 Jan 5;373(6509):37–43. doi: 10.1038/373037a0. [DOI] [PubMed] [Google Scholar]
- Unwin N. Nicotinic acetylcholine receptor at 9 A resolution. J Mol Biol. 1993 Feb 20;229(4):1101–1124. doi: 10.1006/jmbi.1993.1107. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Henderson R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J Mol Biol. 1975 May 25;94(3):425–440. doi: 10.1016/0022-2836(75)90212-0. [DOI] [PubMed] [Google Scholar]
- Zhukovsky E. A., Oprian D. D. Effect of carboxylic acid side chains on the absorption maximum of visual pigments. Science. 1989 Nov 17;246(4932):928–930. doi: 10.1126/science.2573154. [DOI] [PubMed] [Google Scholar]