Abstract
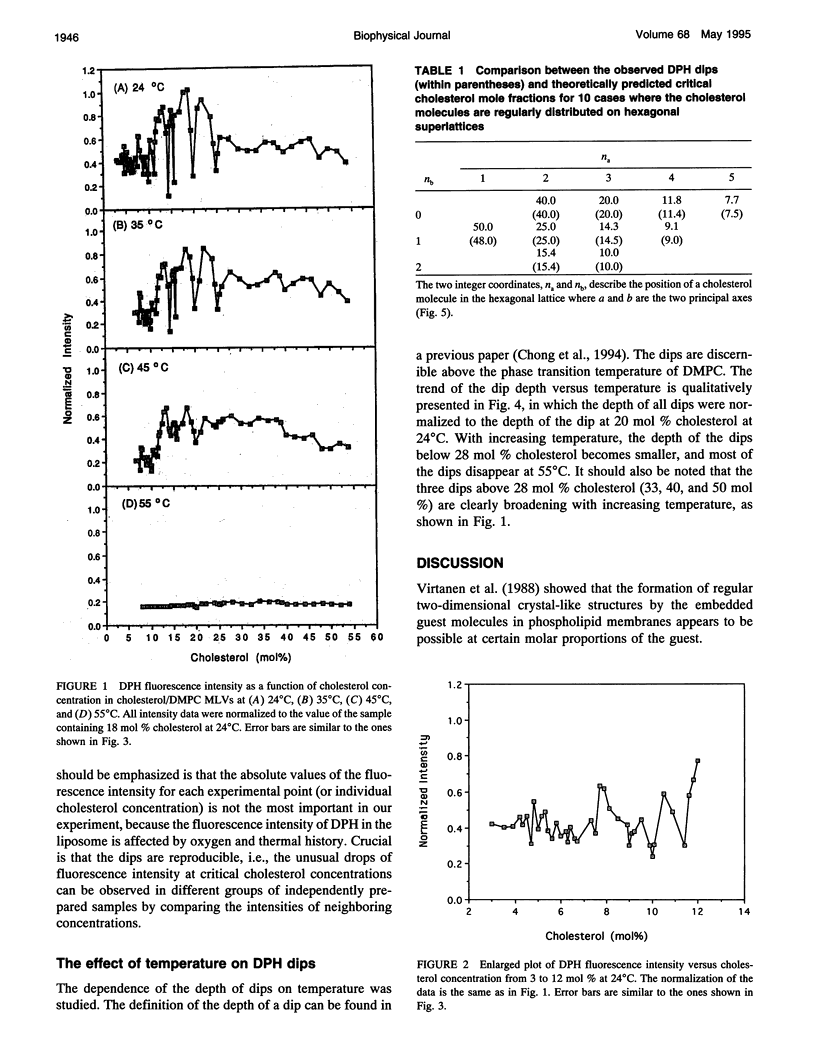
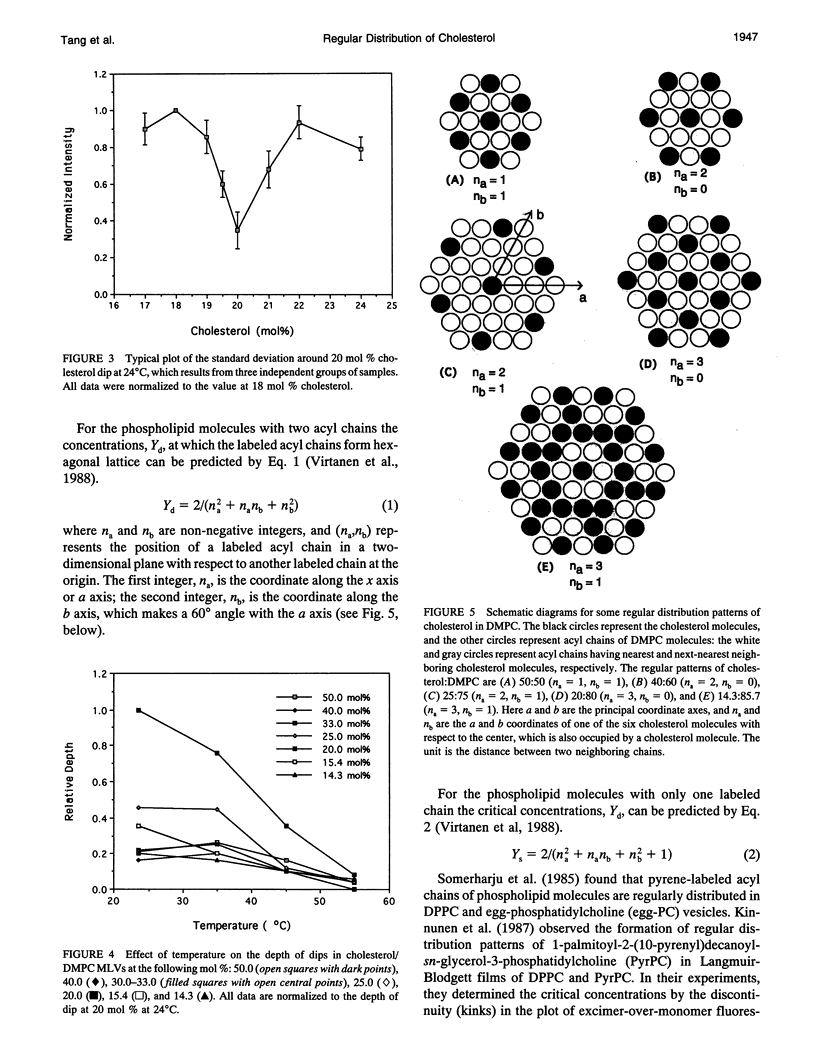
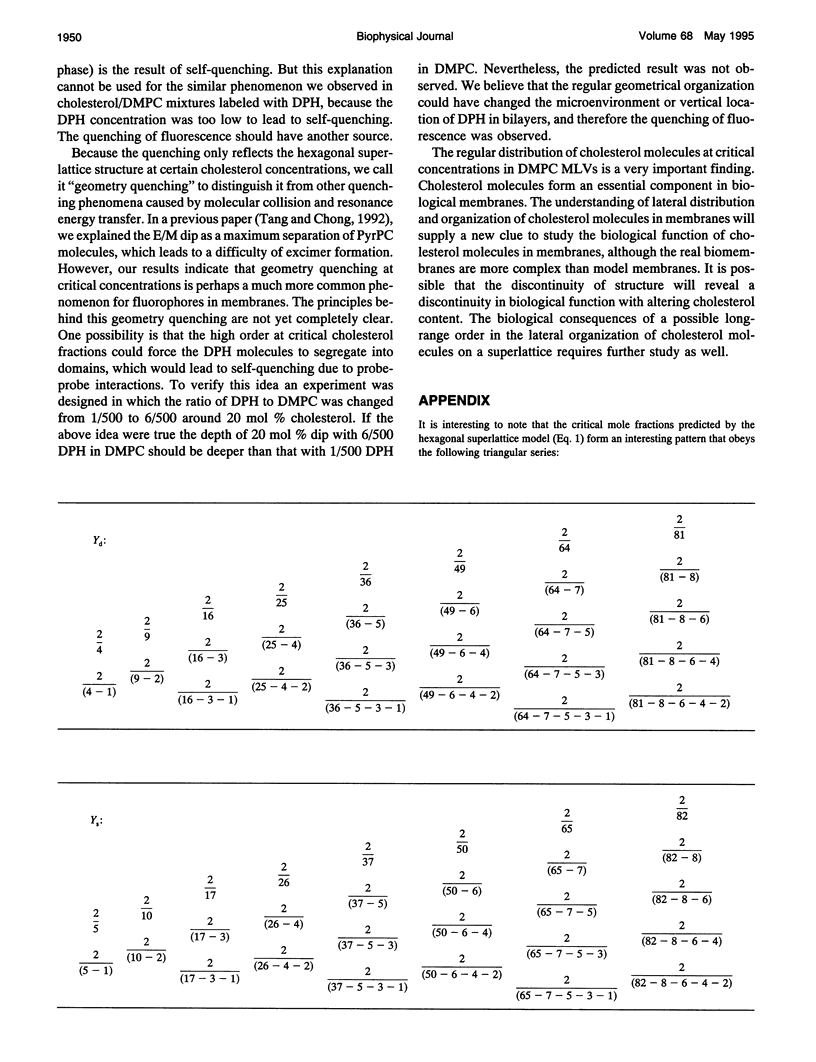
Cholesterol/dimyristoylphosphatidylcholine (DMPC) multilamellar vesicles were studied by steady-state fluorescence using diphenylhexatriene (DPH) as a probe. A series of dips were found in the plot of DPH fluorescence intensity versus cholesterol concentration at certain specific cholesterol concentrations. This observation indicates that there are dominant domains in which cholesterol molecules are regularly distributed on a hexagonal superlattice in the acyl chain matrix of DMPC at critical cholesterol concentrations. These concentrations can be predicted by an equation or a mathematical series, except the one at 33 mol %. These dips of DPH fluorescence intensity are temperature dependent. The excellent agreement between experimental data and calculated values as well as similar previous findings of dips and/or kinks in the excimer-over-monomer fluorescence in pyrenephosphatidylcholine/phospholipid mixtures confirm our conclusion about lateral organizations of cholesterol and acyl lipid chains in cholesterol/phospholipid multilamellar vesicles. The regular distribution model at critical concentration is consistent with the phase diagram of cholesterol/DMPC. Using the model of regular distribution, the physical origin of the liquid-disordered (Ld) phase, liquid-ordered phase (Lo), and coexistence of liquid-disordered phase and Lo phase (Lo + Ld) is discussed on the molecular level.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida P. F., Vaz W. L., Thompson T. E. Lateral diffusion in the liquid phases of dimyristoylphosphatidylcholine/cholesterol lipid bilayers: a free volume analysis. Biochemistry. 1992 Jul 28;31(29):6739–6747. doi: 10.1021/bi00144a013. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Chong P. L. Evidence for regular distribution of sterols in liquid crystalline phosphatidylcholine bilayers. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10069–10073. doi: 10.1073/pnas.91.21.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong P. L., Tang D., Sugar I. P. Exploration of physical principles underlying lipid regular distribution: effects of pressure, temperature, and radius of curvature on E/M dips in pyrene-labeled PC/DMPC binary mixtures. Biophys J. 1994 Jun;66(6):2029–2038. doi: 10.1016/S0006-3495(94)80996-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland B. R., McConnel H. M. The rippled structure in bilayer membranes of phosphatidylcholine and binary mixtures of phosphatidylcholine and cholesterol. Biochim Biophys Acta. 1980 Jun 20;599(1):95–109. doi: 10.1016/0005-2736(80)90059-0. [DOI] [PubMed] [Google Scholar]
- Cruzeiro-Hansson L., Ipsen J. H., Mouritsen O. G. Intrinsic molecules in lipid membranes change the lipid-domain interfacial area: cholesterol at domain interfaces. Biochim Biophys Acta. 1989 Feb 27;979(2):166–176. doi: 10.1016/0005-2736(89)90432-x. [DOI] [PubMed] [Google Scholar]
- Darke A., Finer E. G., Flook A. G., Phillips M. C. Nuclear magnetic resonance study of lecithin-cholesterol interactions. J Mol Biol. 1972 Jan 28;63(2):265–279. doi: 10.1016/0022-2836(72)90374-9. [DOI] [PubMed] [Google Scholar]
- De Kruyff B., Van Dijck P. W., Demel R. A., Schuijff A., Brants F., Van Deenen L. L. Non-random distribution of cholesterol in phosphatidylcholine bilayers. Biochim Biophys Acta. 1974 Jul 12;356(1):1–7. doi: 10.1016/0005-2736(74)90288-0. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Rothman J. E. The planar organization of lecithin-cholesterol bilayers. J Biol Chem. 1972 Jun 10;247(11):3694–3697. [PubMed] [Google Scholar]
- Estep T. N., Mountcastle D. B., Biltonen R. L., Thompson T. E. Studies on the anomalous thermotropic behavior of aqueous dispersions of dipalmitoylphosphatidylcholine-cholesterol mixtures. Biochemistry. 1978 May 16;17(10):1984–1989. doi: 10.1021/bi00603a029. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Sturtevant J. M. Calorimetric investigation of the influence of cholesterol on the transition properties of bilayers formed from synthetic L- -lecithins in aqueous suspension. J Biol Chem. 1972 Jun 10;247(11):3697–3700. [PubMed] [Google Scholar]
- Hui S. W., He N. B. Molecular organization in cholesterol-lecithin bilayers by X-ray and electron diffraction measurements. Biochemistry. 1983 Mar 1;22(5):1159–1164. doi: 10.1021/bi00274a026. [DOI] [PubMed] [Google Scholar]
- Hyslop P. A., Morel B., Sauerheber R. D. Organization and interaction of cholesterol and phosphatidylcholine in model bilayer membranes. Biochemistry. 1990 Jan 30;29(4):1025–1038. doi: 10.1021/bi00456a027. [DOI] [PubMed] [Google Scholar]
- Kleemann W., McConnell H. M. Interactions of proteins and cholesterol with lipids in bilayer membranes. Biochim Biophys Acta. 1976 Jan 21;419(2):206–222. doi: 10.1016/0005-2736(76)90347-3. [DOI] [PubMed] [Google Scholar]
- Lecuyer H., Dervichian D. G. Structure of aqueous mixtures of lecithin and cholesterol. J Mol Biol. 1969 Oct 14;45(1):39–57. doi: 10.1016/0022-2836(69)90208-3. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., Barrow D. A., Hoechli M. Cholesterol-phosphatidylcholine interactions in multilamellar vesicles. Biochemistry. 1980 Apr 29;19(9):1943–1954. doi: 10.1021/bi00550a034. [DOI] [PubMed] [Google Scholar]
- Mabrey S., Mateo P. L., Sturtevant J. M. High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl- and dipalmitoylphosphatidylcholines. Biochemistry. 1978 Jun 13;17(12):2464–2468. doi: 10.1021/bi00605a034. [DOI] [PubMed] [Google Scholar]
- Melchior D. L., Scavitto F. J., Steim J. M. Dilatometry of dipalmitoyllecithin-cholesterol bilayers. Biochemistry. 1980 Oct 14;19(21):4828–4834. doi: 10.1021/bi00562a018. [DOI] [PubMed] [Google Scholar]
- Mortensen K., Pfeiffer W., Sackmann E., Knoll W. Structural properties of a phosphatidylcholine-cholesterol system as studied by small-angle neutron scattering: ripple structure and phase diagram. Biochim Biophys Acta. 1988 Nov 22;945(2):221–245. doi: 10.1016/0005-2736(88)90485-3. [DOI] [PubMed] [Google Scholar]
- Nagle J. F., Wilkinson D. A. Lecithin bilayers. Density measurement and molecular interactions. Biophys J. 1978 Aug;23(2):159–175. doi: 10.1016/S0006-3495(78)85441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opella S. J., Yesinowski J. P., Waugh J. S. Nuclear magnetic resonance description of molecular motion and phase separations of cholesterol in lecithin dispersions. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3812–3815. doi: 10.1073/pnas.73.11.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi T., Di Stefano M., Loiero M., Ravagnan G., Gratton E. Cholesterol modifies water concentration and dynamics in phospholipid bilayers: a fluorescence study using Laurdan probe. Biophys J. 1994 Mar;66(3 Pt 1):763–768. doi: 10.1016/s0006-3495(94)80852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presti F. T., Chan S. I. Cholesterol-phospholipid interaction in membranes. 1. Cholestane spin-label studies of phase behavior of cholesterol-phospholipid liposomes. Biochemistry. 1982 Aug 3;21(16):3821–3830. doi: 10.1021/bi00259a016. [DOI] [PubMed] [Google Scholar]
- Recktenwald D. J., McConnell H. M. Phase equilibria in binary mixtures of phosphatidylcholine and cholesterol. Biochemistry. 1981 Jul 21;20(15):4505–4510. doi: 10.1021/bi00518a042. [DOI] [PubMed] [Google Scholar]
- Sankaram M. B., Thompson T. E. Cholesterol-induced fluid-phase immiscibility in membranes. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8686–8690. doi: 10.1073/pnas.88.19.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaram M. B., Thompson T. E. Interaction of cholesterol with various glycerophospholipids and sphingomyelin. Biochemistry. 1990 Nov 27;29(47):10670–10675. doi: 10.1021/bi00499a014. [DOI] [PubMed] [Google Scholar]
- Sankaram M. B., Thompson T. E. Modulation of phospholipid acyl chain order by cholesterol. A solid-state 2H nuclear magnetic resonance study. Biochemistry. 1990 Nov 27;29(47):10676–10684. doi: 10.1021/bi00499a015. [DOI] [PubMed] [Google Scholar]
- Schroeder F., Barenholz Y., Gratton E., Thompson T. E. A fluorescence study of dehydroergosterol in phosphatidylcholine bilayer vesicles. Biochemistry. 1987 May 5;26(9):2441–2448. doi: 10.1021/bi00383a007. [DOI] [PubMed] [Google Scholar]
- Schroeder F., Jefferson J. R., Kier A. B., Knittel J., Scallen T. J., Wood W. G., Hapala I. Membrane cholesterol dynamics: cholesterol domains and kinetic pools. Proc Soc Exp Biol Med. 1991 Mar;196(3):235–252. doi: 10.3181/00379727-196-43185. [DOI] [PubMed] [Google Scholar]
- Somerharju P. J., Virtanen J. A., Eklund K. K., Vainio P., Kinnunen P. K. 1-Palmitoyl-2-pyrenedecanoyl glycerophospholipids as membrane probes: evidence for regular distribution in liquid-crystalline phosphatidylcholine bilayers. Biochemistry. 1985 May 21;24(11):2773–2781. doi: 10.1021/bi00332a027. [DOI] [PubMed] [Google Scholar]
- Tampé R., von Lukas A., Galla H. J. Glycophorin-induced cholesterol-phospholipid domains in dimyristoylphosphatidylcholine bilayer vesicles. Biochemistry. 1991 May 21;30(20):4909–4916. doi: 10.1021/bi00234a011. [DOI] [PubMed] [Google Scholar]
- Tang D., Chong P. L. E/M dips. Evidence for lipids regularly distributed into hexagonal super-lattices in pyrene-PC/DMPC binary mixtures at specific concentrations. Biophys J. 1992 Oct;63(4):903–910. doi: 10.1016/S0006-3495(92)81672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tson- T. Y. Transport of 8-anilino-1-naphthalenesulfonate as a probe of the effect of cholesterol on the phospholipid bilayer structures. Biochemistry. 1975 Dec 16;14(25):5415–5417. doi: 10.1021/bi00696a005. [DOI] [PubMed] [Google Scholar]
- Vist M. R., Davis J. H. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 1990 Jan 16;29(2):451–464. doi: 10.1021/bi00454a021. [DOI] [PubMed] [Google Scholar]
- Yeager M. D., Feigenson G. W. Fluorescence quenching in model membranes: phospholipid acyl chain distributions around small fluorophores. Biochemistry. 1990 May 8;29(18):4380–4392. doi: 10.1021/bi00470a018. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L. Cholesterol and the cell membrane. Biochim Biophys Acta. 1985 Dec 9;822(3-4):267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]



